Abstract
Purpose.
Oxidative stress and inflammation have key roles in the light damage (LD) model of retinal degeneration as well as in age-related macular degeneration (AMD). We sought to determine if lipoic acid (LA), an antioxidant and iron chelator, protects the retina against LD.
Methods.
Balb/c mice were treated with LA or control saline via intraperitoneal injection, and then were placed in constant cool white light-emitting diode (LED) light (10,000 lux) for 4 hours. Retinas were evaluated at several time points after LD. Photoreceptor apoptosis was assessed using the TUNEL assay. Retinal function was analyzed via electroretinography (ERG). Retinal degeneration was assessed after LD by optical coherence tomography (OCT), TUNEL analysis, and histology. The mRNAs of several oxidative stress, inflammation, and iron-related genes were quantified by quantitative PCR (qPCR).
Results.
The LD resulted in substantial photoreceptor-specific cell death. Dosing with LA protected photoreceptors, decreasing the numbers of TUNEL-positive photoreceptors and increasing the number of surviving photoreceptors. The retinal mRNA levels of genes indicating oxidative stress, inflammation, and iron accumulation were lower following LD in mice treated with LA than in control mice. The ERG analysis demonstrated functional protection by LA.
Conclusions.
Systemic LA is protective against light-induced retinal degeneration. Since this agent already has proven protective in other retinal degeneration models, and is safe and protective against diabetic neuropathy in patients, it is worthy of consideration for a human clinical trial against retinal degeneration or AMD.
Keywords: lipoic acid, oxidative stress, light damage, retinal degeneration
The paper shows for the first time to our knowledge that the antioxidant/iron chelator lipoic acid, a widely-available nutritional supplement, protects against light induced retinal degeneration.
Introduction
Age-related macular degeneration (AMD) is a complex, degenerative, and progressive eye disease that can result in severe loss of central vision. It is one of the major causes of blindness in developed countries. The contribution of oxidative damage to AMD has received considerable attention.1 Light damage (LD) in rodents has been used for over 40 years as a model of photo-oxidative stress-induced retinal degeneration, and has been used to test antioxidants for retinal protection.2,3
One source of oxidative stress in the retina is iron, which accumulates in AMD.4 The possibility that iron contributes to AMD pathogenesis is supported by the finding of AMD-like retinal degeneration in mice and humans with hereditary retinal iron overload.5 The complement cascade, which can be activated by oxidative stress,6 has been strongly linked to AMD by genetic and histologic studies.7,8
Lipoic acid (LA), a naturally occurring enzyme cofactor, antioxidant, and iron chelator, has been used as a dietary supplement to treat diabetics with peripheral neuropathy in Germany for years.9 This supplement also may help protect the brain as it can pass easily across the blood–brain barrier.10 In a number of human and animal studies, LA can protect against ocular and central nervous system (CNS) insults. Preliminary studies suggest it may help treat glaucoma.11 It can improve measures of memory in aged mice with age-associated cognitive decline.12–15 In diabetic animal models, it demonstrated antioxidant activity, decreased the apoptosis of retinal capillary cells, and suppressed the expression of vascular endothelial growth factor.16–28 It also can protect the conjunctiva and cornea against ultraviolet (UV) explosure29 and alter the metabolism of reactive nitrogen species with some benefit for dry eye.30 It protects against retinal ischemia and high IOP in animal models.31–33 In retinitis pigmentosa mouse models, oxidative stress is thought to have a significant role. However, LA, either alone, or in combination with other antioxidants, can diminish the oxidative stress and photoreceptor death in these models.34–36 LA also can inhibit cataract progression in starch-induced diabetic rats, and change iron uptake and storage in lens epithelial cells.37,38 It can protect cultured human fetal RPE cells against a chemical oxidant.39
The goal of the present study was to determine whether LA also might protect against retinal oxidative stress, inflammation, and photoreceptor cell death induced by photo-oxidative (light) damage in wild-type mice. A prior study convincingly showed that treatment with a combination of antioxidants can protect retinas from acute oxidative damage caused by LD.40 The combination of LA with ascorbic acid and manganese [III] tetrakis (4-benzoic acid) porphyrin (MnTBAP) was found protective, but the effect of LA alone was not studied.
We examined light damaged retinas to determine whether systemic administration of LA could protect against light-induced retinal degeneration. We also studied the effect of LA on mRNA levels of genes upregulated by retinal oxidative stress, inflammation, and iron overload.
Materials and Methods
Animals
Adult male albino BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and exposed to LD at age 10 weeks. All mice were maintained in a temperature-controlled room at 21°C to 23°C with a 12-hour:12-hour light-dark photoperiod. Experimental procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmology and Vision Research. All protocols were approved by the animal care review board of the University of Pennsylvania.
LA Administration in Mice
The α-LA was obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA). The LA was dissolved in 0.9% sodium chloride injection USP from B. Braun Medical (Irvine, CA, USA), and brought to a final pH of approximately 7.4 by addition of NaOH. The LA was injected intraperitoneally (IP) at a dose of 100 mg/kg. An equal volume of a pH-matched 0.9% sodium chloride was used as a vehicle-control. Mice received daily IP injections of LA (100 mg/kg) or saline 7 days before LD and also after LD until they were killed.
LD Paradigm
Mice were exposed to 10,000 lux of cool white LED light in a well-ventilated room continuously for 4 hours as we have described previously41 from 5 PM to 9 PM. After the exposure to light, mice either were sacrificed or placed in the normal light/dark cycle for 24 hours or 7 days. Eyes were enucleated immediately after sacrifice 24 hours after LD for analysis by quantitative PCR (qPCR), immunofluorescence, and TUNEL, and at 7 days following LD for morphologic analysis.
