Abstract
Background
Many subjects with asthma exhibit sputum eosinophilia associated with exacerbations. Benralizumab targets eosinophils by binding interleukin-5 receptor alpha, inducing apoptosis via antibody-dependent cell-mediated cytotoxicity.
Objectives
To evaluate the safety of benralizumab in adults with eosinophilic asthma, and its effects on eosinophil counts in airway mucosal/submucosal biopsies, sputum, bone marrow, and peripheral blood.
Methods
In this multicenter, double-blind, placebo-controlled Phase I study, 13 subjects were randomized to single intravenous placebo or benralizumab 1 mg/kg (day 0) [Cohort 1], and 14 subjects were randomized to three monthly subcutaneous doses of placebo or benralizumab 100 or 200 mg (days 0, 28, and 56) [Cohort 2]. Cohorts 1 and 2 were consecutive.
Results
The incidence of adverse events was similar between groups. No serious adverse events related to benralizumab occurred. Cohort 1: intravenous benralizumab produced a median decrease from baseline of 61.9% in airway mucosal eosinophils (day 28; placebo: +19.6%; P = .28), 18.7% (day 21) in sputum and 100% (day 28) in blood. Eosinophils were not detectable in bone marrow of benralizumab-treated subjects (day 28, n=4). Cohort 2: subcutaneous benralizumab demonstrated a combined (100 + 200 mg) median reduction of 95.8% in airway eosinophils (day 84; placebo −46.7%; P = .06), 89.9% (day 28) in sputum and 100% (day 84) in blood.
Conclusion
Single-dose intravenous and multiple-dose subcutaneous benralizumab reduced eosinophil counts in airway mucosa/submucosa and sputum, and suppressed eosinophils in bone marrow and peripheral blood. The safety profile supports further development. Additional studies are needed to assess clinical benefit in asthma.
Keywords: eosinophils, IL-5, IL-5 receptors, asthma, antibody-dependent cell-mediated cytotoxicity
Introduction
Eosinophils are thought to play an important role in the pathogenesis and severity of asthma. The clinical relevance of eosinophils in asthma has been confirmed in longitudinal studies demonstrating a reduction in acute exacerbations in subjects who maintained sputum eosinophils <2%,1 <3%,2 or <8%3 with adjustments in inhaled corticosteroids (ICS), versus those whose ICS were modified per standard clinical asthma guidelines. However, a subset of subjects with refractory asthma has persistent airway eosinophilia despite chronic high-dose ICS.4
Interleukin-5 (IL-5) is a cytokine secreted predominantly by T-lymphocytes, mast cells, and eosinophils, and is involved in regulating the differentiation, proliferation, and activation of eosinophils via the IL-5 receptor (IL-5R).5 Several studies using anti-IL-5 monoclonal antibodies (mAb), in subjects with eosinophilic asthma have shown clinical promise. In a pilot study, twelve monthly infusions of 750 mg mepolizumab reduced severe exacerbations by 41% (P = .02 versus placebo).6 These results were reproduced in a larger Phase IIb study with exacerbations reduced by 39–52% (P < .001 versus placebo).7 Mepolizumab also demonstrated a steroid-sparing effect in a 6-month study allowing subjects with prednisone-dependent eosinophilic asthma to reduce oral prednisone by 84% compared with 48% on placebo (P = .04).8 Though underpowered for this endpoint, a reduction in asthma exacerbations (P = .08) was shown with reslizumab, another anti-IL-5 mAb.9 These studies provide compelling evidence that targeting the IL-5 pathway in subjects with eosinophilic asthma has therapeutic potential.
Benralizumab is a humanized, afucosylated mAb, designed to target IL-5Rα expressed on eosinophils and basophils.10,11 Lack of a fucose sugar moiety on the oligosaccharide core enhances the binding affinity of benralizumab to FcγRIIIα and augments antibody-dependent cell-mediated cytotoxicity (ADCC), inducing apoptosis of target cells. 12 In an open-label study in subjects with mild atopic asthma, a single intravenous (IV) dose of benralizumab had an acceptable safety profile and resulted in marked reductions of peripheral blood eosinophil counts within 24 hours of dosing.13
This phase I study evaluated single (IV) or multiple subcutaneous (SC) doses of benralizumab in adults with eosinophilic asthma. The primary objectives were to evaluate the safety profile of benralizumab and the effect of benralizumab on eosinophil counts in airway mucosal/submucosal biopsies 28 days after dosing. Exploratory objectives included evaluation of eosinophil counts in sputum and bone marrow, and eosinophil and basophil counts in peripheral blood.
Methods
Study design
This was a multicenter, randomized, double-blind, placebo-controlled study (ClinicalTrials.gov identifier: NCT00659659) conducted from April 2008 through April 2011 (Fig 1). Subjects were recruited from three United States and four Canadian medical centers. All subjects signed an informed consent prior to any study-related activities. The protocol was approved by local ethics committees for each site along with the US Food and Drug Administration and Health Canada.
FIG 1.
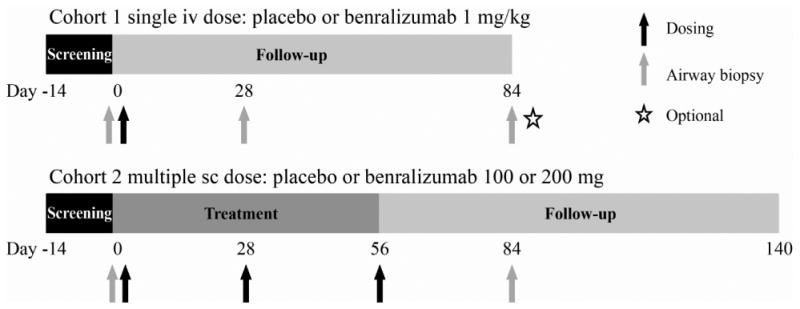
Study design.
Cohorts 1 and 2 were consecutive.
