Abstract
Objective
We developed a novel method to map behavioral effects of deep brain stimulation (DBS) across a 3D brain region and to assign statistical significance after stringent Type I error correction. This method was applied to behavioral changes in Parkinson disease (PD) induced by subthalamic nucleus (STN) DBS to determine whether these responses depended on anatomical location of DBS.
Method
Fifty-one PD participants with STN DBS were evaluated off medication, with DBS off and during unilateral STN DBS with clinically optimized settings. Dependent variables included DBS-induced changes in Unified Parkinson Disease Rating Scale (UPDRS) subscores, kinematic measures of bradykinesia and rigidity, working memory, response inhibition, mood, anxiety, and akathisia. Weighted t-tests at each voxel produced p images showing where DBS most significantly affected each dependent variable based on outcomes of participants with nearby DBS. Finally, a permutation test computed the probability that this p image indicated significantly different responses based on stimulation site.
Results
Most motor variables improved with DBS anywhere in the STN region, but several motor, cognitive and affective responses significantly depended on precise location stimulated, with peak p values in superior STN/zona incerta (quantified bradykinesia), dorsal STN (mood, anxiety), and inferior STN/substantia nigra (UPDRS tremor, working memory).
Interpretation
Our method identified DBS-induced behavioral changes that depended significantly on DBS site. These results do not support complete functional segregation within STN, since movement improved with DBS throughout, and mood improved with dorsal STN DBS. Rather, findings support functional convergence of motor, cognitive and limbic information in STN.
Introduction
Deep brain stimulation of the subthalamic nucleus (STN DBS) reduces motor symptoms in Parkinson disease (PD), but the extent of benefit varies. STN DBS also can induce variable effects on mood and cognition, thereby affecting quality of life1-3. This variability may be due in part to stimulation location in or around the STN, which is anatomically linked to motor, associative and limbic cortical regions4-7. Identifying which anatomical DBS locations most influence specific behavioral outcomes may allow improved prediction of optimal lead placement for motor benefit and side effect reduction in PD patients treated with STN DBS.
This study aimed to extend and improve on previous attempts to link DBS location to behavioral outcomes with a novel method that maps behavior onto stimulation sites in a 3-dimensional (3D) and statistically rigorous manner. Previous attempts to map behavior onto STN DBS sites did not investigate the whole relevant volume of brain8-10, did not test purported relationships between DBS sites and behavior for statistical significance11-14, or did not correct for the multiple comparisons inherent in 3D statistical maps15. Further progress requires a method to map effects of DBS onto anatomy that adequately tests the statistical significance of any link between clinical effect and DBS active contact location.
We present here methods to map at each location in a targeted brain region the expected behavioral effect of DBS and the probability that the effect differs from zero by chance. However, these maps do not directly test whether DBS is superior at one versus another location in the image. They are also susceptible to misinterpretation if most patients respond similarly to DBS on a given measure; we call this the sample mean problem. To understand this problem, imagine that DBS produces some placebo effect in nearly all patients. In this case, any DBS location with adequate data will appear to produce significant benefit, even though the placebo DBS “effect” has no true connection with stimulation site. Similar false positive results can occur for any real outcome on which most patients respond similarly to DBS; consequently, significant local p values alone cannot confirm a true link of DBS site to outcome. We solve this problem and simultaneously correct for multiple comparisons using a permutation test to provide a single global (corrected) p value for each outcome measure.
We applied these novel methods to rigorously investigate the relationships between STN DBS sites and motor, cognitive and mood outcomes in 51 PD participants with bilateral STN DBS. Based on previous findings12,16-18 and functional models of the STN4-7, we predicted that participants with DBS sites within the posterodorsal STN and adjacent zona incerta (ZI) might show greater improvement in motor signs than participants with DBS elsewhere in the STN. We further hypothesized that impaired cognition and improved mood would be significantly related to DBS sites in ventral and not dorsal STN, based on putative functional heterogeneity of the STN4-6 and previous reports8,16-19.
Methods
Participants
The dataset included 51 participants with bilateral STN DBS for treatment of PD recruited from the Washington University in St. Louis Movement Disorders Center (see Table 1 for demographics). The dataset overlaps with that described previously10,20-25, but the analysis strategy applied here is unique. Participants were diagnosed with PD based on established criteria and screened for dementia, stroke, head injury and other neurologic diseases before DBS surgery26. All participants had bilateral Soletra pulse generators with Medtronic DBS leads model 3389 except for two participants who had DBS leads model 3387 (Medtronic Inc., Minneapolis, MN). Guided by MR imaging, stereotactic navigation and microelectrode recording27, DBS leads were implanted in the STN a minimum of 3 months and a median of 8 months prior to participation in the study, and DBS settings were clinically optimized for each participant based on symptoms and motor signs. Only data obtained from monopolar DBS pattern were included. The study was approved by the Washington University Human Research Protection Office at Washington University in St. Louis, and all participants provided written informed consent.
Table 1.
Demographic and clinical characteristics of the 51 Parkinson disease research participants.
| Mean | SD | |
|---|---|---|
| Age (years) | 60.4 | 8.8 |
| Education (years) | 15.0* | 3.0 |
| Disease duration (years) | 13.9 | 4.7 |
| Time since STN DBS surgery (months) |
12.39 | 11.28 |
| Distribution | ||
| Gender | 37 Male, 14 Female | |
| Race and ethnicity | 46 White, 3 Black, 2 Native American | |
| More affected side, by UPDRS III subscore |
22 Right, 27 Left† | |
| Dominant hand | 44 Right, 7 Left | |
| Current PD medicationa,b,c,# | 36 CL Non-extended release /10 CL extended release /31 DA agonist/ 6 MAO inhibitor/16 COMT inhibitor |
|
STN DBS, deep brain stimulation of the subthalamic nucleus; PD, Parkinson disease; CL, carbidopa-levodopa; DA, dopaminergic; MAO, monoamine oxidase; COMT, catechol-O-methyl transferase
Data missing for 1 participant.
No worse side for 2 participants (equivalent OFF DBS left and right total UPDRS III scores)
prior to abstinence on the day of the study
participant may fall in more than one medication category
no participant was taking extended release DA agonists, MAO or COMT inhibitors
data missing: CL type = 5 participants; non-CL dopaminergic agents = 6 participants
Stimulation protocol
On each study day, participants began study participation after taking no antiparkinsonian medications since midnight. Motor, cognitive, and mood measurements were obtained during each of 3 stimulation conditions: DBS OFF, unilateral right DBS ON, and unilateral left DBS ON. Each participant’s clinically optimized DBS contact and stimulation settings were used for each electrode. Across participants, voltage ranged from 1.7 to 3.6 volts, frequency was 145 to185 Hz, and pulse width was 60 or 90 μs. DBS condition order was counterbalanced across participants, and investigators and participants were blind to DBS conditions. Behavioral assessments were performed at least 42 minutes after each change of DBS condition, by which time near-steady-state motor response to DBS is expected28.
Assessments
Movement
During each stimulation condition, motor function was assessed by UPDRS (Part III, motor) by trained clinicians and by quantified kinematic measures of bradykinesia (by measuring speed of hand rotation using a gyroscope at the wrist 3 times consecutively) and rigidity across the elbow joint (by measuring impedance using a rigidity analyzer, 3 times consecutively)23. Dependent variables included change between the OFF and unilateral ON stimulation condition in total UPDRS Part III score or selected item scores including bradykinesia, rigidity, and tremor29 for the side contralateral to DBS. UPDRS items scored separately for left and right extremities were combined to form left and right contralateral hemibody and worse-side UPDRS subscores. The more affected side of the body was defined by comparing left- and right-sided motor scores on the UPDRS Part III rated with participants off medication and OFF DBS.
Cognition
Working memory was assessed by spatial delayed response (SDR) as described previously21,25. The dependent variable was the percent change between OFF and ON stimulation conditions in mean error (distance between a previously presented target cue on a computer screen and recalled location of target cue).
Response selection and inhibition were assessed by the Go/No-Go task as previously described8. Percent change in ability to discriminate between ‘Go’ and ‘No-Go’ stimuli (Pr) between OFF and ON stimulation conditions was treated as the dependent variable. Only data from participants who reached a criterion of Pr > 0.5 during the OFF DBS condition were included in the analyses8.
