Abstract
Vitamin D deficiency may increase the risk for metabolic syndrome. We determined the relationship of serum 25-hydroxyvitamin D (25(OH)D) with metabolic syndrome components in obese adolescent females and assessed whether vitamin D treatment corrects metabolic disturbances. Eighty postmenarchal adolescents (53 African American (AA) and 27 Caucasian American (CA)) were evaluated with blood pressures and fasting measurements of serum 25(OH)D, lipid profile, C-reactive protein, alanine transaminases (ALTs) and aspartate transaminases followed by an oral glucose tolerance test. A subgroup (n = 14) of vitamin D deficient subjects were re-evaluated following vitamin D treatment. Among all subjects, 25(OH)D was inversely associated with fasting glucose (r = −0.28, P = 0.02) and positively associated with low-density lipoprotein (LDL) cholesterol (r = 0.31, P = 0.008), independent of race and BMI. In analyses by race, adjusted for BMI, 25(OH)D was inversely associated with fasting insulin in CA (r = −0.42, P = 0.03) but not AA (r = 0.11, P = 0.43) whereas 25(OH)D was positively associated with ALT in AA, but not CA (r = 0.29, P = 0.04 vs. r = −0.21, P = 0.32). Fasting glucose improved in vitamin D treated subgroup (from 89.07 ± 8.3 mg/dl to 84.34 ± 8.4 mg/dl, P = 0.05). A trend toward improvement in fasting glucose remained after exclusion of four subjects whose serum 25(OH)D2 did not improve following treatment (P = 0.12). In conclusion, serum 25(OH)D was inversely associated with fasting glucose, and vitamin D treatment had beneficial effects on fasting glucose. Relationships of 25(OH)D with fasting insulin and ALT were ethnic specific. The positive relationship with LDL and ALT were suggestive of possible adverse influences of vitamin D.
INTRODUCTION
The prevalence of metabolic syndrome correlates directly with increasing obesity (1). The diagnosis of metabolic syndrome in adolescents modified from the Adult Treatment Panel III guidelines as defined by Cook et al. requires at least three of the following five criteria: elevated systolic or diastolic blood pressure ≥90th percentile for age and sex, a high-density lipoprotein (HDL) cholesterol level ≤40 mg/dl, fasting triglyceride (TG) level ≥110 mg/dl, fasting glucose level >110 mg/dl and waist circumference >90th percentile (2). Subsequently, the fasting glucose was changed to 100 mg/dl (3). Those with metabolic syndrome typically have insulin resistance and elevated liver enzymes, particularly serum transaminases (4). Vitamin D deficiency, as indicated by a serum concentration of 25-hydroxy D level (25(OH)D) below 20 ng/ml (50 nmol/l (5)), is commonly present in obesity and has been implicated as a risk factor for metabolic syndrome (6).
Previous cross-sectional studies in adults have reported that low 25(OH)D concentrations are related to glucose intolerance, diabetes, insulin resistance, hypertension and metabolic syndrome (7,8). Likewise, an association of serum 25(OH)D with insulin sensitivity and fasting glucose has been found in children (9,10). Ethnic differences in the relationship between 25(OH)D and glucose metabolism have been reported, such that the relationships were statistically significant in Caucasian Americans (CAs) but not African Americans (AA (7)). To our knowledge, no study has explored whether ethnic differences exist between serum 25(OH)D and other aspects of the metabolic syndrome. Some, but not all, studies in adults have demonstrated changes in blood glucose (11), insulin sensitivity (7,11), lipid levels (12,13) and BMI (14) with vitamin D treatment. To date, no studies have investigated the effects of vitamin D replacement on the biochemical abnormalities of the metabolic syndrome in adolescents.
Insulin resistance enhances the flux of free fatty acids to the liver, and alters hepatic production of lipoproteins such that concentrations of TG and small dense low-density lipoprotein (LDL) are increased, whereas that of HDL is decreased (15). With increased adiposity there is increased production of inflammatory markers which, in turn, promotes further exaggeration of insulin resistance. Nonalcoholic steatohepatitis associated with metabolic syndrome is thought to be related to insulin resistance and dyslipidemia. However, whether vitamin D status influences LDL or liver enzymes has not been extensively examined, particularly in children.
Our primary objective was to examine the relationship between vitamin D status and components of metabolic syndrome, such as BMI, blood pressure, TG, HDL, and glucose-insulin dynamics and to explore any relationship to low-density lipoprotein cholesterol (LDL) and liver transaminases in obese adolescent females. Further, we determined whether ethnic differences exist in these relationships and/or whether insulin resistance mediates any of the relationships in this population. The secondary aim was to examine the outcome of vitamin D treatment on glucose-insulin metabolism in obese adolescent females.
METHODS AND PROCEDURES
Subjects
Subjects were obese postmenarchal adolescent females attending the weight management clinic at the Children’s Hospital, University of Alabama at Birmingham (Birmingham, AL). All were in Tanner stage ≥4 for breast and pubic hair development (16). Subjects were recruited from a study period of March of 2007 to September of 2009. We have previously reported the results of data on 51 of the AA subjects (17). Race/ethnicity were reported by the subject’s caregiver. Subjects with a diagnosis of overt diabetes, malabsorptive disorders, or metabolic rickets, use of diuretics, systemic glucocorticoids, or anticonvulsant medications; and BMI (kg/m2) <95th centile for age and sex were excluded. Metabolic syndrome was diagnosed according to the Cook’s criteria, with the exception of waist circumference (3). As there are no reference points for waist circumference available for adolescents and BMI >95th percentile is considered an element of metabolic syndrome in adolescents (3), we used latter measurement instead of waist circumference as a criteria for defining metabolic syndrome. This study was approved by the University of Alabama at Birmingham Institutional Review Board for Human Use, and written parental consent and subject assent were obtained prior to testing.