Eligible subjects aged 18–65 years had a documented diagnosis of asthma supported by at least one of the following criteria: (1) ≥12% increase in forced expiratory volume in 1 second (FEV1) after inhalation of 400 μg albuterol during screening, (2) history of ≥12% FEV1 reversibility within 1 year of randomization, or (3) history of 20% reduction in FEV1 in response to a provocative methacholine challenge (PC20) of less than 8 mg/mL within 1 year of randomization. In addition, subjects had a sputum eosinophil count ≥2.5%, post-bronchodilator FEV1 ≥65%, pre-bronchodilator FEV1/forced vital capacity (FVC) ratio below age-adjusted norms,14 and an asthma therapeutic regimen that was unchanged for 4 weeks prior to randomization and maintained from screening to the first follow-up airway mucosal/submucosal biopsy. Key exclusion criteria were lung disease other than asthma, smoking within 2 years of baseline or history of ≥10 pack-years, a clinically significant medical condition or acute infection, current use of immunosuppressive drugs (other than oral corticosteroids), positive serology to HIV, hepatitis, history of tuberculosis or positive tuberculosis test without a complete course of treatment. Subjects were asked to maintain their regular asthma medication during the study.
Subjects were randomized to receive an IV infusion of 1 mg/kg benralizumab or placebo (2:1) on day 0 (Cohort 1); or 100 or 200 mg benralizumab or placebo (1:1:1) delivered in four SC injections on days 0, 28, and 56 (Cohort 2). Group assignment was determined using block randomization through an interactive voice response system. Cohort 2 was recruited subsequent to Cohort 1. Subjects were followed-up for at least 84 days after the last dose of study medication.
Safety assessments
Safety was assessed by monitoring treatment-emergent adverse events (AEs), serious AEs, physical examinations, vital signs, and laboratory test results. Any significant changes identified during physical examinations were considered to be AEs.
Clinical procedures
A baseline bronchoscopy with airway mucosal/submucosal biopsies was performed following standard procedures at least 7 days after screening sputum induction and within 2 days before day 0. Subjects were pretreated with an inhaled β2 agonist. Airway mucosal/submucosal biopsies were repeated on days 28 (Cohort 1) and 84 (Cohort 2; optional for Cohort 1, not reported). The number of eosinophils per mm2 in airway biopsy samples was determined by immunofluorescence assay using anti-major basic protein-1 antibody (US Biological, Texas, USA). Hematoxylin and eosin (H&E) stained sections were also prepared to assess the quality of the biopsy sample.
Sputum samples were induced, collected, and analyzed for eosinophils at screening (within 14 days prior to day 0), and days 21, 56, and 77 (Cohort 1) and days 28, 77, and 140 (Cohort 2).
Subjects who consented had bone marrow aspiration at screening and day 28. Bone marrow aspirates stained with Wright-Giemsa stain were analyzed.
Blood samples were taken at screening, and days 0, 7, 28, 56, and 84 (both cohorts), and at days 21, 119, and 140 (Cohort 2 only) for evaluation of eosinophils via an automated hematology analyzer. Basophils were evaluated from whole blood by flow cytometry utilizing antibodies to immunoglobulin E high affinity receptor (eBiosciences), CD123, CCR3 (CD193), and CRTH2 (CD294) (BD Biosciences) at days 0, 1, 7, 28, 56, 84, and 140 (Cohort 2 only).
Further details of the procedures used to obtain and prepare samples of airway mucosa, peripheral blood, bone marrow, and sputum are provided in the online supplement.
Statistical analysis
A total of 12 randomized subjects per cohort were considered to be sufficiently powered to assess the effect of benralizumab on eosinophil counts in airway mucosal/submucosal biopsies. All subjects randomized into the study were included in the intent-to-treat (ITT) population. The according to protocol (ATP) population was defined as subjects who received all planned doses of study drug, had airway mucosal/submucosal biopsy results at screening and day 28 (Cohort 1) or day 84 (Cohort 2), and did not receive a burst of corticosteroids within 28 days prior to the second airway biopsy. For Cohort 2, subjects were excluded from the ATP population if they had an upper or lower respiratory tract infection requiring treatment within 28 days prior to the second airway biopsy. The safety population included all subjects who received at least one dose of study drug. Cell count evaluations were based on the ATP population and safety evaluations were based on the safety population.
Baseline was defined as the day 0 value prior to administration of study medication. If the day 0 value was missing, invalid, or measured after study drug administration, baseline was determined as the latest assessment prior to day 0.
The primary endpoints were safety and the effect of benralizumab on eosinophil counts in airway mucosal/submucosal biopsies 28 days after completion of dosing. A two-sided Wilcoxon rank sum test (alpha = .05) was used to analyze differences in median percent change in airway mucosal/submucosal absolute eosinophil count between placebo and benralizumab (Cohort 1), and placebo and the combined (100+200 mg) benralizumab groups (Cohort 2).
A post-hoc analysis was performed using the Sign test to compare the within group median percent change from screening to 28 days after the last dose of study medication in airway mucosal absolute eosinophil count. A second post-hoc analysis was conducted using a Wilcoxon rank sum test to compare median percent change from screening to 28 days after the last dose of study medication in airway mucosal/submucosal, sputum, and peripheral blood absolute eosinophil counts between placebo (Cohorts 1 and 2 combined) and benralizumab (Cohorts 1 and 2 combined). All other parameters were summarized descriptively.
Results
Subjects
Twenty-seven adult subjects with asthma were randomized to receive placebo or benralizumab (IV or SC) and all subjects were included in all analyses. Subject disposition is shown in Fig E1 in the online repository. Twenty-six subjects completed the study. One subject randomized to benralizumab 1 mg/kg IV withdrew consent after receiving study drug (no reason was given) but was followed up to day 56; this subject was randomized, received study drug, and had airway biopsies at screening and day 28, and was thus included in all study populations. Subjects were 20 to 62 years old, most were non-Hispanic (88.9%) and White (92.6%), with a slightly higher percentage of females (59.3%) (Table I). Actual and predicted baseline FEV1 values were similar across groups and in both cohorts. ICS were taken at baseline by six of eight benralizumab and four of five placebo subjects in Cohort 1, and six of nine benralizumab and all five placebo subjects in Cohort 2. In addition, one subject (Cohort 2, placebo) was taking oral corticosteroids at baseline.