Mood, anxiety and akathisia
Self-reported emotional state was assessed using visual analog scale (VAS) ratings, converted to valence and arousal scores based on the circumplex model of emotion (arousal is also called “activation”)30-31. Self-rated anxiety, apathy, and akathisia were also assessed by VAS. Changes in each of these scores between OFF and left or right ON DBS conditions were dependent variables.
Univariate statistics
STN DBS-induced changes in motor behavior, cognition, mood, anxiety and akathisia, without regards to anatomy, were tested for significance with paired t-tests.
3-Dimensional location of electrode contacts
Image processing and atlas registration were performed as described previously8,32. Briefly, preoperative MR and postoperative CT images of the head were co-registered as rigid bodies, and the MR image was mapped to the stereotactic atlas of Mai et al.33 based on structural fiducial points surrounding the STN32. This method assigns midbrain locations to atlas space with mean accuracy < 1 mm32. Atlas coordinates are reported herein with positive y values for points anterior to the anterior commissure rather than the opposite convention used in the original atlas publication33.
Mapping DBS effects to 3D anatomy
Statistical inference linking DBS location in 3D to its effects is not a trivial problem. Superficially, the DBS question seems similar to generating 3D statistical images from PET and fMRI data, a problem for which software packages such as SPM (http://www.fil.ion.ucl.ac.uk/spm/) or AFNI (http://afni.nimh.nih.gov/) are routinely used. But data from DBS violates two important assumptions of such software. First, such software generally assumes that the number of participants contributing data does not vary from one analyzed voxel to another, since each PET or fMRI image provides data throughout the brain. By contrast, DBS at one location in a given participant contributes no information to predict what would happen with DBS at a distant location (i.e., a voxel more than a few mm away from the participant’s active contact). Including data from all participants at each voxel analyzed would artificially inflate the degrees of freedom and hence the claimed statistical significance. Second, extant neuroimaging statistical software compares the observed data to a null hypothesis of zero mean change at each voxel, i.e. assumes that under the null hypothesis there is no nonzero bias in the sample. That assumption makes sense for PET or fMRI data, but as described in the introduction, will produce false positives if most participants improve (or worsen) on a given outcome measure regardless of DBS location. Thus a new approach was required.
For each dependent measure, several 3D images with 0.2mm cubic voxels were created as follows:
Mean Effect image
The mean effect image shows at each voxel the weighted mean effect of DBS on the selected outcome measure, where measures from participants stimulated nearer that voxel are given greater weight. Specifically, the value of the effect image at voxel i is the weighted mean of the numerical effect gk of DBS on the selected behavioral measure in participant k. The weighting function wik is a monotonically decreasing function of the distance between voxel i and the contact stimulated in participant k. In other words, the result of DBS in one participant contributes good information about what happens when DBS is administered at the exact location of his/her active contact, reasonable information about what happens when DBS is administered nearby, and progressively less information about what happens with DBS at locations farther and farther from that point. The analyses presented here used a 3D Gaussian function for w with FWHM 3.0 mm. With this weighting function, w at 1.5 mm away is half of w at the active contact itself. Since we are not modeling the electrical effects of DBS but only estimating the location of the stimulated contact, a 3D normal distribution is a reasonable choice of weighting function. Nevertheless, this choice was influenced by an estimate that voltage dropped 50% through ~1.5 mm of gray matter34. In fact, for monopolar stimulation near the STN, a spherical model for contact location actually approximates quite well modeled predictions of activated axons (Fig 3 in McIntyre et al.35 and Fig. 2 in Maggio et al.36). The weighted mean effect image can be interpreted as showing at each point the expected effect of DBS at that point.
N image, t image, and p image
One cannot deduce from the weighted mean effect alone whether DBS-induced outcomes differ significantly from zero at a given point; a probability map is needed. We performed a weighted t test37 at each voxel, again weighting by nearness to each participant’s stimulated contact. To compute and interpret the t statistic, the number of participants contributing data to the voxel must first be considered. To avoid artificially inflating the degrees of freedom for interpreting the t statistic, participants were considered to have contributed data to a voxel only if their active contact in the stimulation condition being examined was close enough that its weight was at least 5% of the maximum weight. Bland and Kerry37 do not address this directly, but the 5% threshold was based on the example presented in that publication. Thus an N image was defined as Ni = ∑k {1, if wik ≥ 0.05; 0, elsewhere}; with the weighting function described above, Ni is the number of participants stimulated within 3.1 mm of voxel i. The weighted t statistic ti was set to zero if fewer than 6 participants had contributed data to a given voxel. For Ni ≥ 6, ti was computed as follows:
Since the significance of a t statistic depends on the degrees of freedom (d.f.), and the d.f. was not constant across the image space, a probability image p was computed whose value pi at each voxel i was the one-tailed probability of exceeding the given t statistic with d.f. = Ni −1. The values in the t image can be viewed as effect sizes for DBS behavioral outcome, and corresponding p values are found in the p image. We refer to the most significant p value in the p image for a given dependent variable as the “peak p”.
Type I error correction for multiple comparisons and sample bias
As we noted, statistical images of this type from DBS data do not directly test whether stimulation is better at one site that at another, and cannot be corrected for false positives merely by requiring a high statistical threshold at the voxel level or by applying typical neuroimaging software. To address the multiple comparisons problem and the sample bias problem discussed above, we implemented a permutation analysis inspired by SnPM38 (http://go.warwick.ac.uk/tenichols/snpm). This approach provides a single global p value testing whether DBS effects differ significantly based on active contact location.
For each dependent measure, the permutation test first reduces the original statistical image containing over 2 million voxels to a single summary statistic Q, defined as
Effectively Q reflects the collective size and magnitude of “hot spots” in the probability image p computed from the real data, i.e. by pairing the quantitative outcome gj in each participant with the location xj of that participant’s active contact. The null hypothesis is that one could obtain an equal or greater value of Q by randomly pairing the outcomes and active contact locations without regard to which participant actually contributed each datum. The statistical significance is determined by comparing the true value of Q to 200 false Q values, one from each of 200 false p images generated by randomly pairing outcomes with active contact locations. If 10 or fewer of these 200 false Qs are as big as the true Q, then the overall (corrected) p value is ≤ 10/200 = 0.05, rejecting the null hypothesis. To address multiple comparisons and the sample bias problem, we had to sacrifice the focality of our conclusion; i.e. the corrected p value refers to the whole probability image, not to any one of its voxels. Nevertheless, the p image provides reasonable clues as to the locations that may drive the significant association between DBS location and behavioral outcome.
Visualization
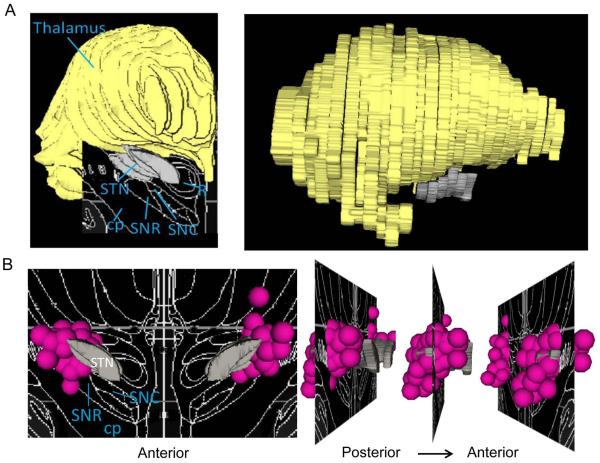
We created 3D images of the left and right thalamus and left and right STN from the Mai et al.33 atlas of the human brain using methods described by Zhang et al.39. The program 3D Slicer40(www.slicer.org) was used to create 3D models from these images and to display statistical images relative to these Mai33 atlas-defined structures. The white-on-black 2D atlas tracings shown in some figures were created as described in Videen et al.32. A 3D image that includes the thalamus, STN and nearby regions is shown for reference (Fig 1A).
Figure 1. 3D models of the STN, thalamus, and DBS electrode contacts.
