Abstract
Objective
Children with Neurofibromatosis-1 (NF1) are at risk for developing numerous nervous system abnormalities, including cognitive problems and brain tumors (optic pathway glioma). Currently, there are few prognostic factors that predict clinical manifestations or outcomes in patients, even in families with an identical NF1 gene mutation. In this study, we leveraged Nf1 genetically-engineered mice (GEM) to define the potential role of sex as a clinically-relevant modifier of NF1-associated neuronal dysfunction.
Methods
De-identified clinical data were analyzed to determine the impact of sex on optic glioma-associated visual decline in children with NF1. In addition, Nf1 GEM were employed as experimental platforms to investigate sexually dimorphic differences in learning/memory, visual acuity, retinal ganglion cell (RGC) death, and Nf1 protein (neurofibromin)-regulated signaling pathway function (RAS activity, cyclic AMP and dopamine levels).
Results
Female patients with NF1-associated optic glioma were twice as likely to undergo brain MRI for visual symptoms and three times more likely to require treatment for visual decline than their male counterparts. As such, only female Nf1 GEM exhibited a decrement in optic glioma-associated visual acuity, shorter RGC axons, and attenuated cAMP levels. In contrast, only male Nf1 GEM showed spatial learning/memory deficits, increased RAS activity, and reduced dopamine levels.
Interpretation
Collectively, these observations establish sex as a major prognostic factor underlying neuronal dysfunction in NF1, and suggest that sex should be considered when interpreting future preclinical and clinical study results.
Keywords: neurofibromin, cyclic AMP, dopamine, Ras, NF1, learning, vision, central nervous system
Introduction
Neurofibromatosis type 1 (NF1) is a clinically heterogeneous neurologic disorder characterized by germline NF1 gene mutations1, such that children with NF1 are prone to the development of brain tumors (optic gliomas)2,3, cognitive problems4,5, and attention deficits6. While affected individuals are at risk for all of these abnormalities, it is currently not possible to predict who will develop which neurologic problem and what clinical outcome will ensue. This challenge is further underscored by the observation that individuals from the same family (with the identical NF1 gene mutation) can exhibit significantly different clinical features and disease severities.
Recent studies using Nf1 genetically-engineered mice (GEM) have begun to reveal potential genomic loci that influence tumor susceptibility7,8. For example, astrocytoma resistance is conferred by a modifier gene (Arlm1) located on mouse chromosome 12, which operates in a sex-specific manner. The finding that sex interacts with this genomic modifier to influence gliomagenesis raises the intriguing possibility that other NF1 clinical abnormalities may also be sexually dimorphic. To define the role of sex as a modifier for neurologic dysfunction in NF1, we leveraged Nf1 GEM that develop optic glioma and learning/memory abnormalities. In this report, we establish that sex is a major determining factor underlying learning/memory deficits and optic glioma-associated visual decline in Nf1 mutant mice through sexually-dimorphic effects on Nf1 protein (neurofibromin)-mediated neuronal cyclic AMP (cAMP), Ras and dopamine signaling.
Materials and Methods
Human subjects
De-identified data from individuals ≤18 years with an established diagnosis of NF19 managed in the St. Louis Children’s Hospital Neurofibromatosis Clinical Program (1994-2013) were collected under an approved Human Studies Protocol at the Washington University School of Medicine.
Mice
Nf1+/-GFAPCKO (Nf1flox/mut;GFAP-Cre, Nf1-CKO) and littermate control (Nf1flox/flox; CTL) mice were maintained on an inbred C57BL/6 background with ad libitum access to food and water. All experiments were performed on 3-4-month-old mice, unless otherwise stated, under an approved Animal Studies Committee protocol at the Washington University School of Medicine.
Immunostaining
Western blots were performed using pDARPP32 (1:500, Cell Signaling), DARPP32 (1:1000, Cell Signaling), pERK1/2 (1:1000, Cell Signaling), and ERK1/2 (1:1000, Cell Signaling) primary antibodies. Ras activation (Millipore), cAMP (New East Biosciences), TUNEL (Roche Diagnostics), and dopamine (Rocky Mountain Diagnostics) measurements were determined following manufacturer’s protocols. Each experiment was performed with samples from at least three independently-generated cohorts.