Table I. Subject demographics and baseline characteristics (ITT population).
| Characteristica | Cohort 1 (single-dose IV)b N=13 | Cohort 2 (multiple-dose SC)b N=14 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Placebo n = 5 |
Benralizumab 1 mg/kg n= 8 |
Placebo n= 5 |
Benralizumab 100 mg n= 4 |
Benralizumab 200 mg n= 5 |
Benralizumab total n= 9 |
|
| Age, y, mean (SD) | 34.4 (9.7) | 38.9 (14.7) | 40.2 (14.5) | 41.0 (14.9) | 37.2 (14.4) | 38.9 (13.8) |
| Sex, n, Male/Female | 3 / 2 | 2 / 6 | 1 / 4 | 3 / 1 | 2 / 3 | 5 /4 |
| Race, n (%) | ||||||
| White | 4 (80.0) | 8 (100) | 5 (100) | 4 (100) | 4 (80.0) | 8 (88.9) |
| Other | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (11.1) |
| Weight, kg, mean (SD) | 72.2 (10.8) | 72.9 (17.5) | 74.6 (19.9) | 92.4 (14.5) | 80.4 (12.6) | 85.7 (14.1) |
| ≥1 asthma controller medication, n | 4 | 6 | 5 | 2 | 4 | 6 |
| ICS dose (budesonide equivalent μg/day), median (range) | 400 (0–2000) | 550 (0–1300) | 800 (400–3520) | 400 (0–2400) | 800 (0–1600) | 800 (0–2400) |
| Forced expiratory volume (FEV) | ||||||
| FEV1 (L), mean (SD) | 2.5 (0.8) | 2.3 (0.6) | 1.9 (0.4) | 3.0 (1.2) | 2.2 (0.6) | 2.6 (1.0) |
| Predicted FEV1 (%)c, mean (SD) | 67.2 (9.1) | 70.5 (15.6) | 60.5 (6.6) | 72.5 (13.1) | 65.7 (10.2) | 68.7 (11.4) |
| FEV1/FVC (%)c, mean (SD) | 66.7 (10.0) | 70.7 (9.8) | 67.0 (12.7) | 69.6 (5.9) | 67.2 (4.2) | 68.3 (4.8) |
| Sputum eosinophil counts %, mean (SD) | 13.9 (10.3) | 6.6 (2.2) | 34.1 (32.7) | 10.5 (7.7) | 4.9 (2.4) | 7.4 (5.8) |
Abbreviations: FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; ICS = inhaled corticosteroid; ITT = intent to treat; IV = intravenous; SC = subcutaneous; SD = standard deviation.
Subject baseline values were recorded at the visit occurring just prior to receiving the first dose of study medication; this may have been day 0 or screening.
Note that cohorts were consecutive (non-parallel) in this study.
Screening postbronchodilator FEV1 predicted (%) and screening prebronchodilator FEV1/ FVC (%) were used as part of the eligibility criteria.
Safety
The incidence of all AEs was similar between placebo and benralizumab following both IV and SC administration (Table II). The most common AEs in the benralizumab groups were nasopharyngitis (25.0%) and headache (25.0%) in Cohort 1, and nasopharyngitis (22.2%) and nausea (22.2%) in Cohort 2. All AEs reported in the placebo and benralizumab groups in Cohort 1, and all those in the placebo group and approximately two-thirds of those in the benralizumab groups in Cohort 2 were mild-to-moderate in severity.
Table II. Overall summary of adverse events (safety population).
| Adverse Events | Cohort 1 (single-dose IV)a N=13 | Cohort 2 (multiple-dose SC)a N=14 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Placebo n=5 |
Benralizumab 1 mg/kg n=8 |
Placebo n=5 |
Benralizumab 100 mg n=4 |
Benralizumab 200 mg n=5 |
Benralizumab total n=9 |
|
| Total number of AEs | 17 | 27 | 20 | 15 | 10 | 25 |
|
| ||||||
| Subjects reporting ≥1 AE, n (%) | 5 (100) | 5 (62.5) | 5 (100) | 4 (100) | 2 (40.0) | 6 (66.7) |
|
| ||||||
| AEs reported by ≥2 subjects, n (%) | 5 (100) | 5 (62.5) | 5 (100) | 4 (100) | 2 (40.0) | 6 (66.7) |
| Nasopharyngitis | 0 (0.0) | 2 (25.0) | 4 (80.0) | 2 (50.0) | 0 (0.0) | 2 (22.2) |
| Nausea | 1 (20.0) | 1 (12.5) | 0 (0.0) | 1 (25.0) | 1 (20.0) | 2 (22.2) |
| Asthma | 0 (0.0) | 1 (12.5) | 1 (20.0) | 0 (0.0) | 1 (20.0) | 1 (11.1) |
| Headache | 0 (0.0) | 2 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cough | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (11.1) |
| Dyspnea | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (11.1) |
| Fatigue | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (11.1) |
| Oropharyngeal pain | 0 (0.0) | 1 (12.5) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (11.1) |
| Tremor | 1 (20.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anemia | 0 (0.0) | 0 (0.0) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: AE = adverse event; IV = intravenous; SC = subcutaneous;
Cohorts 1 and 2 were consecutive.
The incidence of AEs was generally higher in the benralizumab groups than placebo and in Cohort 1 than Cohort 2. In Cohort 1 there were no AEs in the placebo group and 17 reported by three of eight subjects in the benralizumab IV group. One subject who received 1 mg/kg IV benralizumab reported 15 of these AEs (chills, headache, asthenia [loss of energy and weakness], nausea, dysgeusia, tremor, dizziness, hot flush, hyperhidrosis, swelling on day 0, with decreased white blood cell count [2.3 ×103/μL], decreased neutrophil count [1.1 ×103/μL], increased C-reactive protein [1.61 mg/dL] measured on day 1 after dosing). All these AEs were considered to be moderate in severity apart from decreased neutrophil count, which was mild, and most resolved the following day. In Cohort 2, six AEs occurred in one of five subjects in the placebo group; and one occurred in one of nine subjects in the combined benralizumab SC group. One subject with a prior history of hyperthyroidism who received benralizumab 200 mg SC presented with symptoms consistent with a thyroid storm, was hospitalized for 8 days, and subsequently completed the study. The incident occurred 50 days after the first dose and 23 days after the last dose. The serious AE was considered to be severe, but not treatment-related. No discontinuations due to AEs or deaths occurred.