A.) Brain regions are overlaid on the Mai atlas (18.2 mm posterior to AC) in coronal (left) and sagittal (right) views. B.) The 3D distribution of clinical STN DBS electrode contacts are presented coronally (left) and sagittally (right), overlaid on the structures from the Mai atlas at 16.8 mm posterior to AC. Most clinical DBS contacts fall in the STN. STN, subthalamic nucleus; cp, cerebellar peduncle; SNR, substantia nigra pars reticulata; SNC, substantia nigra pars compacta; R, red nucleus. Gray = STN; pink spheres = approximate contact location, 1.5 mm radius for display.
Relationships between STN DBS-induced changes in non-motor and motor function
In non-motor measures where DBS-induced changes were significantly associated with DBS location, relationships with ipsilateral DBS-induced changes in motor function were assessed with Pearson’s r or Spearman’s ρ.
Results
Mean effects of unilateral STN DBS (without reference to anatomy)
Group mean effects of unilateral DBS on movement, cognition and emotion are summarized in Tables 2-3. The n varies across measures for the following reasons: Two participants did not have a worse side for motor function as determined by OFF DBS, off PD medication UPDRS III. One of these and six other participants had non-monopolar STN DBS clinical settings on the left side of the brain. Other reasons include incomplete (data not obtained for DBS OFF condition or obtained for only one unilateral DBS condition) or no data due to data loss, software malfunction or difficulty on behalf of the participant in completing the condition (UPDRS motor n = 5; kinematics motor n = 11; SDR n = 6; Pr n = 20; VAS valence, arousal and anxiety, n = 7 each; VAS apathy = 5). For SDR, one participant’s behavioral data was an extreme outlier. For Pr, 6 participants did not meet criteria of Pr > 0.5 during the OFF DBS condition. The VAS ratings were introduced later in the study so that approximately half of the 51 participants never completed VAS.
Table 2.
Mean change in motor measures from OFF induced by worse-side, right and left brain subthalamic deep brain stimulation (STN DBS).
|
Outcome Measure and DBS Side |
Number of Participants with improved scores |
Number of Participants |
Mean difference from OFF DBS |
Standard deviation |
t statistic |
p- value |
|---|---|---|---|---|---|---|
| Contralateral UPDRS Total | ||||||
| Worse | 44 | 46 | −5.88 | 3.76 | −10.6 | <0.001 |
| Right | 39 | 48 | −4.70 | 4.59 | −7.09 | <0.001 |
| Left | 39 | 42 | −4.52 | 3.76 | −7.79 | <0.001 |
| Contralateral Hand Rotation Velocity (deg/s) | ||||||
| Worse | 33 | 39 | 147.19 | 154.16 | 5.96 | <0.001 |
| Right | 36 | 40 | 178.75 | 126.56 | 8.93 | <0.001 |
| Left | 28 | 35 | 94.93 | 204.54 | 2.75 | 0.01 |
| Contralateral Impedance (Nm/deg) | ||||||
| Worse | 30 | 39 | −0.55 | 0.82 | −4.22 | <0.001 |
| Right | 36 | 41 | −0.63 | 0.69 | −5.87 | <0.001 |
| Left | 26 | 35 | −0.48 | 1.15 | −2.47 | 0.02 |
| Contralateral UPDRS Bradykinesia | ||||||
| Worse | 38 | 46 | −0.66 | 0.59 | −7.69 | <0.001 |
| Right | 37 | 50 | −0.52 | 0.69 | −5.35 | <0.001 |
| Left | 31 | 43 | −0.43 | 0.55 | −5.17 | <0.001 |
| Contralateral UPDRS Rigidity | ||||||
| Worse | 42 | 46 | −1.84 | 1.11 | −11.3 | <0.001 |
| Right | 36 | 48 | −1.39 | 1.35 | −7.11 | <0.001 |
| Left | 35 | 42 | −1.56 | 1.57 | −6.44 | <0.001 |
| Contralateral UPDRS Tremor | ||||||
| Worse | 24 | 47 | −0.99 | 1.67 | −4.07 | <0.001 |
| Right | 25 | 50 | −0.85 | 1.57 | −3.84 | <0.001 |
| Left | 15 | 43 | −0.48 | 1.61 | −1.94 | 0.06** |
UPDRS, Unified Parkinson Disease Rating Scale; deg/s, degrees per second; Nm/deg, Newton · meter/degree.
, p = 0.01 among participants with nonzero tremor at baseline (n = 20).
Table 3.
Mean change in non-motor measures from OFF induced by worse-side, right and left brain subthalamic nucleus (STN DBS).
|
Outcome Measure and DBS Side |
Number of Participants with improved scores |
Number of Participants |
Mean difference from OFF DBS |
Standard deviation |
t statistic |
p- value |
|---|---|---|---|---|---|---|
|
Cognition
| ||||||
| SDR (error in mm) | ||||||
| Worse | 20 | 39 | 0.09 | 0.30 | 1.93 | 0.061 |
| Right | 19 | 44 | 0.08 | 0.38 | 1.37 | 0.180 |
| Left | 17 | 39 | 0.01 | 0.23 | 0.30 | 0.764 |
| Go/No-Go (Pr) | ||||||
| Worse | 11 | 23 | −0.04 | 0.26 | −0.74 | 0.47 |
| Right | 13 | 27 | −0.01 | 0.17 | −0.43 | 0.67 |
| Left | 13 | 22 | −0.11 | 0.29 | −1.83 | 0.08 |
|
| ||||||
|
Psychiatric
| ||||||
| Valence | ||||||
| Worse | 18 | 23 | 0.24 | 0.35 | 3.33 | 0.003 |
| Right | 16 | 24 | 0.13 | 0.34 | 1.86 | 0.076 |
| Left | 18 | 22 | 0.27 | 0.36 | 3.54 | 0.002 |
| Arousal | ||||||
| Worse | 9 | 23 | 0.01 | 0.39 | 0.11 | 0.910 |
| Right | 13 | 24 | −0.04 | 0.21 | −0.87 | 0.400 |
| Left | 8 | 23 | 0.00 | 0.40 | −0.003 | 1.000 |
| Anxiety | ||||||
| Worse | 19 | 25 | −10.57 | 25.58 | −2.07 | 0.050 |
| Right | 19 | 26 | −6.41 | 21.52 | −1.52 | 0.140 |
| Left | 16 | 23 | −13.20 | 25.05 | −2.53 | 0.020 |
| Apathy a | ||||||
| Worse | 16 | 25 | 11.64 | 29.59 | 1.97 | 0.060 |
| Right | 15 | 27 | 0.26 | 25.57 | 0.05 | 0.958 |
| Left | 14 | 24 | 12.38 | 35.25 | 1.72 | 0.100 |
| Akathisia b | ||||||
| Worse | 14 | 25 | −4.20 | 27.08 | −0.78 | 0.446 |
| Right | 16 | 27 | 1.37 | 18.26 | 0.39 | 0.700 |
| Left | 13 | 24 | −5.96 | 28.08 | −1.04 | 0.309 |
SDR, spatial delayed response task; mm, millimeter; Pr, ability to discriminate between ‘Go’ and ‘No-Go’ stimuli.
Positive mean difference score for apathy indicates decreased apathy/increased motivation.
Negative mean difference score for akithisia indicates decreased restlessness.
Relative to OFF DBS, stimulation of right or left brain, or contralateral to the clinically worse side of the body (hereinafter “worse-brain STN DBS”) improved mean scores on all motor measures (Table 2). STN DBS did not significantly affect mean SDR or Go/No-Go performance. Worse-brain and left-brain STN DBS improved mood as reflected in valence and anxiety scores. STN DBS had no significant effect on apathy (p ≥ 0.06), akathisia (p ≥ 0.45), or affective arousal (p ≥ 0.40) (Table 3).
Distribution of active contacts
Data are from participants with DBS at contacts that cover the posterior 2/3 of the STN in its vertical and left-right extent as well as ventral thalamus, zona incerta (ZI), and substantia nigra (SN) (Fig 1B; see also Fig 2, N image).