Behavioral testing
Morris Water Maze testing for spatial learning and memory was conducted as previously described10. Three days after completing cued (visible but variable platform location; 4 trials/day, 2 consecutive days) trials, spatial learning acquisition was evaluated during the place condition (submerged, hidden platform, constant location; 4 trials/day, 5 consecutive days). Escape path length and latency, and swimming speeds were calculated for all cued and place trials. Retention performance was evaluated during probe trials (platform removed) conducted 1-h after the last place trial on the third and fifth days. Time spent in the target quadrant and spatial bias (time in the target quadrant versus each of the other quadrants) were also analyzed. Visual acuity (n=20 mice per group) was assessed using the virtual optokinetic system (VOS) under photopic conditions (1.8 log cd/m2)10, as previously described. Contrast thresholds were measured at a frequency of 0.128 cycles/degree and a speed of 5.4 degrees/sec.
Results
Sex determines visual outcome in children and mice with NF1
15-20% of children with NF1 develop optic pathway gliomas; however, only one-third to one-half of these children will require treatment, typically as a consequence of visual decline11,12. While previous studies have indicated that glioma location and patient age are negative predictors of visual decline13, we sought to determine whether patient sex influences clinical outcome. From the St. Louis Children’s Hospital NF Clinical Program (1994-2013), 431 individuals younger than 19 years of age were identified who met diagnostic criteria for NF1 (205 males, 226 females) (Fig 1A). Brain magnetic resonance imaging (MRI) was performed on 255 individuals based on clinical indications (Fig 1B; 122 males, 133 females), revealing 96 children with optic gliomas (38%; 40 males, 56 females). While boys and girls with NF1 exhibited similar frequency of optic glioma, girls with NF1-associated optic gliomas were twice as likely to have undergone neuroimaging for visual symptoms (p=0.005, Fig 1B) and 3 times more likely than boys to require treatment due to visual decline (p=0.0007, Fig 1A). There were no significant differences in the age at optic glioma diagnosis or tumor location between boys and girls (Fig 1A, C). However, it is important to note that females with optic gliomas in all locations, except the post-chiasmal tracts, exhibited higher frequencies of visual decline requiring treatment (Fig 1D). The independent prognostic value of post-chiasmal involvement has been recently reported13.
Figure 1. Optic glioma location and size is not impacted by sex.
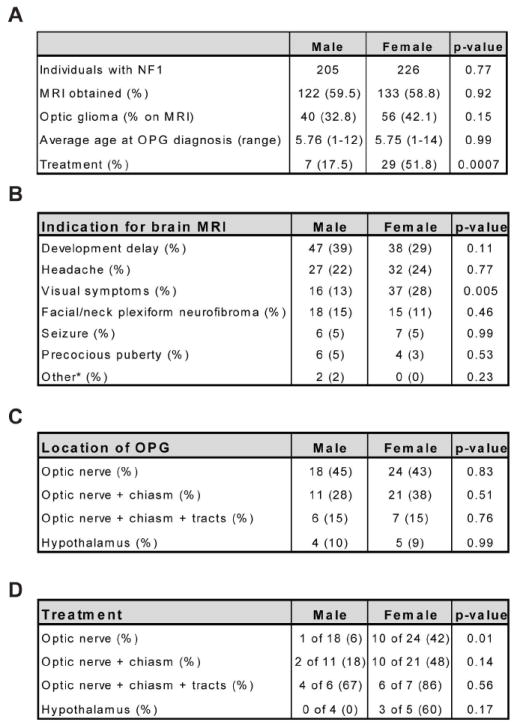
(A) Girls with NF1-associated optic glioma were 3 times more likely to be treated for visual decline than their male counterparts (p=0.0007). (B) Indications for brain MRI. *Other includes one individual each imaged for weakness and the presence of a facial port-wine stain. (C) Locations of optic pathway gliomas (OPGs) in male and female children with NF1 occur at similar frequencies. (D) Frequency of visual decline necessitating treatment in girls and boys stratified by tumor location.
To determine whether sex influences the outcome of murine optic glioma, we leveraged an Nf1 GEM strain that develops optic nerve/chiasmal gliomas (Nf1-CKO)14,15. While optic nerve volumes were indistinguishable between male and female Nf1-CKO mice (Fig 2A), only female Nf1-CKO mice exhibited reduced visual acuity on VOS testing (Fig 2B). Moreover, female Nf1-CKO mice had ~2-fold more retinal ganglion cell death (RGC apoptosis; Fig 2C) relative to their male counterparts. This increase in RGC apoptosis is also observed in vivo, as evidenced by a time-dependent degeneration of optic nerve axons16, 17, as well as reduced RGC neuronal lengths in vitro18. Consistent with these sex-specific observations, reduced axon lengths were only observed in female Nf1-CKO primary RGC neurons relative to controls in vitro (Fig 2D, E).