Objective 1
All subjects had anthropometric evaluation with height, weight, and BMI measurement and assessment of pubertal development. Blood pressure was recorded with an automated blood pressure cuff (Dinamap Pro 200; GE Medical Systems, Milwaukee, WI). After applying a blood pressure cuff appropriate for the subject’s arm size, automatic blood pressure recordings of systolic, diastolic, and heart rate were measured. Following an overnight fast, all subjects underwent oral glucose tolerance test with a standard flavored glucose dose of 75 g given orally over 5 min with measurements of plasma glucose and insulin at fasting state (0 min), 30, 60, and 120 min. Serum 25(OH)D, intact parathyroid hormone, serum calcium, hemoglobin A1C, serum C-reactive protein, lipid profile and transaminases (aspartate aminotransferase and alanine aminotransferase (ALT)) were also measured with the 0-min sampling. All blood samples were obtained between 8:00 am and 12:00 pm.
Objective 2
All subjects identified with vitamin D deficiency (defined as serum 25(OH)D concentration below 20 ng/ml (50 nmol/l (5))) were subsequently treated with vitamin D (50,000 IU of ergocalciferol orally, once a week for 8 weeks (18)) as part of their clinical care. A subgroup of these subjects consented to participate in the second part of study and underwent a follow-up oral glucose tolerance test, as described in part 1 of protocol, along with measurements of serum 25(OH)D, parathyroid hormone and serum calcium. This was a convenience sample.
Laboratory analyses
Plasma glucose concentration was determined using the glucose oxidase method, using the Vitros 5, 1 (Ortho Clinical Diagnostics, Raritan, NJ). The assay coefficients of variations were 1.5% at a glucose level of 83 mg/dl and 1.2% at a glucose level of 292 mg/dl. Serum insulin was determined using two-site immunoenzymometric assay (AIA-PACK IRI; Tosoh, Tokyo, Japan). C-reactive protein was measured with VITROS Chemistry Systems (Ortho Clinical Diagnostics) using a “MicroSlide” method. Serum 25(OH)D was analyzed at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA), using liquid chromatography-tandem mass spectrometry methodology (19). Serum parathyroid hormone was also measured at Quest Diagnostics with a two-site immunoradiometric assay (normal range 10–65 pg/ml). Measurements of total cholesterol and direct HDL, direct LDL and TG were determined by VITROS 5.1 FS Chemistry System (Ortho Clinical Diagnostics). Total cholesterol and TG were determined by standard enzymatic methods, the HDL by modified enzymatic assay and LDL by an elimination-detergent and selective assay. The intra and inter assay coefficient of variation of total cholesterol is 1.8% and 1.5%, of HDL 2.9% and 3.0%, of LDL 2.2% and 3.5% and that of TG 1.4% and 0.9%, respectively.
Assessment of insulin resistance/sensitivity
The whole body insulin sensitivity index was calculated using the formula (whole body insulin sensitivity index = 10,000/√ (fasting glucose × fasting insulin) × (mean glucose × mean insulin during oral glucose tolerance test)) proposed by Matsuda et al. (20). Whole body insulin sensitivity index has been validated for use in obese children and correlates with insulin sensitivity derived from the hyperinsulinemic-euglycemic clamp (1,21). The homeostatic model assessment of basal insulin resistance (HOMAIR) was calculated using the formula: HOMAIR = (fasting insulin (µU/ml) × fasting glucose (mmol/l))/22.5 (21). Post-oral glucose tolerance test area under the curve (AUC) and incremental AUC for insulin and glucose were calculated using the trapezoidal method (22). Hepatic insulin resistance and skeletal muscle insulin sensitivity indexes were calculated as previously described (23).
Statistical analyses
Baseline descriptive characteristics (mean ± standard deviation) were determined by ethnic group for all variables of interest. The distributions of continuous variables were examined, and outliers, defined as points greater than 3 standard deviations above or below the mean, were excluded. TG, C-reactive protein, ALT, aspartate aminotransferase, and alkaline phosphatase were log10-transformed for analyses to obtain a normal distribution. A two-group t-test was used to examine ethnic differences. Pearson correlation analysis was used to examine the relationship of 25(OH)D with other variable of interests among all subjects. Partial correlation analysis was used to examine these relationships after adjustments for race and BMI. Multiple linear regression analysis was used to examine potential interactions between race and 25(OH)D. Variables identified to have significant interaction terms were analyzed by race with partial correlations analysis (adjusting for BMI). Indexes of insulin resistance/sensitivity were investigated as potential confounders in relationships of metabolic syndrome components found to be significantly associated with 25(OH)D. In a subgroup of subjects, paired t-tests and repeated measures analysis of covariance were used to compare variables of interest before and after vitamin D treatment. Statistical analyses were performed using the SAS software package (version 9.1; SAS Institute, Cary, NC). A two-sided P value < 0.05 was considered statistically significant.