There were no apparent trends or markedly abnormal findings from serum chemistry, hematology or laboratory tests in either cohort except for the expected low blood eosinophil counts in subjects receiving benralizumab. Details of safety variables are provided in the online supplement.
Airway mucosal/submucosal eosinophils
Airway mucosal/submucosal eosinophil counts decreased from screening to endpoint for most subjects who received benralizumab [Cohort 1: 6/8, 75.0%; Cohort 2: 8/9, 88.9%] (Figs 2a and b). Eosinophil counts in biopsy samples determined using immunofluorescence at screening and day 84 are shown in Fig 2c. The photomicrographs demonstrate increased mucosal and submucosal eosinophils in the placebo-treated subject. In contrast, no eosinophils are observed at day 84 in the benralizumab-treated subject. Corresponding H&E photomicrographs from the same biopsy sections can be found in the online repository (Figure E2).
FIG 2.
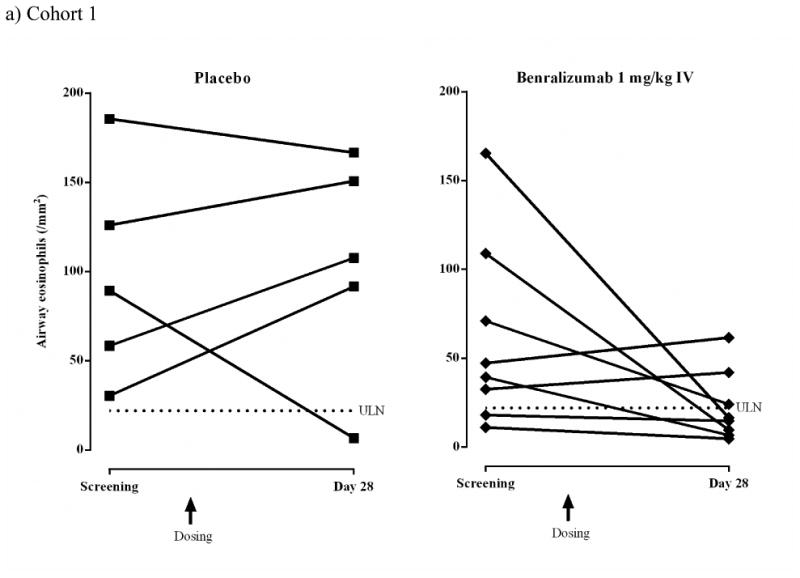
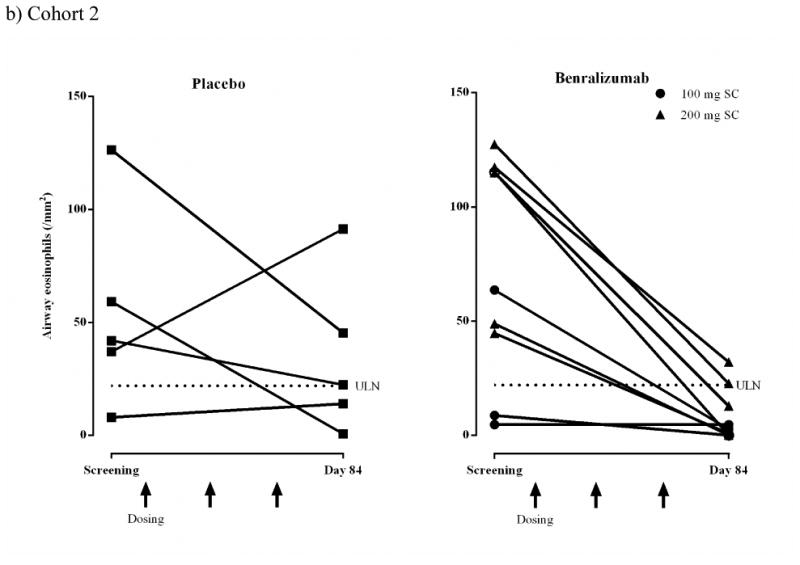

Airway eosinophils (/mm2) for individual subjects (ATP population).
a) Cohort 1 IV: placebo, n = 5; benralizumab 1 mg/kg, n = 8.
b) Cohort 2 SC: placebo, n = 5; benralizumab 100 mg, n = 4; benralizumab 200 mg, n = 5. Dashed lines represent upper limit of normal (ULN) for airway eosinophil counts (22/mm2).4,15
c) Cohort 2 SC. Representative immunofluorescence photomicrographs showing mucosal and submucosal tissue sections obtained from one placebo subject and one benralizumab subject at screening and day 84. Green indicates anti-major basic protein-1-stained eosinophils and blue indicates 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei. The inserts show sections at x400 magnification.
In Cohort 1, median (range) absolute eosinophil count increased in the placebo group from 89.3 (30.4–185.6)/mm2 at screening to 107.6 (6.7–166.7)/mm2 at day 28 (median change +19.6%) compared with a decrease in the benralizumab IV group from 43.3 (11.0–165.3)/mm2 at screening to 15.6 (4.7–61.6)/mm2 at day 28 (median change −61.9%). The difference in median percent change from screening to day 28 between placebo and benralizumab was not statistically significant (Table III).
Table III. Airway mucosal eosinophils % change from baseline (ATP population).
| Cohort | Group, Endpoint | N | Median % Change from baseline | P Value Benralizumab vs. placebo | P Value Baseline vs. endpoint | |
|---|---|---|---|---|---|---|
| 1 (IV) | Placebo, Day 28 | 5 | 19.6 | 1 | ||
| Benralizumab 1 mg/kg, Day 28 | 8 | −61.9 | .28a | .29b | ||
|
| ||||||
| 2 (SC) | Placebo, Day 84 | 5 | −46.7 | 1 | ||
| Benralizumab 100+200 mg, Day 84 | 9 | −95.8 | .06a | .01b | ||
|
| ||||||
| 1+2 | Placebo, 28 days after last dose | 10 | 4.7 | 1 | ||
| Benralizumab, 28 days after last dose | 17 | −83.1 | .02c | 0.0023b | ||
ATP = according to protocol; IV = intravenous; SC = subcutaneous.