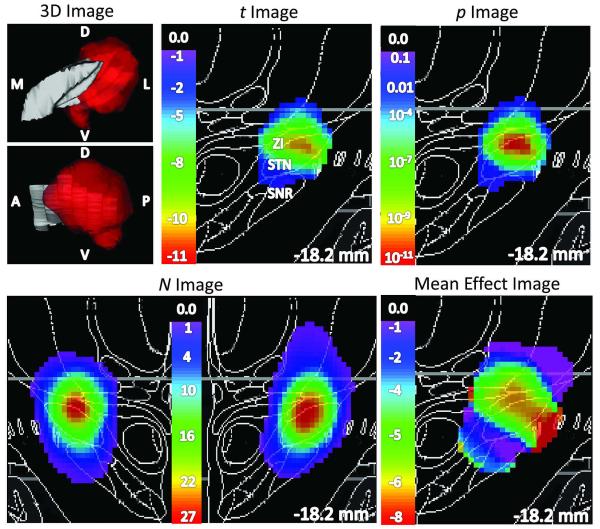
Figure 2. STN DBS-induced improvement in UPDRS III motor scores did not differ significantly by DBS site.
The left panel shows 3D views of the left STN in gray, with color indicating voxels for which p < 0.05 by weighted t test (upper image: viewed from a point anterior to STN; lower image: viewed from a point lateral to STN). Statistical images in color, laid over coronal slices from the atlas, are shown in the top center panel (t image, thresholded at N ≥ 6), top right panel (p image, thresholded at p < 0.05), bottom left panel (N image) and bottom right panel (weighted mean effect image, thresholded at N ≥ 6). Notably, although the p image includes values as low as 10−11, the improvement did not differ significantly by contact location (p = 0.12 by permutation test; see Results). D, dorsal; V, ventral; A, anterior; P, posterior; M, medial; L, lateral. ZI, zona incerta; SNR, substantia nigra.
STN DBS effects: Dependence on anatomical location
One analysis is presented in detail to demonstrate the new method (see Fig 2). Measures with significant relationships between DBS site and behavioral outcome (corrected p ≤ 0.05 by permutation test, Tables 4 and 5, last columns) are shown in Fig 3 and 4 and discussed briefly. All results are summarized in Tables 4 and 5.
Table 4.
Effects of anatomical location of active deep brain stimulation (DBS) contact on movement.
|
Outcome Measure and DBS Side |
N | Peak N |
x | y | z | Peak t |
x | y | z | Peak p |
x | y | z | Peak p Region |
Corrected p- value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contralateral UPDRS Total | |||||||||||||||
| Worse | 46 | 32 | 12.0 | −18.0 | −3.0 | −12.5 | 9.00 | −18.5 | −2.5 | 10−13 | 12.0 | −18.0 | −2.5 | STN | 0.235 |
| Right | 48 | 32 | 12.5 | −17.5 | −3.0 | −7.90 | 12.5 | −19 | −2.5 | 10−8 | 12.0 | −19.5 | −2.5 | ZI | 0.080 |
| Left | 42 | 27 | −12.5 | −18.0 | −3.0 | −12.4 | −15.0 | −20.5 | −4.0 | 10−11 | −13.0 | −18.5 | −3.0 | STN/ZI | 0.120 |
| Contralateral Hand Rotation Velocity (deg/s) | |||||||||||||||
| Worse | 39 | 28 | 12.0 | −18.0 | −3.0 | 8.20 | 12.0 | −18 | −2.5 | 10−9 | 12.0 | −18.0 | −2.5 | STN | 0.085 |
| Right | 40 | 28 | 12.5 | −17.5 | −3.0 | 14.4 | 11.5 | −15 | −4.0 | 10−13 | 12.5 | −17.5 | −3.0 | STN/ZI | 0.005 |
| Left | 35 | 24 | −12.5 | −18.5 | −3.5 | 5.70 | −12.5 | −15 | −1.0 | 10−4 | −12.5 | −16.0 | −2.5 | dSTN | 0.720 |
| Contralateral Impedance (Nm/deg) | |||||||||||||||
| Worse | 39 | 28 | 12.0 | −18.0 | −3.0 | −5.00 | 13.5 | −19 | −7.5 | 10−4 | 13.0 | −19.0 | −6.0 | STN/SN | 0.800 |
| Right | 41 | 28 | 12.5 | −17.5 | −3.0 | −5.80 | 13.0 | −18.5 | −7.5 | 10−4 | 14.0 | −19.0 | −3.5 | STN/ZI | 0.835 |
| Left | 35 | 24 | −12.5 | −18.5 | −3.5 | −5.20 | −13.5 | −16.0 | 0.0 | 10−3 | −13.5 | −16.0 | 0.0 | ZI/VLP | 0.545 |
| Contralateral UPDRS Bradykinesia | |||||||||||||||
| Worse | 46 | 32 | 12.0 | −18.0 | −3.0 | −9.80 | 15.0 | −19 | −6.0 | 10−9 | 12.0 | −18.0 | −2.5 | STN | 0.140 |
| Right | 50 | 34 | 12.5 | −17.5 | −3.0 | −8.10 | 13.5 | −19 | −7.0 | 10−6 | 11.5 | −18.5 | −1.5 | ZI/VLP | 0.085 |
| Left | 43 | 27 | −12.5 | −18.0 | −3.0 | −6.50 | −11.5 | −17 | −3.0 | 10−7 | −12.0 | −17.5 | −3.0 | dSTN | 0.265 |
| Contralateral UPDRS Rigidity | |||||||||||||||
| Worse | 46 | 32 | 12.0 | −18.0 | −3.0 | −22.4 | 11.5 | −16.5 | 0.0 | 10−12 | 12.0 | −18.0 | −2.5 | STN | 0.255 |
| Right | 48 | 32 | 12.5 | −17.5 | −3.0 | −8.00 | 10.5 | −17.5 | 0.0 | 10−8 | 12.0 | −18.0 | −2.5 | STN | 0.450 |
| Left | 42 | 27 | −12.5 | −18.0 | −3.0 | −11.7 | −13.5 | −16 | −1.5 | 10−10 | −11.5 | −17.0 | −3.0 | STN/ZI | 0.385 |
| Contralateral UPDRS Tremor | |||||||||||||||
| Worse | 47 | 32 | 12.0 | −18.0 | −3.0 | −5.40 | 9.50 | −19.5 | −3.0 | 10−4 | 9.50 | −19.5 | −3.0 | ZI/H1 | 0.865 |
| Right | 50 | 34 | 12.5 | −17.5 | −3.0 | −4.30 | 9.00 | −19.5 | −2.5 | 10−4 | 11.5 | −20.0 | −3.5 | ZI | 0.925 |
| Left | 43 | 27 | −12.5 | −18.0 | −3.0 | −3.50 | −14.5 | −17.5 | −4.0 | 10−3 | −14.5 | −17.5 | −4.0 | STN/SN | 0.030 |
UPDRS, Unified Parkinson Disease Rating Scale; deg/s, degrees per second; Nm/deg, Newton · meter/degree; STN, subthalamic nucleus; ZI, zonaincerta; dSTN, dorsal STN; SN, substantia nigra; VLP, ventral lateral posterior thalamic nucleus; H1, field H1 of thalamic fasciculus.
Table 5.