TUNEL Analysis
Eyes enucleated 24 hours following LD were immersion fixed in 4% paraformaldehyde for 10 minutes. Cryosections were cut in the sagittal plane through the optic nerve head (ONH). The fluorescein-conjugated TUNEL in situ cell death detection kit (Roche, Mannheim, Germany) was used for these sections, followed by fluorescence microcopy using a Nikon Eclipse TE-300 microscope (Nikon, Inc., Melville, NY, USA). For each retina, the number of TUNEL-positive photoreceptors was counted on both sides of the ONH (n = 3 sections per retina). The number of TUNEL-positive photoreceptors per retina was compared within LA-treated and untreated controls with GraphPad Prism 5.03 (San Diego, CA, USA).
Morphologic Analysis
Eyes enucleated 7 days following LD were immersion fixed in 2% paraformaldehyde/2% glutaraldehyde overnight, and eye cups were made by dissecting away the cornea and lens. The tissues then were dehydrated in increasing concentrations of ethanol, infiltrated overnight, and embedded the next day in plastic (JB4; Polysciences, Inc., Warrington, PA, USA). For standard histology 3-μm thick plastic sections were cut in the sagittal plane and were toluidine blue–stained by incubation of the sections in 1% toluidine blue O and 1% sodium tetraborate decahydrate (Sigma-Aldrich Corp.) for 5 seconds. Stained sections were observed and photographed using brightfield illumination (model TE300; Nikon, Inc.).42 The number of nuclei per column of outer nuclear layer (ONL) photoreceptors was counted in triplicate at 200-μm intervals from the ONH to 2000 μm from the ONH, using image analysis software (ImagePro Plus4.1; Media Cybernetics, Rockville, MD, USA) to calculate distances from manually set lengths.
Optical Coherence Tomography (OCT) Imaging
One day after LD or 7 days after LD, mice were anesthetized as described previously. One drop of 1% tropicamide ophthalmic solution USP was administrated to eyes before examination. We performed OCT using a Bioptigen imager (Durham, NC, USA). One horizontal line scan was saved, corresponding to the region of maximal LD, approximately one disc diameter above the superior edge of the optic disc. Corresponding ONL thicknesses for LA- and saline-treated mice eyes were compared at the same location.
Electroretinography (ERG)
The ERG recordings followed procedures described previously.43,44 In brief, mice were dark-adapted overnight and then anesthetized with a cocktail containing (in mg/kg body weight): 25 ketamine, 10 xylazine, and 1000 urethane. In each mouse, the pupils were dilated with 1% tropicamide saline solution (Mydriacil; Alconox, New York, NY, USA) and the mouse was placed on a stage maintained at 37°C. Two miniature cups made of UV-transparent plastic with embedded platinum wires serving as recording electrodes were placed in electrical contact with the corneas. A platinum wire loop placed in the mouth served as the reference and ground electrode. Then, ERGs were recorded (Espion Electrophysiology System; Diagnosys LLC, Lowell, MA, USA). The apparatus was modified by the manufacturer for experiments with mice by substituting LEDs with emission maximum at 365 nm for standard blue ones. A stage with the mouse was positioned in such a way that the mouse's head was located inside the stimulator (ColorDome; Diagnosys LLC), thus ensuring full-field uniform illumination. Methods for light stimulation and calibration of light stimuli have been described previously.43 The a- and b-wave amplitudes are reported for saturating light stimuli.
Real-Time qPCR
Gene expression was analyzed in the neurosensory retina (NR) and RPE samples obtained from LA- and control saline-treated mice after LD at the indicated time points by quantitative RT-PCR as we have described.42 Probes used were rhodopsin (Rho, Mm00520345_m1), retinal pigment epithelium 65 (Rpe65, Mm00504133_m1), heme oxygenase 1 (Hmox1, Mm00516005_m1), ceruloplasmin (Cp, Mm00432654_1), catalase (Cat, Mm00437992_m1), superoxide dismutase 1 (Sod1, Mm01700393_g1), superoxide dismutase 2 (Sod2, Mm01313000_m1), glutathione peroxidase 1 (Gpx1, Mm00656767_g1), glutathione peroxidase 4 (Gpx4, Mm00515041_m1), ferritin light chain 1 (Ftl1, Mm03030144_g1), transferrin receptor (Tfrc, Mm00441941_m1), allograft inflammatory factor 1 (Aif1, Mm00479862_g1), EGF-like module containing, mucin-like, hormone receptor-like sequence 1 (Emr1, Mm00802529_m1). Eukaryotic 18s rRNA (Hs99999901_s1) was used as an endogenous control. Real-time qPCR (Taqman; Applied Biosystems, Carlsbad, CA, USA) was performed on a sequence detection system (Prism model7500; Applied Biosystems) using the ΔΔCT method, which provides normalized expression values. The amount of target mRNA was compared among the groups of interest. All reactions were performed in biological (seven mice) and technical (three qPCR replicates per biological sample) triplicates.
Statistical Analysis
The mean and the standard error were calculated for each comparison group. Statistical analysis was performed using the Student's two group, 2-sided t-test. P < 0.05 was considered statistically significant. For multiple comparisons with Rho in the NR and Rpe65 in the RPE at different time points (see Fig. 5) we used 1-way ANOVA with post hoc pairwise comparisons using the Tukey method to correct for multiple comparisons. All statistical analysis was performed with GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA).
Figure 5.