Prespecified Wilcoxon rank-sum test of % change from baseline.
Post-hoc Sign test of absolute eosinophil counts.
Post-hoc Wilcoxon rank-sum test of % change from baseline.
In Cohort 2, median (range) absolute eosinophil count decreased in the placebo group from 42.0 (8.0–126.3)/mm2 at screening to 22.4 (0.7–91.3)/mm2 at day 84 (median change − 46.7%), and in the combined 100+200 mg benralizumab SC groups from 63.6 (4.7–127.3)/mm2 at screening to 2.7 (0–32.0)/mm2 at day 84 (median change −95.8%). The difference in median percent change from screening to day 84 between placebo and benralizumab was not statistically significant (Table III).
Data from the post-hoc analysis combining data from Cohorts 1 and 2 are shown in Table III. The difference between placebo and benralizumab in median percent change in airway mucosal/submucosal eosinophil count from screening to 28 days after the last dose was statistically significant (P = .02). For the combined placebo cohorts (n = 10), there was a 4.7% increase in airway mucosal/submucosal eosinophils 28 days after the last dose (interquartile range [Q1–Q3], −64.1% to +84.3%) compared with −83.1% (Q1–Q3: −95.8% to −57.6%) for the combined benralizumab cohorts (n = 17).
Sputum eosinophils
Induced sputum eosinophils were reduced in response to benralizumab in both cohorts (Figs 3a and b). In Cohort 1, median (range) sputum eosinophil counts in the placebo group were 13.0% (4.8–31.0%) at screening and 20.8% (2.5–33.3%) at day 21 (median change +146.2%) but decreased in the benralizumab IV group from 5.7% (4.3–11.0%) at screening to 4.5% (0–16.5%) at day 21 (median change −18.7%). At day 77, median (range) counts were 11.3% (5.0–27.3%, median change −16.7%) for placebo and 4.4% (1.5–18.0%, median change −17.7%) for benralizumab IV. In Cohort 2, median (range) sputum eosinophil counts were reduced in the placebo group from 16.8% (2.9–73.9%) at screening to 6.4% (1.9–20.0%) at day 28 (median change −66.6%) and in the combined 100+200 mg benralizumab SC groups from 4.6% (2.5– 20.8%) at screening to 0.6% (0–3.5%) at day 28 (median change −89.9%).
FIG 3.
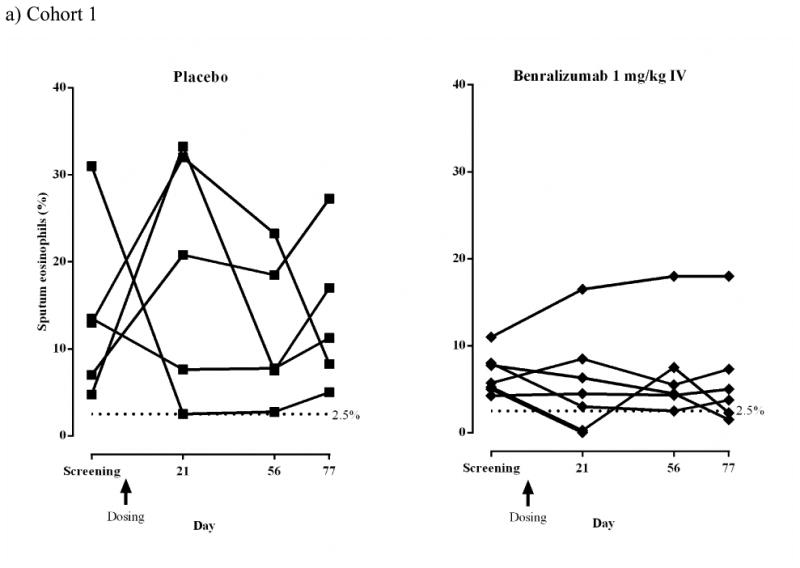
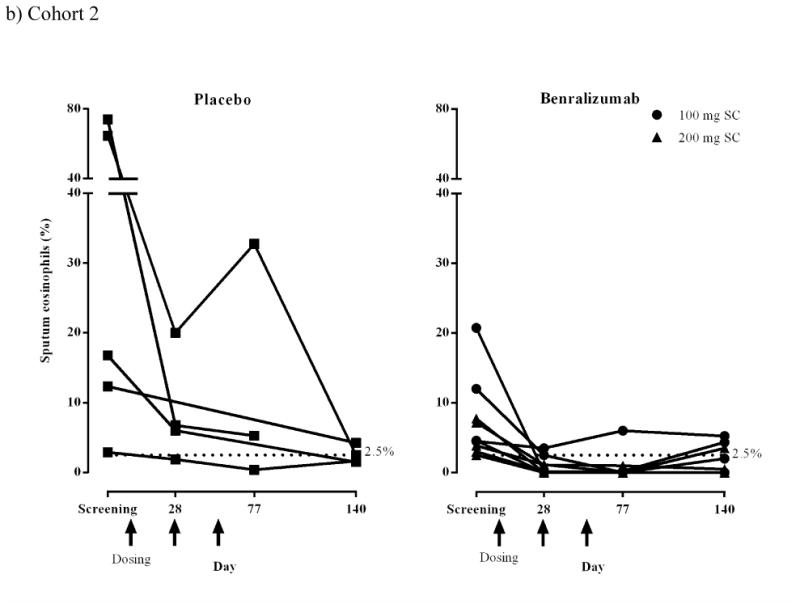
Sputum eosinophils (%) for individual subjects (ATP population).
a) Cohort 1 IV: placebo, n = 5; benralizumab 1 mg/kg, n = 7. One subject in the benralizumab group completed the study but did not have sputum induction after screening and is not included in this figure. An additional subject discontinued the study but provided sputum at screening and day 21; these data are included in this figure.
b) Cohort 2 SC: placebo, n = 5; benralizumab 100 mg, n = 4; benralizumab 200 mg, n = 5. Dashed lines represent the inclusion criteria cut-off point for sputum eosinophil counts (2.5%).