RESULTS
Objective 1
A total of 80 obese adolescent females (53 AA and 27 CA) were studied. Vitamin D <20 ng/ml was observed in 85% AA and 30% of CA subjects. Three subjects (all AA) had impaired fasting glucose, defined as fasting glucose ≥100 mg/dl and <126 mg/ dl (24), and three subjects (all AA) had impaired glucose tolerance, defined as 2-h glucose ≥140 mg/dl (24). A total of 17 participants had systolic hypertension (10 AA and 7 CA) and 1 participant had diastolic hypertension. Eighteen subjects met the criteria for metabolic syndrome, of which 55% had serum 25(OH)D concentration <20 ng/ml and 83% had <30 ng/ml.
Table 1 provides descriptive characteristics of subjects by race. AA had lower serum concentration of 25(OH)D (P < 0.001) and serum TG (P < 0.001) and lower skeletal muscle insulin sensitivity index (P = 0.03) and higher hemoglobin A1C (P = 0.03) and parathyroid hormone (P = 0.03) compared to their CA counterparts.
Table 1.
Descriptive characteristics of obese adolescents based on race
| African American |
Caucasian American |
||
|---|---|---|---|
| Variable | Mean ± s.d. (n = 53) |
Mean ± s.d. (n = 27) |
P |
| Age at test (year) | 14.3 ± 2.3 | 14.8 ± 2.3 | 0.29 |
| Weight (kg) | 101.8 ± 41.0 | 100.3 ± 47.5 | 0.88 |
| Height (cm) | 103.2 ± 71.9 | 112.6 ± 71.0 | 0.58 |
| BMI (kg/m2) | 43.5 ± 9.6 | 41.9 ± 7.8 | 0.46 |
| Heart rate | 78.8 ± 10.0 | 80.5 ± 14.6 | 0.53 |
| SBP | 117.6 ± 14.7 | 118.5 ± 14.9 | 0.80 |
| DBP | 65.8 ± 8.7 | 66.7 ± 9.4 | 0.67 |
| A1C (%) | 5.5 ± 0.4a | 5.3 ± 0.3e | 0.03 |
| Fasting glucose (mg/dl) | 86.7 ± 7.8c | 85.7 ± 8.4 | 0.60 |
| Fasting insulin (µU/ml) | 34.2 ± 14.4 | 33.4 ± 18.7 | 0.85 |
| 2-h insulin (µU/ml) | 136.9 ± 93.0 | 146.9 ± 135.2d | 0.70 |
| 2-h glucose (mg/dl) | 101.45 ± 23.32b | 97.4 ± 16.1d | 0.43 |
| PTH (pg/ml) | 35.5 ± 14.9g | 27.8 ± 13.7 | 0.03 |
| Serum calcium (mg/dl) | 9.4 ± 0.4 | 9.5 ± 0.4 | 0.14 |
| 25(OH)D (ng/ml) | 14.0 ± 6.6 | 28.4 ± 10.3 | <0.001 |
| TC (mg/dl) | 161.9 ± 24.7b | 162.9 ± 24.5d | 0.87 |
| HDL (mg/dl) | 43.0 ± 8.8c | 41.9 ± 8.5d | 0.61 |
| LDL (mg/dl) | 101.5 ± 26.2c | 101.2 ± 27.7d | 0.95 |
| TG (mg/dl) | 80.1 ± 36.8b | 129.4 ± 56.6d | <0.001 |
| CRP (mg/dl) | 1.0 ± 0.6d | 0.7 ± 0.5f | 0.15 |
| ALT (u/l) | 22.0 ± 8.4h | 22.0 ± 7.8d | 0.90 |
| AST (u/l) | 28.2 ± 8.2c | 26.4 ± 5.9d | 0.40 |
| ALP (u/l) | 134.8 ± 65.4h | 124.9 ± 56.9d | 0.51 |
| HOMAIR | 7.4 ± 3.4g | 7.2 ± 4.4 | 0.82 |
| WBISI (Matsuda Index) | 1.7 ± 0.9g | 1.9 ± 1.2d | 0.48 |
| Inc insulin AUC (µU/ml × 120 min) |
15,965 ± 8,858g | 18,271 ± 10,527e | 0.32 |
| Inc glucose AUC (mg/dl × 120 min) |
3,318 ± 2,785 | 3,996 ± 2,274d | 0.28 |
| Hepatic IR | 1.3 × 107 ± 6.1 × 106g |
1.3 × 107 ± 7.4 × 106d |
0.65 |
| Muscle Si | 0.3 ± 0.3a | 0.5 ± 0.3e | 0.008 |
Values indicated in boldface are signifcantly different (P < 0.05).
25(OH)D, 25-hydroxyvitamin D; A1C, hemoglobin A1C; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; AUC, area under the curve; CRP, C-reactive protein; DBP, diastolic blood pressure; HDL, high-density lipoprotein; hepatic Si, hepatic insulin sensitivity index; HOMAIR, homeo-static model assessment of basal insulin resistance; LDL, low-density lipoprotein; hepatic IR, hepatic insulin resistance; muscle Si, skeletal muscle insulin sensitivity index; PTH, parathyroid hormone; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WBISI, whole body insulin sensitivity index.
n = 48,
n = 51,
n = 50,
n = 26,
n = 25,
n = 13,
n = 52,
n = 49.