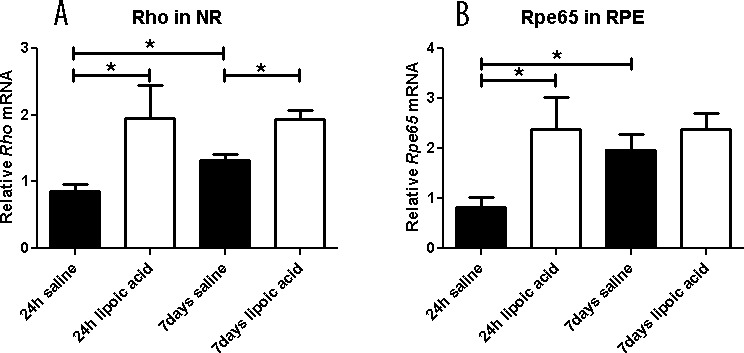
Graphs showing relative mRNA levels measured by qPCR. The LA treatment results in higher Rho mRNA level in NR at 1 and 7 days after LD (A) and RPE65 mRNA levels in RPE at 1 day after LD (B). In the saline group Rho mRNA levels in NR show recovery comparing 24 hours and 7 days. Similarly, in the saline group RPE65 mRNA levels in RPE show recovery comparing 24 hours and 7 days. Numbers represent mean values (±SEM).
Results
LA Diminishes the Number of TUNEL-Positive Photoreceptors Following LD
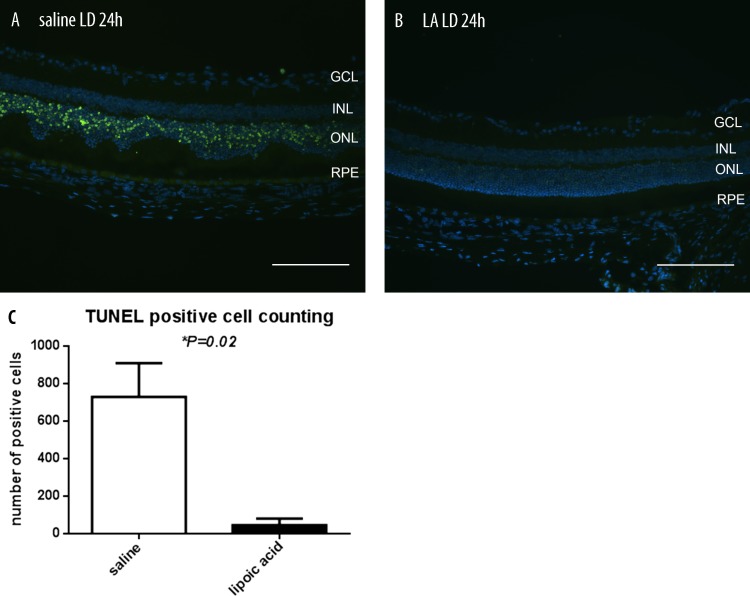
As an initial assessment of the photoreceptor-protective activity of LA, we quantified the number of TUNEL-positive photoreceptors in LD mice with or without LA treatment. The TUNEL reaction detects DNA fragmentation, a step in the apoptotic program. At 24 hours following LD, many TUNEL-positive photoreceptors were present in the ONL of LD retinas without LA treatment, but only a few TUNEL-positive photoreceptors were present in retinas of LA-treated mice (Figs. 1A, 1B). To quantify the difference in cell death, the number of TUNEL-positive photoreceptors was counted in entire sagittal sections crossing through the optic nerve head (n = 3). The LA-treated mice had significantly fewer TUNEL-positive photoreceptors than control (Fig. 1C).
Figure 1.
Fluorescence photomicrographs showing TUNEL label in mouse retinas. There are more TUNEL-positive photoreceptor nuclei (green) 24 hours following LD in mice treated with saline (A) compared with mice treated with LA (B). Histogram comparing numbers of TUNEL-positive photoreceptors from saline + LD (n = 3) and LA + LD (n = 3) mice. The histogram displays the mean (±SEM) of total numbers of TUNEL-positive photoreceptors counted in whole sections (C). *Significant difference (P < 0.05). GCL, ganglion cell layer; INL, inner nuclear layer. Scale bars in (A) and (B) represents 100 μm.
Preservation of Photoreceptor Nuclei by LA
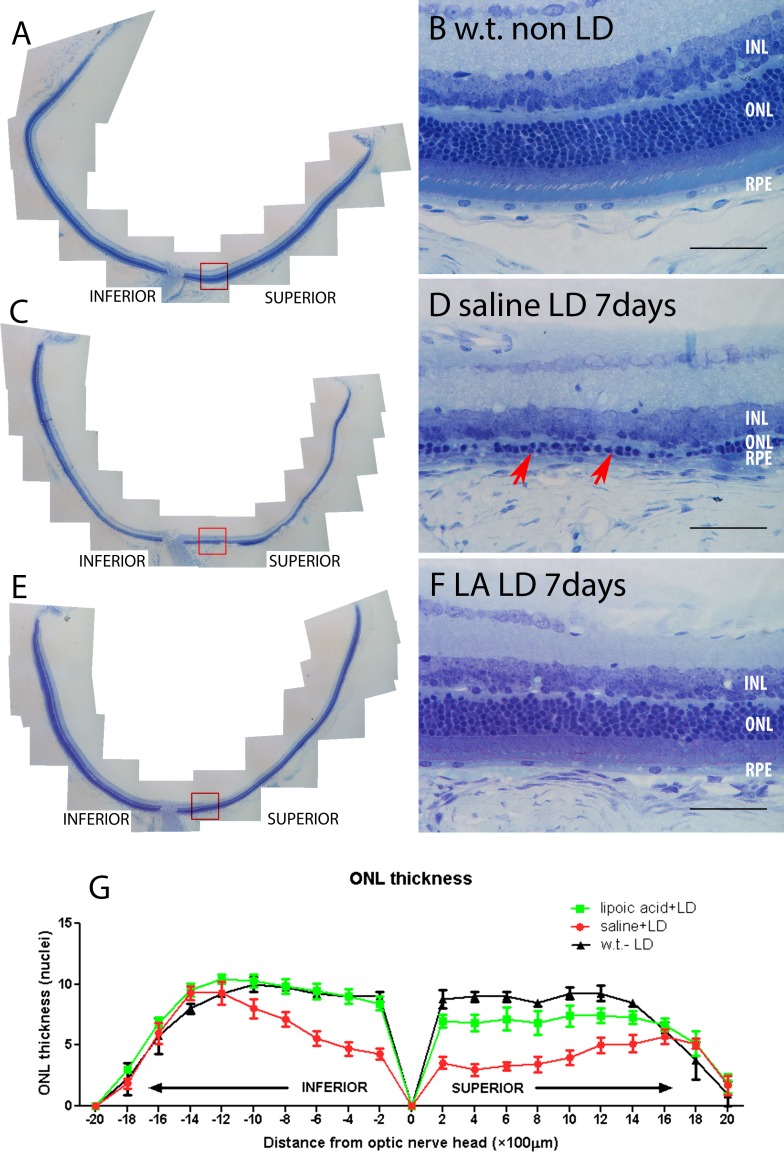