Effects of anatomical location of active deep brain stimulation (DBS) contact on non-motor measures.
|
Outcome Measure and DBS Side |
N | Peak N |
x | y | z | Peak t |
x | y | z | Peak p |
x | y | z | Peak p Region | Corrected p- value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cognition
| |||||||||||||||
| SDR (error in mm) | |||||||||||||||
| Worse | 39 | 26 | 12.0 | −17.5 | −3.0 | 4.90 | 10.0 | −16.0 | −1.5 | 10−4 | 11.0 | −17.5 | −2.0 | STN/ZI | 0.060 |
| Right (improvement) |
44 | 29 | 12.5 | −17.5 | −3.0 | −4.50 | 15.5 | −14.5 | −4.0 | 10−3 | 14.5 | −15.0 | −4.0 | comb | 0.030 |
| Right (impairment) | 3.60 | 10.5 | −16.5 | −5.5 | 0.01 | 10.5 | −16.5 | −5.5 | STN/SN | ||||||
| Left | 39 | 25 | −12.5 | −17.5 | −3.0 | 3.30 | −10.5 | −21.5 | −4.0 | 0.01 | −11.5 | −21.0 | −3.0 | VPL/PBP | 0.405 |
| Go/No-Go (Pr) | |||||||||||||||
| Worse | 23 | 16 | 12.0 | −17.5 | −3.0 | 2.20 | 12.0 | −17.0 | −2.5 | 0.01 | 12.0 | −17.0 | −2.5 | STN/ZI | 0.730 |
| Right | 27 | 18 | 12.5 | −17.5 | −3.0 | −3.50 | 13.0 | −19.0 | −7.0 | 0.01 | 13.0 | −19.0 | −7.0 | SN | 0.970 |
| Left | 22 | 16 | −12.0 | −18.5 | −5.0 | −4.80 | −12.0 | −16.5 | −2.5 | 10−3 | −12.0 | −16.5 | −2.5 | dSTN | 0.300 |
|
| |||||||||||||||
|
Psychiatric
| |||||||||||||||
| Valence | |||||||||||||||
| Worse | 23 | 18 | 12.2 | −17.8 | −3.2 | 4.83 | 14.4 | −17.4 | −2.0 | 10−4 | 14.0 | −17.0 | −3.0 | dSTN | 0.650 |
| Right | 24 | 17 | 12.6 | −17.6 | −3.2 | 4.00 | 14.0 | −17.0 | −3.0 | 10−3 | 13.2 | −17.2 | −3.2 | dSTN | 0.220 |
| Left | 22 | 13 | −12.0 | −18.5 | −4.0 | 7.52 | −13.0 | −19.0 | −4.5 | 10−5 | −13.0 | −18.5 | −4.5 | dSTN | 0.015 |
| Arousal | |||||||||||||||
| Worse | 23 | 18 | 12.2 | −17.8 | −3.2 | 2.66 | 12.4 | −20.4 | −6.4 | 0.01 | 12.4 | −20.4 | −6.4 | SN | 0.870 |
| Right | 24 | 17 | 12.6 | −17.6 | −3.2 | −3.30 | 14.8 | −19.2 | −2.0 | 0.01 | 14.4 | −19.8 | −2.2 | ZI | 0.740 |
| Left | 23 | 13 | −12.8 | −17.4 | −2.8 | −2.30 | −11.8 | −17.8 | −4.2 | 0.01 | −11.8 | −17.8 | −4.2 | STN/SN | 0.400 |
| Anxiety | |||||||||||||||
| Worse | 25 | 19 | 12.2 | −18.2 | −3.2 | −5.00 | 14.0 | −20.0 | −3.0 | 10−4 | 14.2 | −18.2 | −2.4 | dSTN | 0.650 |
| Right | 26 | 18 | 12.4 | −18.0 | −3.2 | −7.70 | 14.0 | −19.4 | −1.0 | 10−4 | 14.0 | −19.4 | −1.0 | VPL | 0.540 |
| Left | 23 | 14 | −12.6 | −18.0 | −2.4 | −5.88 | −14.2 | −19.2 | −3.0 | 10−4 | −14.2 | −18.8 | −3.2 | dSTN | 0.005 |
| Apathy | |||||||||||||||
| Worse | 25 | 19 | 12.2 | −18.2 | −3.2 | 4.30 | 14.2 | −17.2 | −1.8 | 10−3 | 14.2 | −17.2 | −1.8 | STN/ZI | 0.850 |
| Right | 27 | 19 | 12.2 | −18.0 | −3.0 | 2.80 | 11.6 | −17.2 | 0.2 | 0.01 | 11.6 | −17.2 | 0.2 | VLA+E/ZI | 0.190 |
| Left | 24 | 14 | −12.6 | −18.0 | −2.4 | −5.00 | −11.0 | −21.0 | −3.0 | 10−3 | −11.0 | −20.8 | −2.8 | ZI | 0.240 |
| Akathisia | |||||||||||||||
| Worse | 25 | 19 | 12.2 | −18.2 | −3.2 | −4.00 | 14.0 | −20.0 | −2.0 | 10−3 | 13.0 | −20.0 | −2.0 | ZI/VPL | 0.110 |
| Right | 27 | 19 | 12.2 | −18.0 | −3.0 | −5.00 | 14.0 | −20.0 | −1.0 | 10−3 | 14.2 | −19.8 | −2 | ZI/VPL | 0.190 |
| Left | 24 | 14 | −12.6 | −18.0 | −2.4 | −5.00 | −11.0 | −21.0 | −3.0 | 10−3 | −11.0 | −20.8 | −2.8 | ZI | 0.240 |
SDR, spatial delayed response task; mm, millimeter; Pr, ability to discriminate between ‘Go’ and ‘No-Go’ stimuli; STN, subthalamic nucleus; ZI, zona incerta; SN, substantia nigra; comb, comb system (SN fragments in white matter lateral to the STN); VPL, ventral posterior lateral thalamic nucleus; PBP, parabrachial pigmented nucleus; dSTN, dorsal STN; VLA+E, ventral lateral anterior thalamic nucleus and ventral lateral posterior thalamic nucleus – external part.
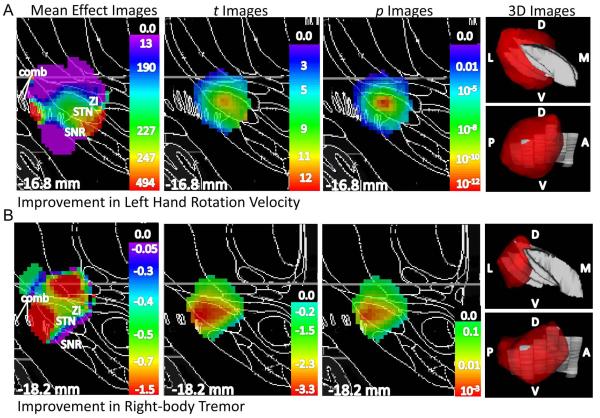
Figure 3. STN DBS-induced improvements in hand rotation velocity and tremor significantly differed by DBS site.
A.) Left hand rotation velocity: mean effect, t, and p images are shown left to right, with the p image thresholded at p ≤ 0.05, on a Mai atlas section close to the peak p value in black and white at the indicated distance from the plane through the anterior commissure and normal to the bicommissural line; the rightmost panels show 3D representation of voxels for which p ≤ 0.05 in color, with the STN in gray (top left panel: viewed from the front, bottom left panel: viewed from the side). B.) Right-side tremor: same conventions as in 3A. Images are horizontally flipped for clarity. Color scales indicate weighted mean, t, and p values. D, dorsal; V, ventral; A, anterior; P, posterior; M, medial; L, lateral; ZI, zona incerta; STN, subthalamic nucleus; SNR, substantia nigra pars reticulata; comb, comb system (SNR fragments in white matter)
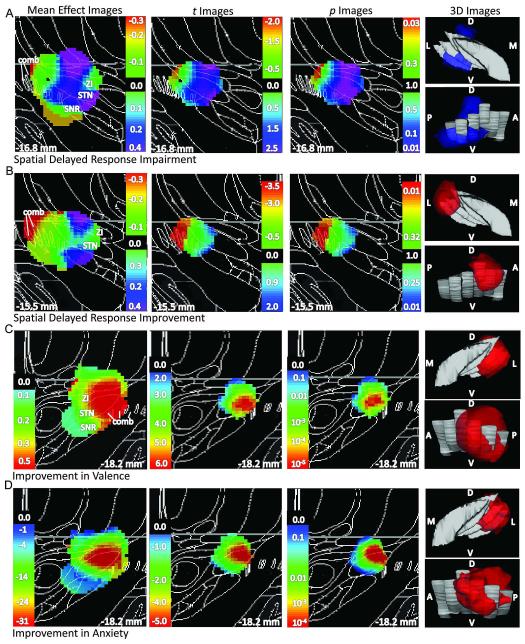
Figure 4. STN DBS-induced alterations in non-motor outcomes significantly differed by DBS site.