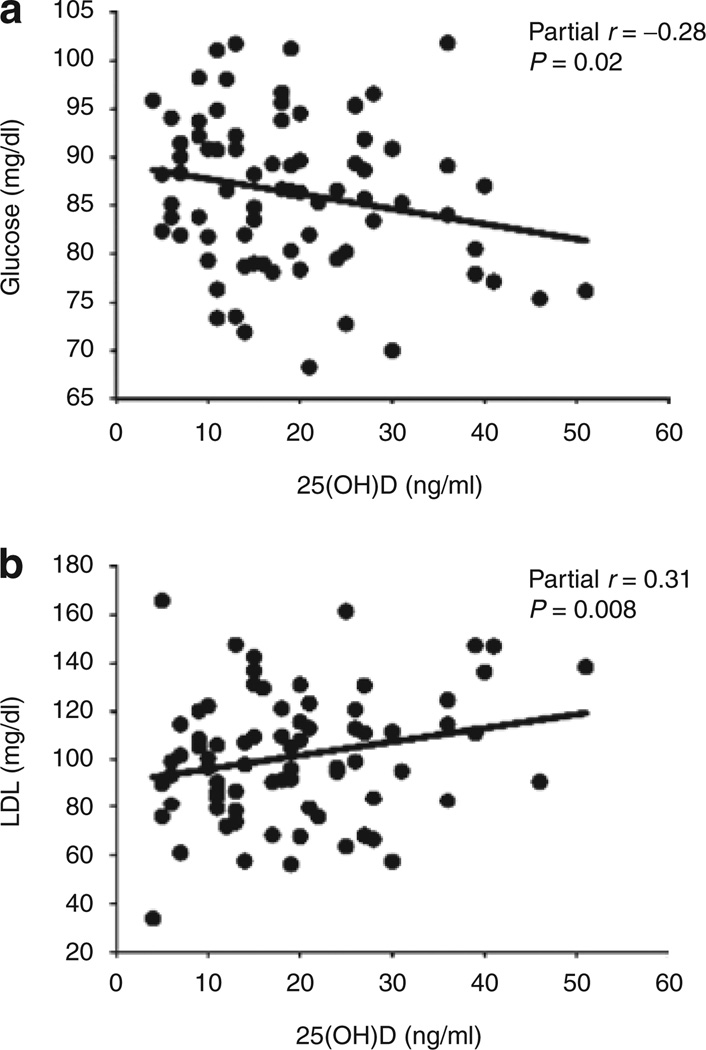
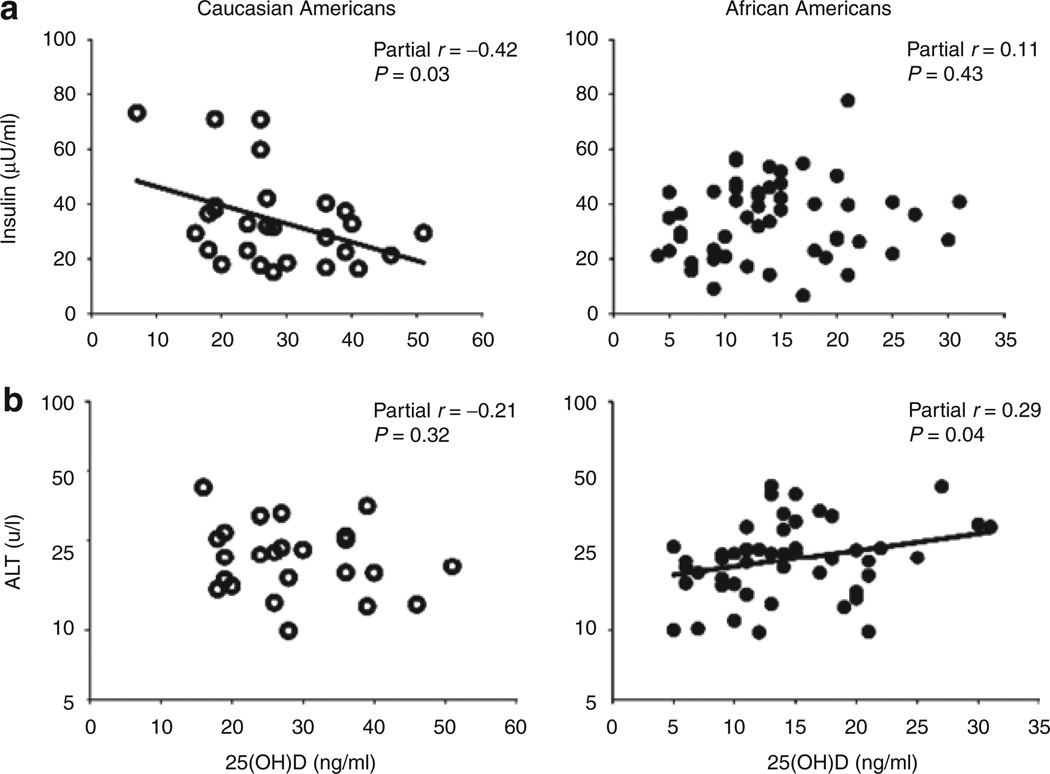
The partial correlations of serum 25(OH)D against other metabolic variables, adjusted for race and BMI, are reported in Table 2. Among all subjects, 25(OH)D was inversely associated with fasting glucose (P = 0.02, Figure 1a) and positively associated with LDL (P = 0.008, Figure 1b) independent of race and BMI. The relationship between 25(OH)D and LDL remained significant after further adjustment for indexes of insulin resistance (P = 0.02). There was no statistically significant association between 25(OH)D and systolic or diastolic BP. Statistically significant interactions between race and 25(OH)D were apparent for fasting insulin (P = 0.02) and ALT (P = 0.03). In correlation analyses by race, after adjusting for BMI, 25(OH)D was inversely associated with fasting insulin in CA (r = −0.42, P = 0.03) but not in AA (r = 0.11, P = 0.43, Figure 2a) whereas 25(OH)D was positively associated with ALT in AA, but not CA (r = 0.29, P = 0.04 vs. r = −0.21, P = 0.32, Figure 2b). The relationship between 25(OH)D and ALT among AA generally remained after further adjustment for indexes of insulin resistance (P = 0.008–0.06).