For the combined cohorts (post-hoc analysis), the median percent change from baseline to 21 days after the last dose was −46.4% (Q1–Q3: −89.5%, +171.7%) for placebo (n = 8) and − 95.1% (Q1–Q3 −100% to −6.6%) for benralizumab (n = 16; P = .04 vs. placebo).
Bone marrow eosinophils and neutrophils
Five subjects in Cohort 1 (1 placebo; four benralizumab 1 mg/kg IV) and one subject in Cohort 2 (benralizumab 100 mg SC) consented to bone marrow aspiration and provided evaluable samples at screening and day 28 (Cohort 1) or day 84 (Cohort 2). Eosinophil precursors and mature eosinophils were not detectable at day 28 in the bone marrow of all subjects who received benralizumab IV or at day 84 in the subject who received benralizumab 100 mg SC (Fig 4a). Benralizumab IV produced no clear effect on neutrophils (Fig 4b).
FIG 4.
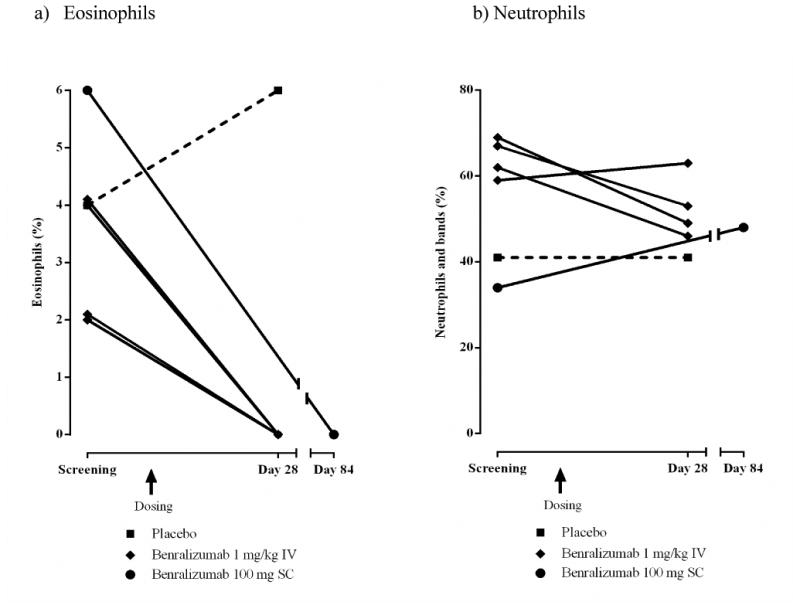
Bone marrow eosinophil and neutrophils (%) for individual subjects (ATP population). Only subjects who consented to bone marrow aspiration and provided evaluable samples at screening and endpoint are included.
a) Eosinophils. Cohort 1 IV: placebo n = 1, square with dashed line; benralizumab 1 mg/kg n = 4, diamond with solid line. Cohort 2 SC: benralizumab 100 mg n = 1, circle with solid line.
b) Neutrophils and bands. Cohort 1 IV: placebo, n = 1; benralizumab 1 mg/kg, n = 4. Cohort 2 SC: benralizumab 100 mg n = 1.
Peripheral blood eosinophils and basophils
Peripheral blood eosinophils were below the level of detection by 1 day after administration of benralizumab, and remained depleted through the end of the study in both cohorts (Figs 5a and 5b). Within Cohort 1, median (range) peripheral blood eosinophil counts did not change in the placebo group: 0.40 (0.2–0.7) ×103/μL at baseline (day 0) and day 28 [0.40 (0.3–0.5) ×103/μL]; and decreased in the benralizumab IV group from 0.15 (0.1–0.6) ×103/μL at baseline to 0 (0–0) ×103/μL at day 28. This effect continued to day 84. In Cohort 2, median (range) peripheral blood eosinophil counts for placebo were 0.30 (0.1–0.6) ×103/μL at baseline and 0.20 (0.1–0.8) ×103/μL at day 84. For the combined benralizumab SC groups, there was a decrease from 0.40 (0.2–0.9) ×103/μL at baseline to 0 (0–0) ×103/μL at day 84.
FIG 5.
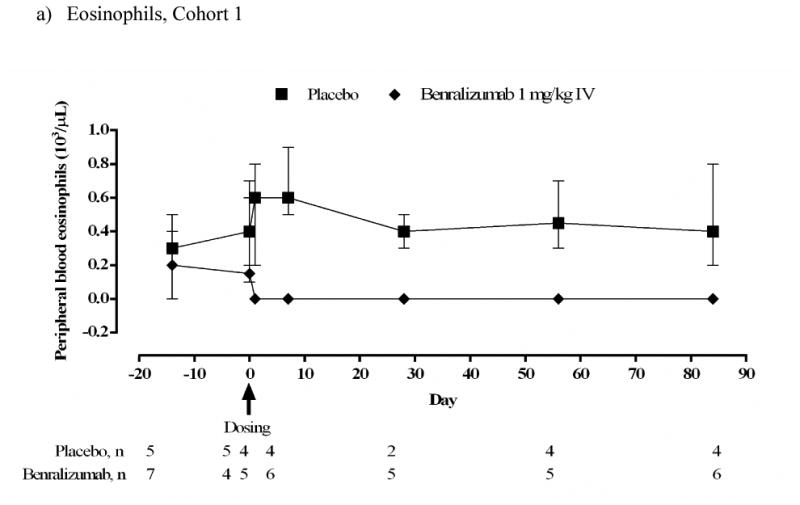
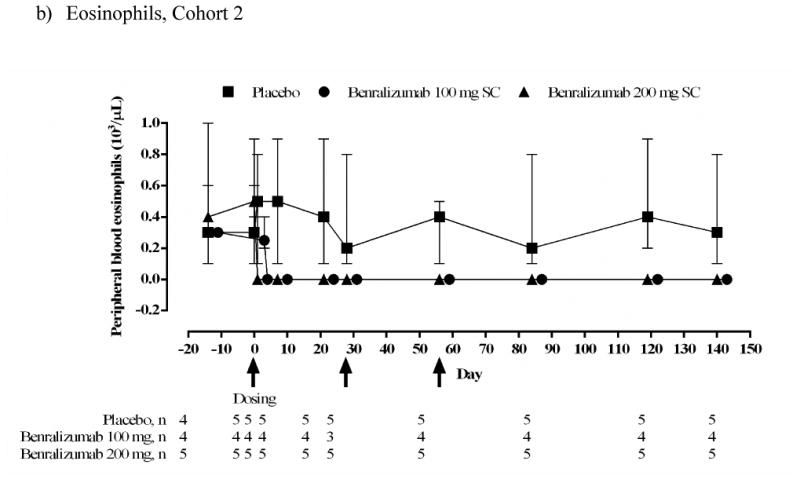

Median (range) peripheral blood eosinophil and basophil counts (103/μL) (ATP population). Subject numbers vary between timepoints depending on availability of data. At some timepoints all subjects in the benralizumab groups had the same value.