A.) Impairment in SDR performance. B.) Improvement in SDR performance. Same conventions as in Fig 3 except that weighted mean, t, and p values are shown on all 2D panels for both impairment (aquamarine-violet) and improvement (green-red). C.) Improvement in valence: same conventions as in Fig 3. D.) Improvement in anxiety: same conventions as in Fig 3.
Change in right-side UPDRS III scores with left-brain DBS
Left-brain STN DBS significantly improved mean right-side UPDRS III scores (Table 2). The peak value in the t-image, −12.4, is at (−15, −20.5, −4.0) (Fig 2, t image). The number of participants contributing data at each voxel was at least 13 throughout much of the STN, but varied substantially across the region analyzed, with a peak of 27 participants at (−12.5, −18, −3.0) (Fig 2, N image). Thus the p image differed somewhat from the t image; the p image has its peak value, corresponding to p ≈ 10−11, at (−13, −18.5, −3.0) (STN/ZI, 0.71 mm from the peak of the N image) (Fig 2, p image). A 3D view of voxels at which p < 0.05 by weighted t-test appears in (Fig 2, left-most top panel).
Note that the p image differs importantly from the image of weighted mean effect. For instance, stimulation throughout most of the STN is estimated to produce a 5- to 7-point improvement in the contralateral UPDRS subscore (Fig 2, mean effect image). However, due to differences in variance and/or N, the statistical significance varies substantially across the STN, and the most significant difference from zero falls in dorsal STN/ZI (Fig 2, p image).
The key question is whether improvement in motor function with DBS depended significantly on the specific anatomical location stimulated. For this measure, the data does not support such a conclusion; improvement occurred regardless of the precise anatomical location of the active contact (p = 0.12 by permutation testing, Table 4, last column of row 3). The p image does include values as low as p < 10−11 for participants with DBS near the dorsal STN and ZI, but the p image includes low values in many locations, and may simply reflect the fact that most participants (39 of 42) improved with DBS. Traditional neuroimaging statistical software, or in fact any approach that tests the t or p image against the null hypothesis of zero mean change, would falsely conclude that this result was significant (see Appendix for an example). By contrast, the permutation analysis rejects significance in this case by showing that a result of this size and magnitude (i.e., the true Q value) also occurs in ~12% of p images made by incorrectly (randomly) assigning this sample’s generally positive outcome data to this sample’s set of active contact locations (Table 4, last column of row 3). (The true Q was no bigger than 24 of the 200 false pairings of DBS site to behavioral outcome.) This case demonstrates that simply examining mean effects or voxelwise p values can yield misleading conclusions about the association between DBS site and behavioral outcome.
Other motor outcomes
Right-brain STN DBS-induced improvement in left hand rotation velocity did depend significantly on DBS location (corrected p = 0.005, n = 40, Table 4, last column of row 5). The region of the brain most strongly associated with improvement was the dorsal STN/ZI border. Fig 3A presents a 3D view of voxels where p < 0.05 by t-test and 2D sections through the mean effect, t, and p images for one atlas slice nearest to the peak p-value. Tremor ratings on the right side of the body improved in 75% of participants during left-brain DBS relative to DBS OFF (15 of 20 participants who had substantial right hand tremor when OFF STN DBS). However, the degree of improvement depended significantly on DBS location (corrected p = 0.03, n = 43, Table 4, last column of the last row). The region of the brain most strongly associated with improvement, the STN/SN border, was lateral to that for improvement in left hand rotation velocity (peak p-value in p image, Fig 3B).
Several other motor responses indicated a trend (0.05 < p < 0.10) for dependence on DBS location (see Table 4).
Cognition
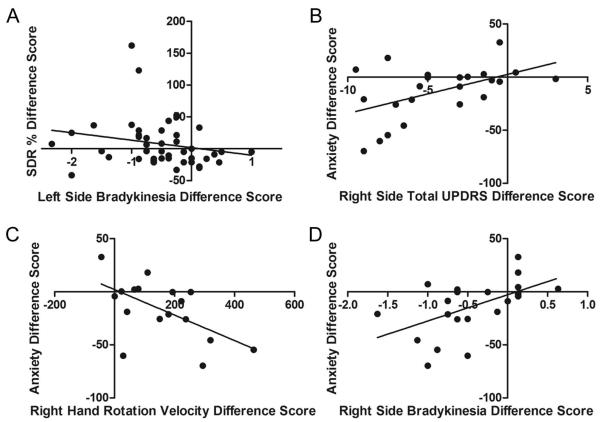
STN DBS-induced effects on working memory performance depended significantly on active contact location (corrected p = 0.03, n = 44) in the right hemisphere, with the peak p value for worsened performance in the right STN/SN and the peak p value for improved performance in the comb system (SN fragments in white matter lateral to STN) (Table 5, Fig 4A to B). The right brain STN DBS-induced change in SDR performance was related to right brain STN DBS-induced changes in left side bradykinesia (ρ44 = −0.33, p = 0.03, Fig 5A) but not left side total UDPRS score (ρ 43 = −0.06, p = 0.72), left hand rotation velocity (r35 = −0.07, p = 0.33), left side impedance (r35 = 0.02, p = 0.93), left side rigidity (ρ 43 = 0.11, p = 0.48), or left side tremor (ρ 44 = 0.08, p = 0.61). The effect of STN-DBS on response selection and inhibition as measured by the Go/No-Go task was not significantly affected by active contact location (corrected p ≥ 0.30).
Figure 5. STN DBS-induced changes in cognition and anxiety are related to changes in motor function.
A.) Right-brain STN DBS-induced percent change in SDR error was negatively related to right-brain STN DBS-induced improvement in UPDRS subscore bradykinesia. Left-brain STN DBS-induced decreases in anxiety were associated with left-brain STN DBS-induced improvements in B.) right side total UPDRS scores, C.) right hand rotation velocity and D.) right side UPDRS subscore bradykinesia. Except for hand rotation velocity, decreasing difference scores signify improvement in that measure.
Mood, anxiety, and akathisia
STN DBS-induced improvement in valence and anxiety depended on stimulation site for left STN DBS (valence: corrected p = 0.015, n = 22; anxiety: corrected p = 0.005, n = 23). Peak p values occurred with stimulation in the dorsal left STN for both measures (Table 5, Fig 4C to D). STN DBS-induced alterations in self-rated apathy, akathisia and affective arousal were not significantly related to contact location (corrected p ≥ 0.11).
The left brain STN DBS-induced change in valence was not correlated with left brain STN DBS-induced changes in right side total UDPRS score (ρ 21 = −0.29, p = 0.20), right hand rotation velocity (r16 = 0.13, p = 0.64), right side impedance (r16 = −0.30, p = 0.27), right side bradykinesia (ρ 21 = −0.37, p = 0.10), right side rigidity (ρ 21 = −0.26, p = 0.25), or right side tremor (ρ 21 = −0.25, p = 0.27).
Left brain STN DBS-induced improvement in anxiety was significantly correlated with left brain STN DBS-induced improvement in right side total UDPRS score (ρ 23 = 0.51, p = 0.01, Fig 5B), right hand rotation velocity (r17 = −0.58, p = 0.02, Fig 5C), and right side bradykinesia (ρ 23 = 0.54, p = 0.01, Fig 5D) but not right side impedance (r17 = 0.41, p = 0.10), right side rigidity (ρ 23 = 0.36, p = 0.09), or right side tremor (ρ 23 = 0.25, p = 0.26).
Discussion
STN DBS improved motor function, tended to impair cognition, and improved mood and anxiety, in accordance with previous literature10,18,20,23,25,41-42. We further determined that DBS- induced changes in several behaviors depended on specific anatomical DBS site using a 3D analysis. The novel method solves problems that have hindered efforts to convincingly connect DBS effects to precise anatomical locations stimulated. It maps expected DBS effects at each point as well as the probability that the result at each point occurred by chance. A second step corrects rigorously for false positive results. Our method separates outcomes improved by DBS anywhere in or near the STN from outcomes that depend strongly on the anatomical subregion stimulated.