Since the decrease in the number of TUNEL-positive photoreceptors after LA treatment implies a prosurvival effect, we sought to confirm this by determining whether the reduction of TUNEL-positive cells corresponded to preservation of photoreceptors. Morphologic analysis was performed 7 days following LD and the numbers of photoreceptor nuclei were counted in sagittal sections through the ONH (n = 3). The LA provided significant preservation of photoreceptors. The mice treated with LA had a thicker ONL, and better preserved photoreceptor inner and outer segments compared to mice without LA treatment (Figs. 2C–F). The most severely damaged part of the retina was located centrally near the ONH, especially on the superior side, with relative preservation of the peripheral retina (Fig. 2G).
Figure 2.
Photomicrographs of plastic sections of mouse retinas and plots showing morphologic protection 7 days following LD in LA + LD retinas. Retinas from mice receiving no LD (A, B), saline + LD (C, D), LA + LD (E, F). (B, D, F) Higher magnification views equidistant from the optic nerve and indicated by the red box in (A), (C), and (E), respectively. Red arrows in (D) show the thinning ONL. Plot of the thickness of the ONL 7 days after LD, measured in numbers of photoreceptor nuclei per column (G). Measurements are made in triplicate every 200 μm from the ONH. No LD (n = 4, black), saline + LD (n = 7, red), LA + LD (n = 7, green). Numbers represent mean values (±SEM). Scale bars: 100 μm (B, D, F).
OCT In Vivo Retinal Imaging 24 Hours and 7 Days After LD
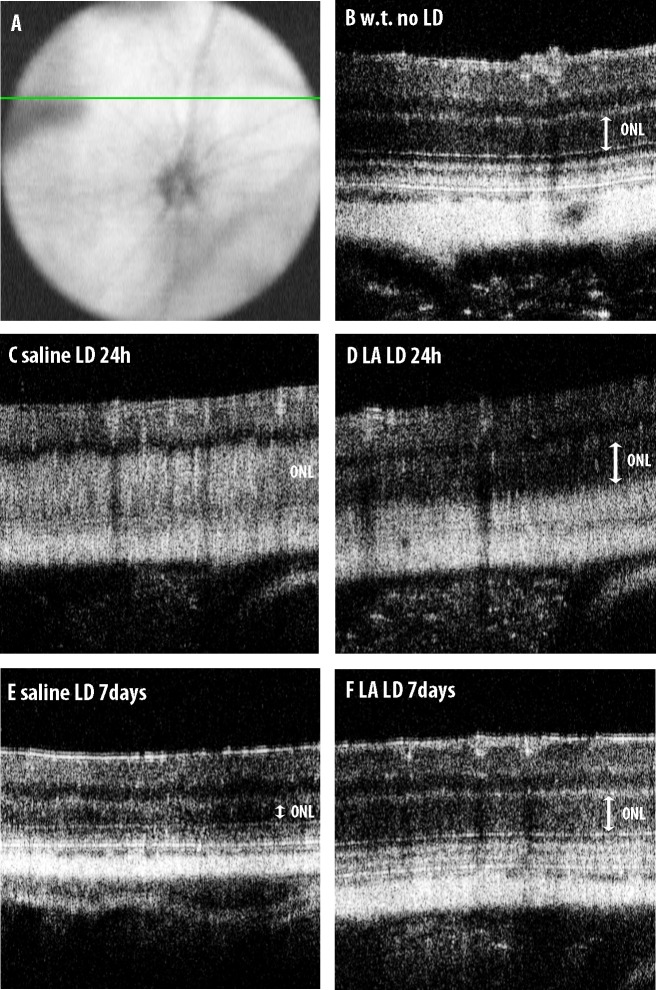
We used OCT imaging to obtain in vivo images showing the ONL thickness. In mice not exposed to LD, a line scan superior to the ONH (position indicated in Fig. 3A) showed a region of low reflectivity corresponding to the ONL (Fig. 3B). At 1 day after LD, OCT images showed increased reflectivity in the ONL in mice treated with control saline and LD. The entire region from the outer plexiform layer down to the RPE is highly reflective. This reflectivity most likely represents a change in the structure of photoreceptor nuclei or cell bodies (Fig. 3C). The LA-treated mice had less of this abnormal ONL reflectivity; the ONL remains visible as a low reflectivity band (Fig. 3D). Control mice receiving saline showed severe ONL thinning 7 days after LD (Fig. 3E). The LA-treated mice retained a thicker ONL (Fig. 3F).
Figure 3.