a) Eosinophils. Cohort 1 IV.
b) Eosinophils. Cohort 2 SC.
c) Basophils. Cohort 2 SC.
For the combined cohorts (post-hoc analysis), the difference in median (Q1–Q3) percent change from baseline to 28 days after the last dose was significantly lower for benralizumab vs. placebo (P < .0001): 0% (Q1–Q3: −50.0% to +20.3%) for placebo (n = 7); −100% (−100% to − 100%) for benralizumab (n = 14).
Median [range] estimated basophil counts in the placebo group were similar at baseline (0.075 [0.054–0.089 ×103/μL) and day 84 (0.073 [0.029–0.081] ×103/μL, median change +5.9%), but decreased from 0.026 [0.011–0.081] ×103/μL at baseline to 0.006 [0.001–0.030] ×103/μL at day 84 in the combined benralizumab SC group (median change −74.2%) (Fig 5c).
Discussion
This study evaluated the safety profile of benralizumab and its effects on eosinophils in the airways, sputum, bone marrow, and peripheral blood in subjects with eosinophilic asthma. Eosinophil counts were reduced in the airway mucosa following benralizumab IV and SC compared with placebo. Differences between benralizumab and placebo in percent change in airway eosinophil count were not statistically significant within cohorts, but reached statistical significance when the two cohorts were combined in a post-hoc analysis. Moreover, benralizumab administration resulted in reduction of sputum eosinophils and complete suppression of bone marrow and peripheral blood eosinophils. Benralizumab had an acceptable safety profile, though larger studies will be needed to fully assess potential safety issues.
The effect of anti-IL-5 mAb therapy on airway mucosal eosinophils was first described in steroid-naïve subjects with mild atopic asthma.16 In that study, three monthly 750 mg mepolizumab IV infusions resulted in a median decrease in airway mucosal eosinophils of 55% (P < .01 versus placebo). In a subsequent study, when subjects with refractory eosinophilic asthma received twelve monthly IV infusions of 750 mg mepolizumab, the subepithelial airway eosinophil geometric mean between group difference was 48% vs. placebo (P = .68) suggesting that dosing of mepolizumab beyond 3 months may not provide additional airway eosinophil depletion.6 Our study extends previous work by demonstrating that benralizumab, which binds with high affinity to IL-5Rα and depletes eosinophils and basophils by inducing apoptosis via enhanced ADCC, can produce a 96% median reduction in mucosal airway eosinophils after three 100 or 200 mg SC doses.
After administration of benralizumab, median blood and bone marrow eosinophil levels were reduced by 100% (undetectable) in both cohorts. Median blood eosinophil counts were undetectable from 7 days after the first dose through the end of the study. Previous studies using mepolizumab16 and reslizumab9 also reported median blood eosinophil reductions of 100% and 80%, respectively. Of the five subjects who received benralizumab and consented to bone marrow aspirates, all had undetectable levels of eosinophils and eosinophil precursors at study endpoint. Whereas, 3 mepolizumab IV doses resulted in a 52% median reduction in bone marrow eosinophils.16
In this study, subjects were required to have ≥2.5% eosinophils in sputum for inclusion. After benralizumab administration, sputum eosinophil percentages were reduced but the results were more variable than in other compartments: median reductions of 18.7% in Cohort 1, 89.9% in Cohort 2, and 95.1% in Cohorts 1 and 2 combined. Results from the combined cohorts are similar to those reported for mepolizumab8 and reslizumab.9
Basophils also express the IL-5Rα chain12 making this cell lineage another potential target of benralizumab. Basophils have been shown to be involved in asthma allergen challenges,17,18 cold air challenges,19 asthma exacerbations,20-22 and fatal asthma.23,24 In an exploratory analysis, estimated blood basophil counts determined using flow cytometry were reduced by 74%. Assessment of changes in basophil numbers in airway biopsies, sputum, and bone marrow were not feasible due to baseline values of 0 or near 0 (data not shown).
This study has some limitations. The sample size of the study was smaller than published suggested sample sizes for airway biopsy studies.25,26 However, post-hoc analyses performed on combined Cohorts 1 and 2 resulted in statistically significant reductions in airway and sputum eosinophils. Sputum analyses were performed locally at each site, which may have increased variability of the results. However, all sites had experience in performing induced sputum analysis, and cell counts were performed by experienced cytotechnologists. Concomitant corticosteroid therapy was not standardized: with doses ranging from none to high-dose ICS plus oral corticosteroids (one placebo subject), though the median ICS budesonide equivalent dose was 800 μg for the benralizumab groups in Cohorts 1 and 2. This contrasts to mepolizumab airway biopsy studies that allowed no ICS16 or required high dose ICS +/- oral corticosteroids.6 Lastly, documentation of adherence to ICS prior to and during the screening/run-in period was not obtained. Any changes in ICS usage during this period could have had an effect on tissue and sputum eosinophil numbers.
In conclusion, these results suggest eosinophils and possibly basophils are effectively depleted after administration of benralizumab, a mAb that functions via an ADCC pathway. A potential clinical response to benralizumab was demonstrated in a recent phase II study in which the number and severity of exacerbations were reduced in subjects with acute severe asthma.27 Confirmation of these results and determination of the target population are being explored in an ongoing Phase IIb study.