Location-specific DBS effects on movement
DBS effects on hand rotation velocity (bradykinesia) and tremor depended significantly on DBS location. DBS in posterior dorsomedial STN/ZI provided maximal reduction of bradykinesia, whereas maximal reduction of tremor occurred with DBS in posterolateral STN/SN border. Previous studies suggest that motor function improves with DBS in dorsal STN and regions superior to the STN such as ZI12,17-18. Our 3D analysis extends these results by identifying a relatively more inferior and lateral STN/SN region as most strongly related to improvement in tremor. Given that dorsolateral STN is embedded in the basal ganglia-thalamocortical motor loop5, these locations are not surprising. Improvement did not depend on DBS site for other motor outcomes.
The nonsignificant corrected p values for several motor measures may initially seem surprising, but actually highlight the value of the new statistical approach and are consistent with the within-subjects DBS study of Frankemolle et al.11, in which limiting DBS to dorsal STN reduced cognitive side effects, but did not change motor responses. Almost all participants’ movement improved with DBS, and if DBS anywhere in the region produces similar improvement, precise location is not significant. In fact, most PD patients benefit from STN DBS even though DBS settings are not usually based on precise active contact location. However, since DBS sites were not chosen randomly, we cannot exclude an alternative interpretation of the nonsignificant results; namely, that for almost any DBS location near STN there may exist some patients who will benefit, even if on a population level certain sites improve movement more than others.
Location-specific DBS effects on cognition
DBS effects on working memory, but not response selection and inhibition, depended on stimulation location; performance tended to worsen with DBS in the right brain posterior ventrolateral STN/SN and improve with DBS in the comb system, a white matter region that contains fragments of substantia nigra pars reticulata (SNr). This result provides evidence that DBS location may explain variance in response across subjects; without contact location, DBS’s effect on SDR performance would not have appeared significant. STN DBS may affect performance on SDR via projections from STN to internal segment of the globus pallidus and SNr. These structures, via the thalamus43, influence dorsolateral prefrontal cortex, a region that supports working memory44 and where blood flow induced by STN DBS negatively correlates with change in SDR21. As noted above and consistent with our results, DBS-induced cognitive impairment is reversed when predicted suprathreshold current spread is confined to dorsal STN11.
Location-specific DBS effects on emotion and akathisia
Mood and anxiety improvements related most strongly to DBS sites within left dorsal STN. STN anatomy4-7 might predict that mood would be influenced most by stimulation of ventromedial or ventral STN due to afferent and efferent connections to limbic regions45. Accordingly, reports of mania with STN DBS have mostly occurred with stimulation in or near ventromedial STN46-47. However, mania is distinct from milder, beneficial elevations in mood and decreases in anxiety that apparently occur with acute dorsal STN DBS. Anteroventral and ventromedial STN were not represented in our sample of active DBS sites, preventing us from inferences about these regions. Our findings add to current knowledge of the functional heterogeneity of the STN: they support the hypothesis that limbic, cognitive and motor circuitry somewhat overlap in STN6-7 rather than strictly segregate into dorsal and ventral regions. Significant associations of electrode location with akathisia, arousal or apathy were not found.
STN DBS-induced changes in working memory and anxiety, but not valence, correlated with STN DBS-induced alterations in movement. From our data, it cannot be ascertained whether DBS effects on non-motor behaviors are completely or partially dependent on, or completely separate from but parallel to, those on motor function. Future studies of neural circuitry underlying cognition, anxiety, and motor responses to DBS should help answer this important question.
Other approaches to 3D prediction of DBS effects
Butson et al.48 developed a “Probabilistic Stimulation Atlas” (PSA) to map DBS effects onto neuroanatomy. Patient-specific computer models of predicted volume of tissue activated (VTA) were generated for 163 different locations and DBS parameter settings in 6 patients. The PSA shows at each point the fraction of participants whose VTA included that point. This method is elegant but does not test for statistical significance. The differences between the effect and p images in Fig 2, and the non-significant global p value for contralateral UPDRS, demonstrate the importance of this limitation.
Hilliard et al.15 did create a p image for the link between stimulation location and motor outcomes in PD by shifting a 3.5 mm cube iteratively across anatomical space, and for each cube location using a one-way ANOVA to compare the efficacy of contacts within versus outside the cube. While this method reports a probability and addresses the sample bias problem, it does not correct for multiple comparisons but labels a point as significant if even one of the 2744 ANOVAs with a test cube containing that point is positive. Finally, the Hilliard and Butson methods require binary rather than continuous outcome variables, whereas the method presented here can use either.
Limitations
As described above, an unavoidable limitation of clinical data is the targeting bias toward the dorsal posterolateral STN during surgical implantation of the electrodes which curtails collection of responses from stimulation of anterior or medial-ventral STN, a current research focus in our laboratory. Another limitation of this study is possible Type II error. For example, some motor results were significant for only one side of the brain, most likely because of inadequate power on the non-significant side. Several other motor measures approached significance (corrected p < 0.10), and may prove significant with a larger sample. Regarding possible Type I error, we report corrected p values for 36 tests, and a few of those tests would be expected to appear positive by chance. However, 5 of our tests were positive, which is not likely to occur by chance (p = 0.04, binomial distribution, n = 36, k ≥ 5). Nevertheless, replication is the ideal way to validate our results.
Obvious motor function improvement by STN DBS may have compromised blinding of participants and examiners. However, neither the examiners nor participants knew the precise locations of their active contacts. Therefore, the study was blinded for the relevant topic of investigation – location of the active contact. Since our method identified significant relationships between several behavioral measures and DBS site, DBS-induced changes in behavior are unlikely to be entirely due to inadequate blinding or placebo-induced improvement in motor function. In addition, the OFF vs. ON DBS, off PD medication experimental design is the best possible method to study STN DBS-induced alterations in behavior because it controls for surgery-induced placebo effects, lesions, and PD medication effects. Second, we cannot be sure that 42 min is long enough for DBS effects to dissipate when turned OFF. Therefore, our study may have failed to detect longer-term behavioral effects of STN DBS. However, ethical and practical concerns precluded us from keeping participants in the OFF DBS condition longer than this time period.
Finally, for simplicity this study focused on the anatomical location of the stimulated contact, rather than attempting to predict which neurons or fibers of passage are activated. Our approach requires no physiological assumptions about which neurons are stimulated by DBS, but limited this initial report to participants with monopolar stimulation at a single electrode. Fortunately, the statistical method demonstrated here is flexible enough to allow replacing the simple weighting function used here for monopolar stimulation with computer modeling approaches14 to allow including participants with other DBS configurations or voltage settings.
Summary
A new method that allows data-driven 3D analysis of DBS outcomes, including statistically rigorous significance testing, was applied to 51 participants with PD and determined significant relationships between local STN neuroanatomy and DBS effects on movement, cognition and mood. Interestingly, our results do not support complete functional segregation within the STN. For instance, improvements in mood and movement were not correlated but both occurred with stimulation of posterodorsal STN. Rather, our findings support the view of the STN as a convergence site for functionally distinct information arising from and projecting to motor, cognitive and limbic regions6-7. Studies using this method, with larger sample sizes and more varied DBS sites, can further explore the functional organization of the STN and address practical concerns of PD patients, physicians and surgeons.
Acknowledgments
The authors thank Jeanette K. Kenley, B.S., and Tom O. Videen, Ph.D., for their contributions to methods development and Fredy J. Revilla, M.D., for providing STN DBS surgical information. Parts of this work were presented previously (Deep Brain Stimulation: Motor Systems and Beyond, Rochester, NY, 7-8 October 2010; The Movement Disorder Society’s 15th International Congress of Parkinson’s Disease and Movement Disorders, Toronto, Canada, 6 June 2011; Annual meeting, American Neuropsychiatric Association, New Orleans, LA, 21-24 Mar 2012).
Appendix
Zero-mean analysis: method
To show the advantages of the permutation approach presented here, we also implemented a variant of this method that compares the true p image to the null hypothesis assumed by traditional statistical image analysis methods: namely, that on average the behavior change with stimulation near a given voxel is zero. This variant method retains the contact locations and standard deviation of the real data, but in creating the probability distribution, each false permutation subtracts the group mean effect from each participant’s effect so that each permutation sample has a zero mean change.
Result of zero-mean analysis
The zero-mean analysis of right-side total UPDRS reports a “corrected” p < 0.005.