Table 2.
Partial correlations of serum 25(OH)D with descriptive and metabolic variables, adjusted for race and BMI (r (P value))
| Variable | All subjects |
|---|---|
| Age | −0.15 (0.18) |
| Fasting insulin | −0.12 (0.29)* |
| 2-h insulin | −0.06 (0.62) |
| Heart rate | 0.13 (0.26) |
| SBP | −0.07 (0.53) |
| DBP | 0.04 (0.71) |
| HbA1c | −0.02 (0.88) |
| Fasting glucose | −0.28 (0.02) |
| 2-h glucose | −0.06 (0.60) |
| PTH | −0.18 (0.13) |
| TC | 0.17 (0.14) |
| HDL | 0.01 (0.95) |
| LDL | 0.31 (0.008) |
| Triglycerides | −0.01 (0.94) |
| CRP | 0.10 (0.48) |
| ALT | 0.08 (0.51)* |
| AST | −0.05 (0.66) |
| ALP | 0.03 (0.80) |
| HOMAIR | −0.20 (0.08) |
| WBISI | −0.01 (0.97) |
| Inc insulin AUC | −0.04 (0.73) |
| Inc glucose AUC | −0.03 (0.78) |
| Hepatic IR | 0.08 (0.48) |
| Muscle Si | 0.05 (0.70) |
The bold letters show statistical signifcance or a trend toward significance.
25(OH)D, 25-hydroxyvitamin D; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; CRP, C-reactive protein; DBP, diastolic blood pressure; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; hepatic IR, hepatic insulin resistance; hepatic Si, hepatic insulin sensitivity index; HOMAIR, homeostatic model assessment of basal insulin resistance; Inc Glucose AUC, incremental area under the curve for glucose; LDL, low-density lipoprotein; muscle Si, skeletal muscle insulin sensitivity index; PTH, parathyroid hormone; SBP, systolic blood pressure; TC, total cholesterol; WBISI, whole body insulin sensitivity index.
Statistically significant race by 25(OH)D interaction term identified for fasting insulin (P = 0.02) and ALT (P = 0.03).
Figure 1.
Partial correlation analyses of serum 25-hydroxyvitamin D (25(OH)D), adjusted for race and BMI. Serum 25(OH)D was inversely associated with (a) fasting glucose and positively associated with (b) low-density lipoprotein (LDL) independent of race and BMI.
Figure 2.
Correlation analyses by race, after adjusting for BMI. 25-Hydroxyvitamin D (25(OH)D) was inversely associated with (a) fasting insulin in Caucasian American (CA) but not in African American (AA), whereas 25(OH)D was positively associated with (b) alanine transaminase (ALT) in AA, but not CA.
Objective 2
The second part of the study consisted of 14 Vitamin D deficient subjects who received standard treatment. This subset consisted of 13 AA and 1 CA subjects. One AA had impaired fasting glucose. Table 3 describes the changes in the variables before and after treatment of vitamin D deficiency. A course of ergocalciferol (vitamin D2) 50,000 IU for 8 weeks increased the serum 25(OH)D concentration to >20 ng/dl in 64.3% of subjects (n = 9/14). Only 42.9% of subjects (n = 6/14) achieved levels >30 ng/dl with this regimen. Mean concentrations of serum 25(OH)D increased from 10.57 ± 4.4 to 25.50 ±12.06 ng/ dl. Fasting glucose improved after treatment with vitamin D (89.07 ± 8.3 mg/dl before and 84.34 ± 8.4 mg/dl after, P = 0.05). Post hoc analysis excluding the subject with impaired fasting glucose decreased the P value to 0.02. We re-examined the parameters after omission of four subjects who did not have improvement in serum 25(OH)D2: the 25(OH)D2 increased by 19.2 ng/ml (10.6–29.8 ng/ml, P < 0.001). A trend toward a decrease in fasting glucose remained, (91.8–86.5 mg/dl, P = 0.12), whereas there were no statistically significant changes in other outcome variables.
Table 3.