The OCT images of mouse retinas. (A) Indicates the position of the line scan superior to the optic nerve. Compared to mice receiving no light damage (B), mice receiving IP saline showed increased reflectivity in the ONL 1 day after LD (C). Mice receiving IP LA had a more normal-appearing, low reflectivity ONL 1 day after LD (D). The mice receiving saline showed severe ONL thinning 7 days after LD (E), whereas mice receiving LA did not (F).
Retinal Function Assessed 7 Days After LD by ERG
For functional analysis, full-field ERG responses of control saline– or LA-treated mice were compared 7 days after LD. Maximum amplitude cone-b rod-a and rod-b waves are shown (Fig. 4). All three wave amplitudes were significantly higher in the LA-treated mice compared to saline-treated controls.
Figure 4.
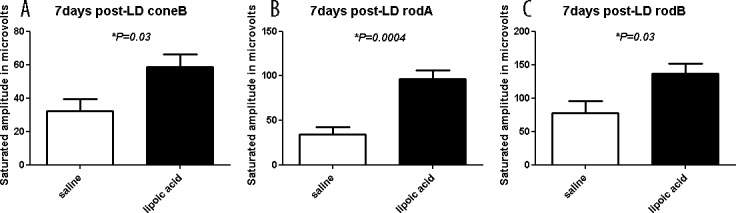
Full-field ERG responses of control saline– or LA-treated mice 7 days after light exposure. Maximum amplitude ERG responses are reported. The LA-treated mice had significantly higher amplitudes for all three wave types compared to control saline treated-animals. Numbers represent mean values (±SEM).
Relative Rho and Rpe65 mRNA Levels at 24 Hours and 7 Days After LD
The relative quantities of mRNAs encoded by a rod-specific gene, rod opsin (Rho) and an RPE-specific gene Rpe65 were used as a measure of the status of these cells. Relative to saline-treated controls, LA-treated mice had higher Rho mRNA levels in the neural retina (Fig. 5A) and Rpe65 mRNA levels in RPE (Fig. 5B). The LA treatment led to higher Rho mRNA levels in neural retina at 24-hour and 7-day time points. The LA treatment also led to higher Rpe65 mRNA levels in RPE at 24 hours. This difference is no longer apparent at 7 days, as the Rpe65 levels in the control saline–treated group had recovered relative to the control group at 1 day.
LA Inhibited the LD-Induction of Oxidative Stress Markers
To investigate the effect of LA on antioxidant gene expression, we performed qPCR with Hmox1, Cp, Cat, Sod1, Sod2, Gpx1, and Gpx4 primers. Within NR samples, LA treatment led to lower levels of Cp, Cat, Sod1, and Gpx1 mRNA after the LD (Figs. 6B–D, 6F). Within enzymatically isolated RPE cells, the treatment with LA led to less upregulation of Hmox1, Gpx4, and Sod2 mRNA compared to saline controls (Figs. 6G, 6H, 6K).
Figure 6.
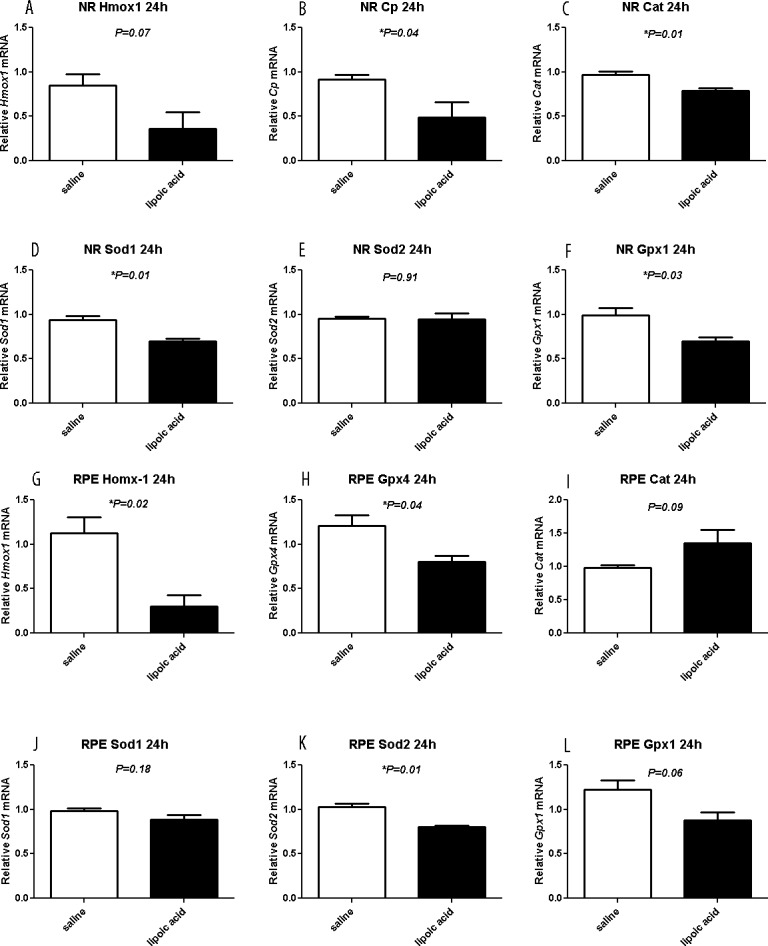
Graphs showing relative mRNA levels measured by qPCR. The LA treatment of LD mice results in lower mRNA levels, compared to saline treatment mice, of oxidative stress markers Cp, Cat, Sod1, and Gpx1 in neural retina (B, C, D, F) and Hmox-1, Gpx4, and Sod2 in RPE (G, H, K). Saline (n = 4) and LA (n = 3) retinas are displayed as mean values (±SEM). *Significant difference (P < 0.05).