Supplementary Material
Clinical Implications.
Benralizumab, a monoclonal antibody that targets the interleukin-5 receptor and induces antibody-dependent cell-mediated cytotoxicity, reduced airway, sputum, bone marrow and blood eosinophils, and may provide therapeutic benefit in eosinophilic asthma.
Acknowledgments
The authors thank Diana Swanson, PhD (MedImmune), Lourdes Briz (MedImmune), and Jennifer Stewart, MSc (QXV Communications, Macclesfield, UK; funded by MedImmune) for assistance with manuscript preparation; Dr. Laura Richman, DVM, PhD (MedImmune), who read the pathology slides; and Steven Eck, PhD (MedImmune) for flow-cytometry analysis of basophils.
Funding: This study was sponsored by MedImmune, LLC.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- AE
adverse event
- ATP
according to protocol
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- H&E
hematoxylin and eosin
- ICS
inhaled corticosteroids
- IL-5
interleukin-5
- IL-5R
interleukin-5 receptor
- ITT
intent-to-treat
- IV
intravenous
- mAb
monoclonal antibody
- SC
subcutaneous
Footnotes
Online repository materials: This article has an online supplement, which is accessible from this issue's table of contents at www.jacionline.org.
References
- 1.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–221. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 2.Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemiere C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483–94. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 3.Chlumský J, Striz I, Terl M, Vondracek J. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J Int Med Res. 2006;34:129–39. doi: 10.1177/147323000603400202. [DOI] [PubMed] [Google Scholar]
- 4.Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. 2005;116:1249–55. doi: 10.1016/j.jaci.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Long AA. Monoclonal antibodies and other biologic agents in the treatment of asthma. mAbs. 2009;1:237–46. doi: 10.4161/mabs.1.3.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavord I, Korn S, Howath P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 8.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Res-5-0010 Study Group Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–32. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 10.Takatsu K, Dickason RR, Huston DP. Interleukin-5. In: Leroith D, Bondy C, editors. Growth Factors and Cytokines in Health and Disease. Vol. 2. Greenwich: JAI Press Inc; 1997. pp. 143–200. [Google Scholar]
- 11.Toba K, Koike T, Shibata A, Hashimoto S, Takahashi M, Masuko M, et al. Novel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophils. Cytometry. 1999;35:249–59. [PubMed] [Google Scholar]
- 12.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–53. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–44. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 14.National Heart Lung and Blood Institute. National Asthma Education and Prevention Program. Guidelines for the Prevention and Diagnosis of Asthma: Expert Panel Report 3. 2007;4051:2007. NIH Publication Number 08-4051. [Google Scholar]
- 15.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes:role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–8. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 17.Guo CB, Liu MC, Galli SJ, Bochner BS, Kagey-Sobotka A, Lichtenstein LM. Identification of IgE-bearing cells in the late-phase response to antigen in the lung as basophils. Am J Respir Cell Mol Biol. 1994;10:384–90. doi: 10.1165/ajrcmb.10.4.7510984. [DOI] [PubMed] [Google Scholar]
- 18.Gauvreau GM, Lee JM, Watson RM, Irani AM, Schwartz LB, O'Byrne PM. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med. 2000;161:1473–8. doi: 10.1164/ajrccm.161.5.9908090. [DOI] [PubMed] [Google Scholar]
- 19.Kauffmann F, Nuekirsch F, Annessi I, Korobaeff M, Doré MF, Lellouch J. Relation of perceived nasal and bronchial hyperresponsiveness to FEV1, basophil counts, and methacholine challenge. Thorax. 1988;43:456–61. doi: 10.1136/thx.43.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura I, Moritani Y, Tanizaki Y. Basophils in bronchial asthma with reference to reagin-type allergy. Clin Allergy. 1973;3:195–202. doi: 10.1111/j.1365-2222.1973.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama N, Tamura G, Aizawa T, Ohrui T, Shimura S, Shirato K, et al. Accumulation of basophils and their chemotactic activity in the airways during natural airway narrowing in asthmatic individuals. Am J Respir Crit Care Med. 1994;150:1086–93. doi: 10.1164/ajrccm.150.4.7921441. [DOI] [PubMed] [Google Scholar]
- 22.Ono E, Taniquichi M, Mita H, Kajiwara K, Yamaguchi H, Tatsuno S, et al. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2010;125:483–9. doi: 10.1016/j.jaci.2009.10.074. e3. [DOI] [PubMed] [Google Scholar]
- 23.Koshino T, Teshima S, Fukushima N, Takaishi T, Hirai K, Miyamoto Y, et al. Identification of basophils by immunohistochemistry in the airways of post-mortem cases of fatal asthma. Clin Exp Allergy. 1993;23:919–25. doi: 10.1111/j.1365-2222.1993.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 24.Kepley CL, McFeeley PJ, Oliver JM, Lipscomb MF. Immunohistochemical detection of human basophils in postmortem cases of fatal asthma. Am J Respir Crit Care Med. 2001;164:1053–8. doi: 10.1164/ajrccm.164.6.2102025. [DOI] [PubMed] [Google Scholar]
- 25.Sont JK, Willems LNA, Evertse CE, Hooijer R, Sterk PJ, van Krieken JHJM. Repeatability of measures of inflammatory cell number in bronchial biopsies in atopic asthma. Eur Respir J. 1997;10:2602–8. doi: 10.1183/09031936.97.10112602. [DOI] [PubMed] [Google Scholar]
- 26.Richmond I, Booth H, Ward C, Walters EH. Intrasubject variability in airway inflammation in biopsies in mild to moderate stable asthma. Am J Respir Crit Care Med. 1996;153:899–903. doi: 10.1164/ajrccm.153.3.8630570. [DOI] [PubMed] [Google Scholar]
- 27.Molfino NA, Nowak R, Silverman R, Rowe B, Smithline H, Kahn F, et al. Reduction in the number and severity of exacerbations following acute severe asthma: results of a placebo-controlled, randomized clinical trial with benralizumab. Am J Respir Crit Care Med. 2012;185:A2753. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