Variables before and after treatment of vitamin D deficiency
| Before treatment |
After treatment |
||
|---|---|---|---|
| Variable | Mean ± s.d. (n = 14) |
Mean ± s.d. (n = 14) |
P |
| Age at test (year) | 14.9 ± 1.8 | 15.6 ± 1.7 | |
| Weight (kg) | 113.5 ± 29.4 | 114.0 ± 26.4 | 0.76 |
| Height (cm) | 162.2 ± 8.0 | 163.4 ± 7.4 | 0.27 |
| BMI (kg/m2) | 43.1 ± 9.8 | 42.8 ± 9.6 | 0.51 |
| Fasting insulin (µU/ml) | 33.7 ± 13.2 | 28.1 ± 7.4a | 0.14 |
| 2-h insulin (µU/ml) | 148.9 ± 11.5 | 163.6 ± 134.3a | 0.87 |
| Fasting glucose (mg/dl) | 89.1 ± 8.3 | 84.4 ± 8.4 | 0.05d |
| 2-h glucose (mg/dl) | 105.5 ± 29.7 | 105.1 ± 30.8 | 0.96 |
| PTH (pg/ml) | 36.5 ± 17.1 | 42.2 ± 20.3a | 0.39 |
| Serum calcium (mg/dl) | 9.4 ± 0.5 | 9.3 ± 0.4 | 0.59 |
| 25(OH)D (ng/ml) | 10.6 ± 4.4 | 25.5 ± 12.1 | <0.001 |
| 25(OH)D2 (ng/ml)c | 4 ± 0 | 14.29 ± 11.46 | 0.005 |
| 25(OH)D3 (ng/ml) | 11.1 ± 4.7 | 12.0 ± 6.2 | 0.96 |
| CRP (mg/dl) | 0.8 ± 0.6 | 0.8 ± 0.5 | 0.30 |
| HOMAIR | 7.4 ± 3.3 | 5.9 ± 1.8a | 0.12 |
| WBISI (Matsuda Index) | 1.6 ± 0.6 | 1.8 ± 0.6a | 0.13 |
| Inc insulin AUC (µU/ml x120 min) |
16,962.4 ± 7,530.7 |
17,480.2 ± 8,397.9a |
0.87 |
| Inc glucose AUC (mg/dl × 20 min) |
3,726.4 ± 3,123.9 |
4,192.1 ± 2,827.1a |
0.74 |
| Hepatic IR | 1.2 × 107 ± 4.6 × 106 |
1.1 × 107 ± 4.2 × 106a |
0.21 |
| Muscle Si | 0.38 ± 0.33 | 0.30 ± 0.15b | 0.54 |
| SBP (mm Hg) | 123 ± 14 | 118 ± 15 | 0.41 |
| DBP (mm Hg) | 65 ± 9 | 64 ± 8 | 0.72 |
| Heart rate (beats/min) | 82 ± 11 | 77 ± 10 | 0.15 |
After adjusting for BMI and time (the weeks between testing) the trend for improvement remained (P = 0.05). Values indicated in boldface are statistically different.
n = 13,
n = 12.
For statistical analyses, a value of 4 was assigned to D2 levels reported as <4 ng/ml.
Exclusion of four nonresponders attenuates the P value for glucose to 0.12.
DISCUSSION
Serum 25(OH)D status has been implicated as a risk factor for both diabetes and metabolic syndrome in adults (7,25,26). Of the components of metabolic syndrome, only fasting glucose had an independent association with serum 25(OH)D in obese adolescent females in this study. We also found that treatment with vitamin D improved fasting glucose in a subset of participants. There were ethnic specific relationships between serum 25(OH)D with fasting insulin, LDL, and ALT.
Vitamin D and glucose metabolism
We found a negative relationship between serum 25(OH)D and fasting glucose, which is in concordance with another pediatric study by Johnson et al. (10). In addition, there was a trend for improvement in fasting glucose in obese, predominantly AA adolescents after treatment of vitamin D deficiency. Fasting glucose reflects endogenous hepatic glucose production and also reflects hepatic insulin resistance, and the inability to regulate the hepatic glucose output is considered to be the key defect in type 2 diabetes. However, sites other than the liver may be involved in 25(OH)D-mediated reduction in glucose. To our knowledge, this is the first pediatric study which demonstrates an improvement in serum glucose after correction of vitamin D deficiency. Of note, the majority of our treated obese subjects (64%) reached a serum 25(OH)D concentration of >20 ng/ml with the conventional regimen. Additional studies are warranted to establish whether a sustained serum 25(OH)D level >20 ng/dl will prevent against the development of diabetes. Although the exact mechanism through which vitamin D modulates glucose metabolism is not completely understood, a growing body of evidence indicates that serum 25(OH)D status influences fasting glucose. Our finding of glucose improvement after vitamin D treatment in a predominantly AA population adds to the previous cross-sectional observations. This finding supports the need for prospective interventional studies to assess whether vitamin D replacement will have a potential impact on patient care in terms of glycemic status.
An inverse association between serum 25(OH)D and fasting insulin was observed in CA only. This ethnic difference in the relationship of insulin sensitivity with vitamin D has been previously reported (27). Our findings that fasting insulin was related to 25(OH)D in CA but not AA, suggest that serum 25(OH)D may modulate glucose metabolism differentially in AA vs. CA. Alternatively, the high prevalence of vitamin D deficiency in AA and the profound insulin resistance of AA adolescents could be masking a true relationship.
Vitamin D and lipids
Metabolic syndrome and obesity are associated with reduced HDL cholesterol concentrations, elevations of TG, as well as increased concentrations of small dense LDL particles (28). Serum 25(OH)D concentrations reportedly correlate positively with HDL levels in normal (10) and obese children (9). We did not find this in our population which consisted of predominantly AA, severely obese adolescent females. Historically, AA children are known to have higher HDL than CA (29). Yet, in our study sample of obese adolescents (Table 1), we did not find a higher HDL in AA children. It is plausible that obesity negates the beneficial effect of AA ethnicity on HDL. It is also possible that due to the ubiquity of severe vitamin D deficiency in AA subjects, the true relationship of serum 25(OH)D with HDL is not conspicuous. Further studies involving a population with more heterogeneous concentrations of serum 25(OH)D and a wide range of adiposities may discern significant associations.