LA Inhibited LD-Induction of Inflammation Gene Markers
Also, LA diminished upregulation of inflammation markers. Gene Aif1, which encodes the protein Iba-1, is expressed in microglia, and is upregulated in activated microglia.45,46 Another macrophage/microglia marker is F4/80. Levels of Aif1 and F4/80 mRNA in neural retina (Figs. 7A, 7B) were significantly lower in the LA-treated group than the saline-treated group 7 days after LD.
Figure 7.
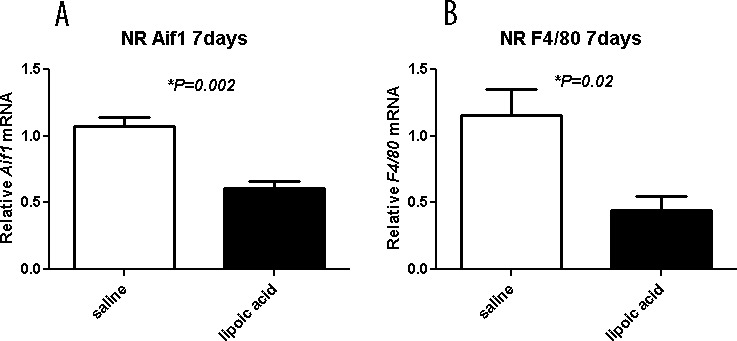
Graphs showing relative mRNA levels measured by qPCR. The LA decreases inflammation markers. Levels of Aif1 and F4/80 mRNA in neural retina are significantly diminished by the LA treatment 7 days after light exposure. Neural retina mRNA levels for the indicated genes in saline- (n = 4) and LA- (n = 4) treated mice are displayed as mean values (±SEM). *Significant difference (P < 0.05).
LA Diminishes Upregulation of Ferritin
In our previous LD studies, we found that L-ferritin was upregulated.47 Ferritin and transferrin receptor levels can be used as an indirect measure of iron levels. Treatment with LA results in lower L-ferritin mRNA levels in neural retina at 1 (Fig. 8A) and 7 (Fig. 8B) days. The Tfrc mRNA levels in the NR of LA-treated mice showed a trend toward higher levels at 24 hours (Fig. 8C) and 7 days (Fig. 8D) compared to saline-treated mice. Together, these results suggested that labile iron levels are lower in the NR of LD mice-treated with the iron chelator LA.
Figure 8.
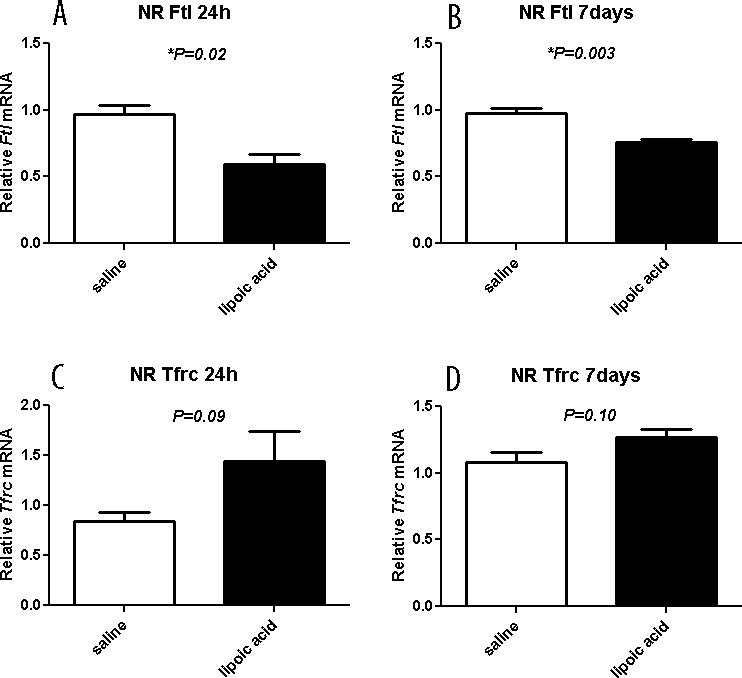
Graphs showing relative mRNA levels of iron handling genes measured by qPCR. The LA results in lower L-ferritin mRNA levels in neural retina at 24 hours (A) and 7 days (B) after LD. The Tfrc mRNA levels in neural retina of LA-treated mice at 24 hours (C) and 7 days (D) are not significantly different in saline- versus LA-treated mice. Saline (n = 4) and LA (n = 3) retinas are displayed as mean values (±SEM). *Significant difference (P < 0.05).
Discussion
In this study, we investigated whether systemic administration of LA, an antioxidant and iron chelator, is an effective prevention for light-induced retinal degeneration. The LA reduced the number of TUNEL-positive photoreceptors induced by LD. Morphological analysis 7 days after LD demonstrated that LA treatment protected against ONL thinning and preserved photoreceptor morphology relative to saline control mice. Furthermore, the genes upregulated by oxidative stress, inflammation, and iron overload were expressed at lower levels in the LA-treated mice. Retinal function also was protected by LA, as indicated by electroretinography. These results suggested that LA may protect the retina from LD through multiple mechanisms: antioxidant, anti-inflammatory, and iron chelation to prevent iron toxicity. The Lipoic acid has been found to inhibit iron-mediated oxidative damage in the test tube48,49 and to inhibit excess iron accumulation in animal models.50,51 Our results with Tfrc and Ftl mRNA levels support a role for LA in controlling iron accumulation in vivo.
