Abstract
The adult pancreas lack stem cells, and consequently, differentiation of pancreatic endocrine cells has been restricted to embryonic development or experimental manipulation. In this issue, Sancho et al. (2014) show that pancreas-specific loss of the ubiquitin ligase Fbxw7 stabilizes an endocrine-specific transcription factor, Ngn3, thus inducing in vivo β-cell neogenesis.
The pancreas is composed of exocrine (acinar and ductal) cells and endocrine (α-,β-, δ-, pp, and ε) cells, which function by secreting enzymes and hormones (Edlund, 2002). Pancreatic β-cells secrete insulin, and their destruction in patients with Type I diabetes mellitus renders the patients hyperglycemic (Atkinson et al., 2011). While treatment options include insulin injections or potential cell replacement therapies with in vitro generated β-cells (Pagliuca and Melton, 2013), β-cell neogenesis has only been accomplished in vivo under limited circumstances using experimental manipulation, such as pancreatic duct ligation (Inada et al., 2008) or adenoviral overexpression of transcription factors (Zhou et al 2008). In this issue, Sancho et al. report a new mechanism for generating β-cells in vivo: genetic deletion of the ubiquitin ligase Fbxw7, which normally destabilizes the transcription factor Ngn3, stimulates the direct conversion of a pancreatic ductal cells into an endocrine β cells (Figure 1).
Figure 1. Control of adult pancreatic cell reprogramming by SCFFbxw7-mediated ubiquitination.
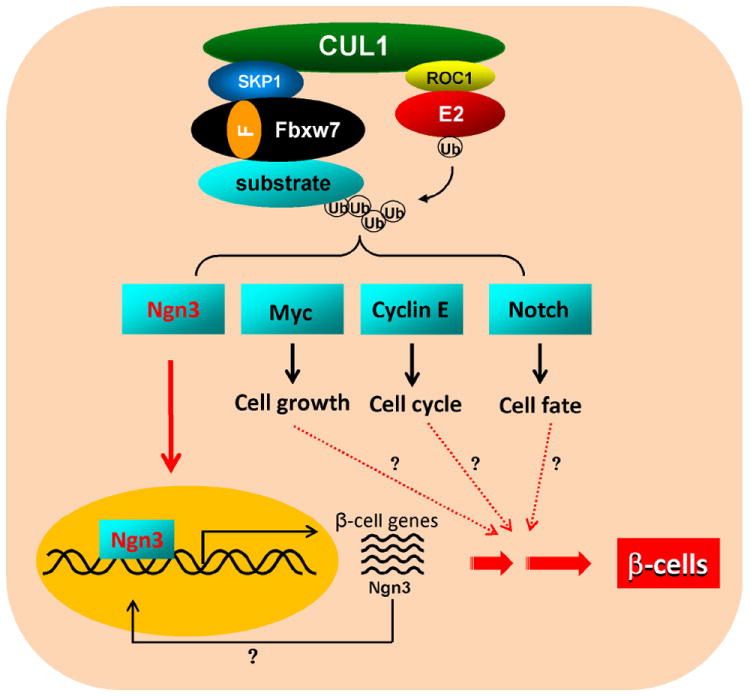
In the control pancreatic duct, SCFFbxw7 polyubiquitylates and targets Ngn3 for proteasome degradation. In the Fbxw7-deleted pancreatic duct, Ngn3 is stabilized and activates transcription of targets that function in β-cell differentiation. SCFFbxw7 also controls the ubiquitination of proteins involved in cell growth, cell cycle and cell fate. As indicated by question marks, whether Fbxw7 controls adult pancreatic cell reprogramming through coordinated regulation of multiple substrates and cellular processes remains to be determined.
Ubiquitination regulates a diverse range of cellular processes to maintain cellular homeostasis. The process includes activation by an E1 enzyme, transfer of ubiquitin to an E2 conjugating enzyme, and lastly ubiquitin ligation onto a substrate protein via an E3 ligase (Pickart, 2001). Cullin proteins, constitute the largest family of E3 ligases, and function by binding a small RING protein, either ROC1 or ROC2 (also known as Rbx), which interacts with the E2 enzyme to comprise the catalytic moiety. A remarkable feature of cullin proteins, which include 9 in human cells, is that individual cullins selectively interact with different motifs, such as the F-box, SOCS box, BTB domain and WD40 repeat that are present in many proteins, which allows cullins to recruit specific substrates for ubiquitination by the RING-E2 catalytic moiety. One such factor, Fbxw7, is an F-box protein that recruits substrates to CUL1. This E3 complex, referred to as SCF/CRL1Fbxw7, mediates proteasomal degradation of proteins involved in cell growth (c-Myc), cell cycle regulation (Cyclin E), and cell fate and differentiation (Notch) (Welcker and Clurman, 2008).