Implications
When the permutation method is weakened to match the assumptions of traditional statistical image analysis methods, it gives similar results (by losing its advantage in reducing Type I error).
Footnotes
Author Contributions: S.A.E. participated in data analysis, editing, and writing the manuscript. J.M.K. and K.D.B. participated in design of the study, data analysis and editing. T.H. and J.S.P. participated in design of the study and editing. M.C.C., M.U., S.D.T., and M.K. participated in data analysis and editing. H.M.L. participated in data analysis. K.J.B. participated in conception and design of the study, data analysis, editing and writing the manuscript. All authors approved the final manuscript and have no conflicts of interest to report.
References
- 1.Foltynie T, Hariz MI. Surgical management of Parkinson’s disease. Expert Rev Neurother. 2010;10:903–914. doi: 10.1586/ern.10.68. [DOI] [PubMed] [Google Scholar]
- 2.Funkiewiez A, Ardouin C, Caputo E, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smeding HM, Speelman JD, Koning-Haanstra M, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: A controlled study. Neurology. 2006;66:1830–1836. doi: 10.1212/01.wnl.0000234881.77830.66. [DOI] [PubMed] [Google Scholar]
- 4.Parent A, Hazrati LN. Functional anatomy of the basal ganglia I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 5.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995b;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 6.Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershey T, Campbell MC, Videen TO, et al. Mapping Go-No-Go performance within the subthalamic nucleus region. Brain. 2010;133:3625–3634. doi: 10.1093/brain/awq256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeely ME, Hershey T, Campbell MC, et al. Effects of deep brain stimulation on dorsal versus ventral subthalamic nucleus regions on gait and balance in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82:1250–1255. doi: 10.1136/jnnp.2010.232900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell MC, Black KJ, Weaver PM, et al. Mood response to deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2012;24:28–36. doi: 10.1176/appi.neuropsych.11030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankemolle AM, Wu J, Noecker AM, et al. Reversing cognitive-motor impairments in Parkinson’s disease patients using a computational modelling approach to deep brain stimulation programming. Brain. 2010;133:746–761. doi: 10.1093/brain/awp315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maks CB, Butson CR, Walter BL, et al. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry. 2009;80:659–666. doi: 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikos A, Bowers D, Noecker AM, et al. Patient-specific analysis of the relationship between the volume of tissue activated during DBS and verbal fluency. Neuroimage. 2011;54:S238–S246. doi: 10.1016/j.neuroimage.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilliard JD, Frysinger RC, Elias WJ. Effective subthalamic nucleus deep brain stimulation sites may differ for tremor, bradykinesia and gait disturbances in Parkinson’s disease. Stereotact Funct Neurosurg. 2011;89:357–364. doi: 10.1159/000331269. [DOI] [PubMed] [Google Scholar]
- 16.Greenhouse I, Gould S, Houser M, et al. Stimulation at dorsal and ventral electrode contacts targeted at the subthalamic nucleus has different effects on motor and emotion functions in Parkinson’s disease. Neuropsychologia. 2011;49:528–534. doi: 10.1016/j.neuropsychologia.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- 18.Burrows AM, Ravin PD, Novak P, et al. Limbic and motor function comparison of deep brain stimulation of the zona incerta and subthalamic nucleus. Neurosurgery. 2012;70:125–130. doi: 10.1227/NEU.0b013e318232fdac. [DOI] [PubMed] [Google Scholar]
- 19.Eitan R, Shamir RR, Linetsky E, et al. Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Front Syst Neurosci. 2013;7:69. doi: 10.3389/fnsys.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hershey T, Wu J, Weaver PM, et al. Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Exp Neurol. 2008;210:402–408. doi: 10.1016/j.expneurol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell MC, Karimi M, Weaver PM, et al. Neural correlates of STN DBS-induced cognitive variability in Parkinson disease. Neuropsychologia. 2008;46:3162–3169. doi: 10.1016/j.neuropsychologia.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karimi M, Golchin N, Tabbal SD, et al. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain. 2008;131:2710–2719. doi: 10.1093/brain/awn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabbal SD, Ushe M, Mink JW, et al. Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp Neurol. 2008;211:234–242. doi: 10.1016/j.expneurol.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershey T, Revilla FJ, Wernle AR, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- 25.Hershey T, Revilla FJ, Wernle A, et al. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;13:1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- 26.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32:S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 27.Tabbal SD, Revilla FJ, Mink JW, et al. Safety and efficacy of subthalamic deep brain stimulation performed with limited intraoperative mapping for treatment of Parkinson’s disease. Neurosurgery. 2007;61:119–127. doi: 10.1227/01.neu.0000289725.97211.51. [DOI] [PubMed] [Google Scholar]
- 28.Temperli P, Ghika J, Villemure JG, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 29.Lang AE, Fahn S. Assessment of Parkinson’s disease. In: Munsat TL, editor. Quantification of Neurologic Deficit. Butterworths; Boston: 1989. [Google Scholar]
- 30.Larsen RJ, Diener E. Problems and promises with the circumplex model of emotion. J Pers Soc Psychol. 1992;62:480–488. [Google Scholar]
- 31.Limsoontarakul S, Campbell MC, Black KJ. A perfusion MRI study of emotional valence and arousal in Parkinson’s disease. Parkinsons Dis. 2011;2011:742907. doi: 10.4061/2011/742907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Videen TO, Campbell MC, Tabbal SD, et al. Validation of a fiducial-based atlas localization method for deep brain stimulation contacts in the area of the subthalamic nucleus. J Neurosci Methods. 2008;168:275–281. doi: 10.1016/j.jneumeth.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- 34.Butson CR, McIntyre CC. Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng. 2006;3:1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIntyre CC, Miocinovic S, Butson CR. Computational analysis of deep brain stimulation. Expert Rev Med Devices. 2007;4:615–622. doi: 10.1586/17434440.4.5.615. [DOI] [PubMed] [Google Scholar]
- 36.Maggio F, Pasciuto T, Paffi A, et al. Micro vs macro electrode DBS stimulation: a dosimetric study. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2057–2060. doi: 10.1109/IEMBS.2010.5626487. [DOI] [PubMed] [Google Scholar]
- 37.Bland JM, Kerry SM. Statistics notes. Weighted comparison of means. BMJ. 1998;316:129. doi: 10.1136/bmj.316.7125.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols TE, Holmes AP. Nonparametric analysis of PET functional neuroimaging experiments: A primer. Hum Brain Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, Synder AZ, Shimony JS, et al. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gering DT, Nabavi A, Kikinis R, et al. An integrated visualization system for surgical planning and guidance using image fusion and interventional imaging. Int Conf Med Image Comput Comput Assist Interv. 1999;2:809–819. [Google Scholar]
- 41.Funkiewiez A, Ardouin C, Krack P, et al. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson’s disease. Mov Disord. 2003;18:524–530. doi: 10.1002/mds.10441. [DOI] [PubMed] [Google Scholar]
- 42.Czernecki V, Pillon B, Houeto JL, et al. Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2005;76:775–779. doi: 10.1136/jnnp.2003.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 44.Gazzaley A, Nobre AC. Top-down modulation: Bridging selective attention and working memory. Trends Cogn Sci. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander GE, Crutcher M, DeLong M. Basal ganglia-thalamo-cortical circuits: Parallel substrates for motor, oculomotor, “prefrontal”, and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 46.Chopra A, Tye SJ, Lee KH, et al. Underlying neurobiology and clinical correlates of mania status after subthalamic nucleus deep brain stimulation in Parkinson’s disease: A review of the literature. J Neuropsychiatry Clin Neurosci. 2012;24:102–110. doi: 10.1176/appi.neuropsych.10070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raucher-Chene D, Charrel CL, de Maindreville AD, Limosin F. Manic episode with psychotic symptoms in a patient with Parkinson’s disease treated by subthalamic nucleus stimulation: improvement on switching the target. J Neurol Sci. 2008;273:116–117. doi: 10.1016/j.jns.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Butson CR, Cooper SE, Henderson JM, et al. Probabilistic analysis of activation volumes generated during deep brain stimulation. Neuroimage. 2011;54:2096–2104. doi: 10.1016/j.neuroimage.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]