The neuroprotective effect of LA may result, in part, from diminished oxidative stress secondary to reduced iron levels in the retina, We have shown previously that an iron chelator can ameliorate retinal LD,52 but the protection by LA appears to be more complete. Our data showing lower levels of microglia/macrophage-specific mRNA levels in LA-treated than control mice 7 days after light damage suggested that LA is acting as an anti-inflammatory agent and/or is diminishing retinal damage that secondarily incites inflammation. In addition, LA could be affecting signaling pathways upstream of antioxidant and anti-inflammatory genes.
In the diabetic neuropathy clinical trials the oral dose of LA varied among trials (100–1800 mg/d and IV dose varied from 600–1200 mg/d). In nondiabetic neuropathy trials, the dose of LA ranged from 200 to 800 mg/d. In this study, we used a 100 mg/kg dose in mice, which, based on allometric scaling,53 translated to approximately 10 mg/kg in humans. This dose is considered safe in human clinical trials. In this experiment, we used a mixture of R-LA and S-LA. In rats, R-LA was more effective than racemic LA and S-LA in preventing cataracts.54 However, the published studies of LA supplementation in humans have used racemic LA. It is not clear whether R-LA supplements would more effective than racemic LA supplements in humans.
Our study provides evidence that LA can rescue LD-induced photoreceptor death in a mouse model. Based on these data along with the recognized safety and efficacy of LA in clinical trials for diabetic neuropathy, it seems reasonable to consider testing LA in a clinical trial for AMD.
Acknowledgments
Supported by the Pennsylvania Lions Eye Resarch and Sight Conservation Foundation, National Institutes of Health (NIH; Bethesda, MD, USA) Grant EY015240, Research to Prevent Blindness, the FM Kirby Foundation, the Paul and Evanina Bell Mackall Foundation Trust, and a gift in memory of Dr. Lee F. Mauger.
Disclosure: L. Zhao, None; C. Wang, None; D. Song, None; Y. Li, None; Y. Song, None; G. Su, None; J.L. Dunaief, None
References
- 1. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004; 122: 598–614 [DOI] [PubMed] [Google Scholar]
- 2. Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966; 5: 450–473 [PubMed] [Google Scholar]
- 3. Shahinfar S, Edward DP, Tso MO. A pathologic study of photoreceptor cell death in retinal photic injury. Curr Eye Res. 1991; 10: 47–59 [DOI] [PubMed] [Google Scholar]
- 4. Wong RW, Richa DC, Hahn P, Green WR, Dunaief JL. Iron toxicity as a potential factor in AMD. Retina Phila Pa. 2007; 27: 997–1003 [DOI] [PubMed] [Google Scholar]
- 5. He X, Hahn P, Iacovelli J, et al. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 2007; 26: 649–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thurman JM, Renner B, Kunchithapautham K, et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009; 284: 16939–16947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog Retin Eye Res. 2010; 29: 95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer's A?-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002; 99: 11830–11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ziegler D, Reljanovic M, Mehnert H, Gries FA. Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Exp Clin Endocrinol Diabetes. 1999; 107: 421–430 [DOI] [PubMed] [Google Scholar]
- 10. Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001; 40: 959–975 [DOI] [PubMed] [Google Scholar]
- 11. Filina AA, Davydova NG, Endrikhovskiĭ SN, Shamshinova AM. [Lipoic acid as a means of metabolic therapy of open-angle glaucoma]. Vestn Oftalmol. 1995; 111: 6–8 [PubMed] [Google Scholar]
- 12. Farr SA, Poon HF, Dogrukol-Ak D, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003; 84: 1173–1183 [DOI] [PubMed] [Google Scholar]
- 13. Quinn JF, Bussiere JR, Hammond RS, et al. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007; 28: 213–225 [DOI] [PubMed] [Google Scholar]
- 14. Shenk JC, Liu J, Fischbach K, et al. The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer's disease. J Neurol Sci. 2009; 283: 199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stoll S, Hartmann H, Cohen SA, Müller WE. The potent free radical scavenger alpha-lipoic acid improves memory in aged mice: putative relationship to NMDA receptor deficits. Pharmacol Biochem Behav. 1993; 46: 799–805 [DOI] [PubMed] [Google Scholar]
- 16. Bucolo C, Marrazzo G, Platania CBM, Drago F, Leggio GM, Salomone S. Fortified extract of red berry, Ginkgo biloba, and white willow bark in experimental early diabetic retinopathy. J Diabetes Res. 2013; 2013: 432695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnsen-Soriano S, Garcia-Pous M, Arnal E, et al. Early lipoic acid intake protects retina of diabetic mice. Free Radic Res. 2008; 42: 613–617 [DOI] [PubMed] [Google Scholar]
- 18. Berkowitz BA, Roberts R, Stemmler A, Luan H, Gradianu M. Impaired apparent ion demand in experimental diabetic retinopathy: correction by lipoic Acid. Invest Ophthalmol Vis Sci. 2007; 48: 4753–4758 [DOI] [PubMed] [Google Scholar]
- 19. Dene BA, Maritim AC, Sanders RA, Watkins JB., III Effects of antioxidant treatment on normal and diabetic rat retinal enzyme activities. J Ocul Pharmacol Ther. 2005; 21: 28–35 [DOI] [PubMed] [Google Scholar]
- 20. Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004; 53: 3233–3238 [DOI] [PubMed] [Google Scholar]
- 21. Yorek MA, Coppey LJ, Gellett JS, Davidson EP, Lund DD. Effect of fidarestat and alpha-lipoic acid on diabetes-induced epineurial arteriole vascular dysfunction. Exp Diabesity Res. 2004; 5: 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Obrosova IG, Fathallah L, Greene DA. Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of DL-alpha-lipoic acid. Eur J Pharmacol. 2000; 398: 139–146 [DOI] [PubMed] [Google Scholar]