Prompted by the role of Fbxw7 in cell fate determination, Sancho and colleagues generated conditional knockout of Fbxw7 in embryonic pancreatic progenitor cells using a Cre recombinase under the control of the Pdx1 promoter, which is active during pancreas development and in mature β-cells. Surprisingly, they discovered scattered duct cells showing features of functional mature β-cells both morphologically and immunologically. Although some cells in Fbxw7-deleted ducts also expressed glucagon, a marker of α-cells, the majority of the endocrine cells were insulin-positive. Since embryonic progenitors in the pancreas can differentiate into all pancreatic lineages, Sancho and colleagues next deleted Fbxw7 in the adult pancreas using a ubiquitously expressed, tamoxifen-inducible Cre recombinase and found that Fbxw7 deletion in the adult pancreatic ductal cells, but not in acinar cells, induced the conversion into functional β-cells. This finding is particularly interesting since the adult pancreas lacks progenitor cells (Dor et al., 2004).
To investigate which SCFFbxw7 substrates may play a role during β-cell emergence in the Fbxw7-deleted pancreas, the authors analyzed a panel of four transcription factors known to be involved in embryonic β-cell development and found that both the mRNA and protein levels of Ngn3, but not the other three, were strongly increased in Fbxw7-deleted pancreas. They then demonstrated that knockdown of Fbxw7 reduced Ngn3 ubiquitination and increased Ngn3 protein half-life. Fbxw7 binds to Ngn3 and this binding, like the interactions between many other F-box proteins and their substrates, requires the GSK3β-mediated phosphorylation of Ngn3 on two adjacent serine residues, a characteristic feature of phosphodegron motifs. Conditional transgenic expression of a phosphodegron mutant Ngn3, in the presence of endogenously expressed Fbxw7, quickly (within 24 hours) resulted in the emergence of insulinpositive β-cells in the pancreas. Together with the results from the pancreas conditional deletion of Fbxw7, these findings suggest that the β-cells arise through direct conversion of ductal cells, rather than an intermediate progenitor cell that divides prior to differentiation (Sancho et al., 2014).
Aside from identifying Fbxw7 as a regulator of cell fate decision in both embryonic and adult pancreas and showing an example of direct ductal-to-β-cell conversion, the study offers new evidence consistent with the lack of stem/progenitor cells in the adult pancreas. It also highlights the latent plasticity of mature adult cells, which are generally viewed as terminally differentiated. However, there are two important issues that remain to be determined. First, unlike most other E3 substrates whose mRNA levels remain largely unchanged when the E3 function is disrupted, the mRNA level of Ngn3 is also increased in Fbxw7-deleted cells. Although the authors propose a positive feedback loop to explain this perplexing phenomenon, both the direct evidence supporting this loop and its significance are yet to be demonstrated. Second, Fbxw7 also degrades several additional proteins that play a critical role in regulating cell growth, proliferation and fate (Figure 1). Whether these proteins are also regulated by Fbxw7 in the embryonic and adult pancreas has not been examined. Does Fbxw7 control β-cell neogenesis solely through Ngn3 or through coordinated regulation of multiple substrates? Ultimately, the in vivo induction of functional β-cells may be a viable treatment option for patients with Type 1 diabetes mellitus, and the current finding of SCF/CRL1Fbxw7-Ngn3 axis in controlling direct ductal-to-β-cell conversion offers a new target for this exploration.
Acknowledgments
We thank Menxi Liu for helping with figure preparation. Y.X. is supported by NIH grant (GM067113).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, Pietropaolo M, Arvan PR, Von Herrath M, Markel DS, et al. How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes. 2011;60:1370–1379. doi: 10.2337/db10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Edlund H. Pancreatic organogenesis--developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–532. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca FW, Melton DA. How to make a functional β-cell. Dev Camb Engl. 2013;140:2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Rukstalis JM, Habener JF. Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets. 2009;1:177–184. doi: 10.4161/isl.1.3.9877. [DOI] [PubMed] [Google Scholar]
- Sancho R, Gruber R, Gu G, Behrens A. Loss of Fbw7 reprograms adult pancreatic ductal cells into α- and β-cells. Cell Stem Cell. 2014;15 doi: 10.1016/j.stem.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]