In the third National Health and Nutrition Examination Survey (30), adults in the lowest quartile of 25(OH)D had the greatest risk of elevated serum TG (≥150 mg/dl), suggesting a detrimental effect consequent to vitamin D deficiency. Similarly, in obese subjects 25(OH)D <50 nmol/l was associated with lower HDL and high TG (31). BMI (or any other measure of adiposity) was, however, not taken into account in these analyses. We did not find an independent association of serum TG with serum 25(OH)D, in concordance with some of previous reports in obese children and adolescents (17,32) as well as data based on National Health and Nutrition Examination Survey conducted in US adolescents (33).
In this study, we found a positive correlation between serum 25(OH)D and LDL concentrations in obese adolescents, even after adjustments for BMI and indexes of insulin resistance. These findings seem paradoxical to recent cross-sectional studies that have suggested a protective role of vitamin D in cardiovascular disease (12,34). However, they are in accordance with animal studies which have suggested vitamin D is an atherogenic agent (35,36) and the finding of a positive association of 25(OH)D with aorta and carotid artery calcified atherosclerotic plaque in AA adults with type 2 diabetes (37). Previous studies examining the associations of serum 25(OH)D specifically with LDL have shown inconsistent results (12,13,38). Analogous with our findings, in a cohort of British Bangladeshis, serum 25(OH)D was positively correlated with LDL, total cholesterol, apo A1 and apo B (38). Further, vitamin D supplementation without calcium in obese participants resulted in an unexpected increase in LDL cholesterol in a weight loss study (13). Similarly, long-term vitamin D supplementation had adverse effects on serum LDL and HDL concentration during postmenopausal hormone replacement therapy (39).
The physiological basis for the positive association between vitamin D and LDL is not clear, however several possible explanations can be offered. Zitterman et al. (13) postulated that a vitamin D-induced increase in intestinal calcium absorption and subsequent decrease in gut calcium content could reduce the formation of calcium-fatty soaps which may result in increased fat absorption from gut (13). Lower calcium content in the gut may also decrease cholesterol excretion due to reduced binding of calcium to bile acids (13). It was further suggested that a high calcium intake may offset the increase in LDL. The positive relationship observed between 25(OH)D and apo A1 and apo B in Bangladeshis 38, suggests a possible synthetic role of vitamin D on apolipoproteins. Vitamin D reportedly decreases serum triglycerides (13,30), and it is plausible that the clearance of VLDL may result in increased LDL and HDL production. It is also possible that, the binding of 1,25(OH)2D to LDL (40) could potentially attenuate the clearance of LDL from circulation. Finally, insofar as fortified milk is the primary source of dietary vitamin D in adolescents (41), the positive relationship between 25(OH)D and LDL may also simply be a reflection of a diet rich in high-fat dairy products consumed by obese subjects. As the effect of serum 25(OH)D on lipid levels is not clearly understood, more research looking into the mechanistic link between vitamin D and lipid metabolism are required. Furthermore, we used direct LDL measurement which does not provide LDL particle number. Thus, it is not clear whether the relationship applies to the small dense LDL particles reflective of metabolic syndrome, or whether the LDL particles in vitamin D sufficient populations are large, less dense LDL particles. Future vitamin D studies involving qualitative lipoprotein analysis to characterize the LDL subclasses may prove to be clinically meaningful.
Vitamin D and liver function
We report novel findings related to 25(OH)D and liver function wherein 25(OH)D was positively associated with ALT in AA, but not CA. This relationship was not mediated by insulin resistance. The mechanism for the positive relationship or the ethnic difference is uncertain. ALT is a marker for hepatic injury and might be considered a proxy for fatty liver (42). AA generally have lower rates of hepatic steatosis relative to CA, presumably because of lower visceral fat accumulation (43), and they have reduced synthesis of 25(OH)D due to melanin competition with 7-dehydrocholesterol for UV radiation, thus reducing the efficiency of conversion of 7-dehydrocholesterol to pre-vitamin D3.
Correlation does not infer causality, therefore it is possible that the positive relationship between 25(OH)D and ALT is mediated by an unmeasured factor related to both variables. For example, subcutaneous adipose tissue may be protective against lipid accumulation in the liver (44), and vitamin D is hypothesized to become sequestered in subcutaneous adipose stores resulting in lower circulating 25(OH)D (45). The cause of ALT elevation and thus possibly greater hepatocellular stress is pathophysiologically unclear and we do not know what accounts for this association. These findings can not be generalized as our study population is unique in that they are severely obese adolescents. Furthermore, few AA in our study population had 25(OH)D concentrations above 25 ng/ml, as shown in Figure 2, thus it is difficult to assess relationships with ALT within that range of 25(OH)D. Further studies are needed to determine whether this is a true association.
Blood pressure
Both animal and human experiments have shown that vitamin D deficiency is known to upregulate renin–angiotensin system with development of hypertension and left ventricular hypertrophy (8). Our data did not show any association between systolic and diastolic blood pressure with 25(OH)D. Blood pressure could have marked variations and since we have not used ambulatory monitoring, we may not find a significant association. It is also possible that our study subjects are unique from other studies—adolescent female subjects who are mainly normotensive.
The strengths of this study included being the first pediatric study to assess the relationship of serum 25(OH)D with metabolic syndrome components and to report improvement in fasting glucose with correction of vitamin D deficiency. The main limitation of the study was the relatively small sample size involving obese adolescents, limiting the generalizability of study findings to other groups. However, relationships among variables were objectively assessed using serum 25(OH)D concentration. In addition, the sub-study was not placebo-controlled and the duration between the vitamin D treatment and second measurements varied. Assessment of changes in serum 25(OH)D2 indicated that there were some subjects whose circulating 25(OH)D2 did not rise after treatment, which when removed, attenuated the power to detect statistically significant findings in the resultant small sample. These findings are exploratory and hypothesis building.