- 23. Kowluru RA, Zhong Q, Santos JM, Thandampallayam M, Putt D, Gierhart DL. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr Metab. 2014; 11: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SG, Lee CG, Yun IH, Hur DY, Yang JW, Kim HW. Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin Experiment Ophthalmol. 2012; 40: e47–e57 [DOI] [PubMed] [Google Scholar]
- 25. Lin J, Bierhaus A, Bugert P, et al. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia. 2006; 49: 1089–1096 [DOI] [PubMed] [Google Scholar]
- 26. Obrosova IG, Minchenko AG, Marinescu V, et al. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001; 44: 1102–1110 [DOI] [PubMed] [Google Scholar]
- 27. Santos JM, Kowluru RA. Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci. 2011; 52: 8791–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kowluru RA, Atasi L, Ho Y-S. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006; 47: 1594–1599 [DOI] [PubMed] [Google Scholar]
- 29. Chen B-Y, Lin DP-C, Chang L-S, et al. Dietary α-Lipoic acid prevents UVB-induced corneal and conjunctival degeneration through multiple effects. Invest Ophthalmol Vis Sci. 2013; 54: 6757–6766 [DOI] [PubMed] [Google Scholar]
- 30. Andrade AS, Salomon TB, Behling CS, et al. Alpha-lipoic acid restores tear production in an animal model of dry eye. Exp Eye Res. 2014; 120: 1–9 [DOI] [PubMed] [Google Scholar]
- 31. Inman DM, Lambert WS, Calkins DJ, Horner PJ. α-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PloS One. 2013; 8: e65389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nebbioso M, Scarsella G, Tafani M, Pescosolido N. Mechanisms of ocular neuroprotection by antioxidant molecules in animal models. J Biol Regul Homeost Agents. 2013; 27: 197–209 [PubMed] [Google Scholar]
- 33. Block F, Schwarz M. Effects of antioxidants on ischemic retinal dysfunction. Exp Eye Res. 1997; 64: 559–564 [DOI] [PubMed] [Google Scholar]
- 34. Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007; 213: 809–815 [DOI] [PubMed] [Google Scholar]
- 35. Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006; 103: 11300–11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanz MM, Johnson LE, Ahuja S, Ekström PAR, Romero J, van Veen T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience. 2007; 145: 1120–1129 [DOI] [PubMed] [Google Scholar]
- 37. Goralska M, Dackor R, Holley B, McGahan MC. Alpha lipoic acid changes iron uptake and storage in lens epithelial cells. Exp Eye Res. 2003; 76: 241–248 [DOI] [PubMed] [Google Scholar]
- 38. Borenshtein D, Ofri R, Werman M, et al. Cataract development in diabetic sand rats treated with alpha-lipoic acid and its gamma-linolenic acid conjugate. Diabetes Metab Res Rev. 2001; 17: 44–50 [DOI] [PubMed] [Google Scholar]
- 39. Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R)-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2005; 46: 4302–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maeda T, Maeda A, Matosky M, et al. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci. 2009; 50: 4917–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen L, Wu W, Dentchev T, et al. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004; 79: 239–247 [DOI] [PubMed] [Google Scholar]
- 42. Hadziahmetovic M, Song Y, Wolkow N, et al. The oral iron chelator deferiprone protects against iron overload–induced retinal degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN. UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999; 19: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lyubarsky AL, Lem J, Chen J, Falsini B, Iannaccone A, Pugh EN Jr. Functionally rodless mice: transgenic models for the investigation of cone function in retinal disease and therapy. Vision Res. 2002; 42: 401–415 [DOI] [PubMed] [Google Scholar]
- 45. Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. J Pers Soc Psychol. 1998; 75: 887–900 [DOI] [PubMed] [Google Scholar]
- 46. Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002; 40: 164–174 [DOI] [PubMed] [Google Scholar]
- 47. Hadziahmetovic M, Kumar U, Song Y, et al. Microarray analysis of murine retinal light damage reveals changes in iron regulatory, complement, and antioxidant genes in the neurosensory retina and isolated RPE. Invest Ophthalmol Vis Sci. 2012; 53: 5231–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol. 1995; 50: 123–126 [DOI] [PubMed] [Google Scholar]
- 49. Suh JH, Zhu B-Z, deSzoeke E, Frei B, Hagen TM. Dihydrolipoic acid lowers the redox activity of transition metal ions but does not remove them from the active site of enzymes. Redox Rep Commun Free Radic Res. 2004; 9: 57–61 [DOI] [PubMed] [Google Scholar]
- 50. Yamamoto H, Watanabe T, Mizuno H, et al. The antioxidant effect of DL-alpha-lipoic acid on copper-induced acute hepatitis in Long-Evans Cinnamon (LEC) rats. Free Radic Res. 2001; 34: 69–80 [DOI] [PubMed] [Google Scholar]
- 51. Suh JH, Moreau R, Heath S-HD, Hagen TM. Dietary supplementation with (R)-alpha-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep Commun Free Radic Res. 2005; 10: 52–60 [DOI] [PubMed] [Google Scholar]
- 52. Song D, Song Y, Hadziahmetovic M, Zhong Y, Dunaief JL. Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina. Free Radic Biol Med. 2012; 53: 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tang H, Mayersohn MA. Novel model for prediction of human drug clearance by allometric scaling. Drug Metab Dispos. 2005; 33: 1297–1303 [DOI] [PubMed] [Google Scholar]
- 54. Maitra I, Serbinova E, Tritschler HJ, Packer L. Stereospecific effects of R-lipoic acid on buthionine sulfoximine-induced cataract formation in newborn rats. Biochem Biophys Res Commun. 1996; 221: 422–429 [DOI] [PubMed] [Google Scholar]