Conclusion
Serum 25(OH)D concentration was only associated with fasting glucose among the metabolic syndrome components. We found an inverse relationship with fasting glucose and fasting insulin, and a positive relationship with LDL and ALT in obese adolescents. The relationships of 25(OH)D with fasting insulin and ALT were ethnic specific, and those with LDL and ALT were suggestive of unfavorable influences of vitamin D. Nevertheless, repletion of vitamin D status had beneficial effects on plasma glucose in a predominantly AA sample, delineating a possible specific effect of 25(OH)D on endogenous glucose output. Larger controlled studies are required to confirm these findings.
ACKNOWLEDGMENTS
This study was supported by Children’s Center for Research and Innovation (CCRI) grants, The Children’s Hospital of Alabama, Birmingham, AL. A.P.A. is supported in part by the Child Health Research Center Grant K12 HD043397 (T0909180013) and UAB Diabetes Research Training Center P60 DK-079626. J.A.A. is supported by the American Heart Association (Greater Southeast Affiliate).
Footnotes
DISCLOSURE
The other authors declared no conflict of interest.
REFERENCES
- 1.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 2.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 3.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Hanley AJ, Williams K, Festa A, et al. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Boucher BJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome ‘X’? Br J Nutr. 1998;79:315–327. doi: 10.1079/bjn19980055. [DOI] [PubMed] [Google Scholar]
- 7.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metab Clin Exp. 2008;57:183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MD, Nader NS, Weaver AL, Singh R, Kumar S. Relationships between 25-hydroxyvitamin D levels and plasma glucose and lipid levels in pediatric outpatients. J Pediatr. 2010;156:444–449. doi: 10.1016/j.jpeds.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 11.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 12.Major GC, Alarie F, Doré J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85:54–59. doi: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 13.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 14.Caan B, Neuhouser M, Aragaki A, et al. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern Med. 2007;167:893–902. doi: 10.1001/archinte.167.9.893. [DOI] [PubMed] [Google Scholar]
- 15.Reaven P. Metabolic syndrome. J Insur Med. 2004;36:132–142. [PubMed] [Google Scholar]
- 16.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashraf A, Alvarez J, Saenz K, et al. Threshold for effects of vitamin D deficiency on glucose metabolism in obese female African-American adolescents. J Clin Endocrinol Metab. 2009;94:3200–3206. doi: 10.1210/jc.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 19.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–159. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve Methodological aspects. Diabetes Care. 1990;13:172–175. doi: 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- 23.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 24.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 25.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 26.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 27.Scragg R, Sowers M, Bell C. Third National Health and Nutrition Examination Survey Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 28.Carr MC, Brunzell JD. Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab. 2004;89:2601–2607. doi: 10.1210/jc.2004-0432. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Chen W, Srinivasan SR, et al. Race (black-white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima-media thickness in adulthood: the Bogalusa Heart Study. Atherosclerosis. 2007;194:421–425. doi: 10.1016/j.atherosclerosis.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 31.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, et al. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–580. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Smotkin-Tangorra M, Purushothaman R, Gupta A, et al. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20:817–823. doi: 10.1515/jpem.2007.20.7.817. [DOI] [PubMed] [Google Scholar]
- 33.Reis JP, von Mühlen D, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124:e371–e379. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 35.Dalderup LM, Vitamin D. cholesterol, and calcium. Lancet. 1968;1:645–646. doi: 10.1016/s0140-6736(68)91282-8. [DOI] [PubMed] [Google Scholar]
- 36.Hines TG, Jacobson NL, Beitz DC, Littledike ET. Dietary calcium and vitamin D: risk factors in the development of atherosclerosis in young goats. J Nutr. 1985;115:167–178. doi: 10.1093/jn/115.2.167. [DOI] [PubMed] [Google Scholar]
- 37.Freedman BI, Wagenknecht LE, Hairston KG, et al. Vitamin d, adiposity, and calcified atherosclerotic plaque in african-americans. J Clin Endocrinol Metab. 2010;95:1076–1083. doi: 10.1210/jc.2009-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John WG, Noonan K, Mannan N, Boucher BJ. Hypovitaminosis D is associated with reductions in serum apolipoprotein A-I but not with fasting lipids in British Bangladeshis. Am J Clin Nutr. 2005;82:517–522. doi: 10.1093/ajcn.82.3.517. [DOI] [PubMed] [Google Scholar]
- 39.Heikkinen AM, Tuppurainen MT, Niskanen L, et al. Long-term vitamin D3 supplementation may have adverse effects on serum lipids during postmenopausal hormone replacement therapy. Eur J Endocrinol. 1997;137:495–502. doi: 10.1530/eje.0.1370495. [DOI] [PubMed] [Google Scholar]
- 40.Teramoto T, Endo K, Ikeda K, et al. Binding of vitamin D to low-density-lipoprotein (LDL) and LDL receptor-mediated pathway into cells. Biochem Biophys Res Commun. 1995;215:199–204. doi: 10.1006/bbrc.1995.2453. [DOI] [PubMed] [Google Scholar]
- 41.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478–2485. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- 42.Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–4294. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 43.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azuma K, Heilbronn LK, Albu JB, et al. and the Look AHEAD Adipose Research Group Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007;293:E435–E442. doi: 10.1152/ajpendo.00394.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]