Figure 2. Optic glioma-associated vision disturbances are greater in girls with NF1 and in female Nf1-CKO mice.
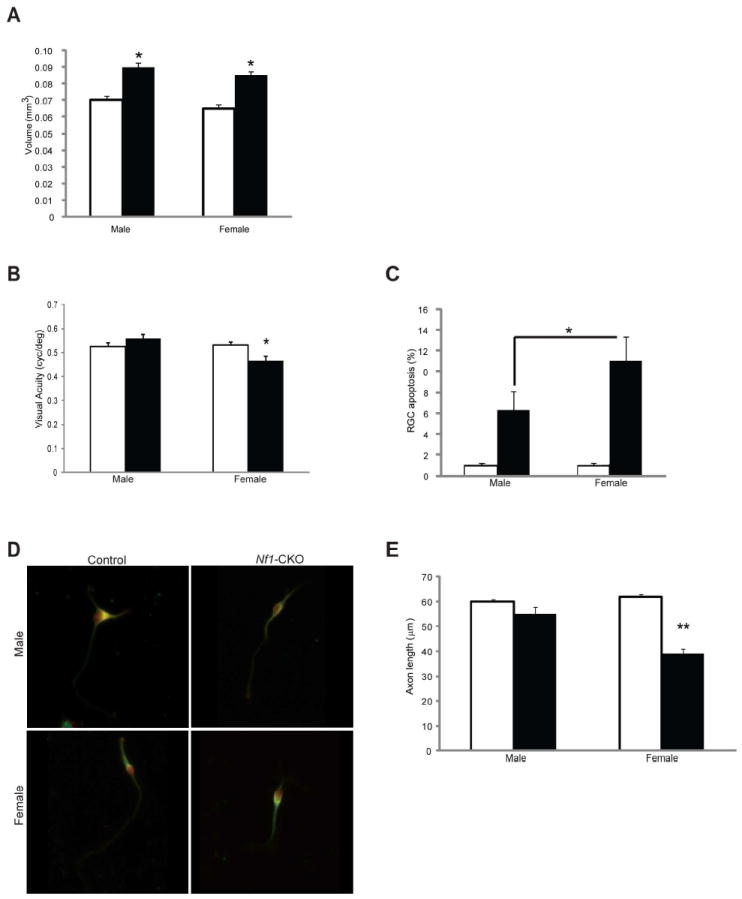
(A) Optic nerve volumes in Nf1-CKO mice (black bars) are larger than those observed in control littermate mice (white bars), regardless of sex. Asterisks denotes p<0.05. (B) Only female Nf1-CKO mice have impaired visual acuity (VOS; cycles/degree) relative to controls. (C) Greater retinal ganglion cell apoptosis (%TUNEL+ cells) was observed in female Nf1-CKO mice (11.5-fold over female controls) relative to Nf1-CKO males (6.2-fold over male controls). (D) Representative images of retinal ganglion cells in culture demonstrate that female, but not male, Nf1-CKO neurons have reduced axon lengths relative to controls. (E) Female, but not male, Nf1-CKO neurons exhibit shorter axon lengths (~50% reduction) relative to controls. Open (white) bars denote control (CTL) mice; closed (black) bars denote Nf1-CKO mice. *p<0.05, **p<0.01.
Spatial learning impairments in male Nf1-CKO mice
While 30-70% of children with NF1 exhibit specific learning deficits, only two reports have specifically examined sex. In these studies, a 2:1 male bias in the prevalence of these NF1-associated learning problems was observed5, 14. To explore the impact of sex on learning/memory in mice, Nf1-CKO mice were evaluated in the Morris Water Maze. Whereas sex did not influence performance during the cued and place trials (Supplementary Fig 1), only Nf1-CKO male mice showed no spatial preference and spent equal time in all quadrants (Fig 3A, B) during both the memory acquisition (probe trial 1) and retention (probe trial 2) trials. Similarly, male Nf1-CKO mice exhibited a 25% reduction in time spent and number of entries into the target quadrant relative to littermate controls (Fig 3C-F). In contrast, Nf1-CKO female mice performed comparably to controls.
Figure 3. Spatial learning impairments in the Morris Water Maze occur only in Nf1-CKO male mice.
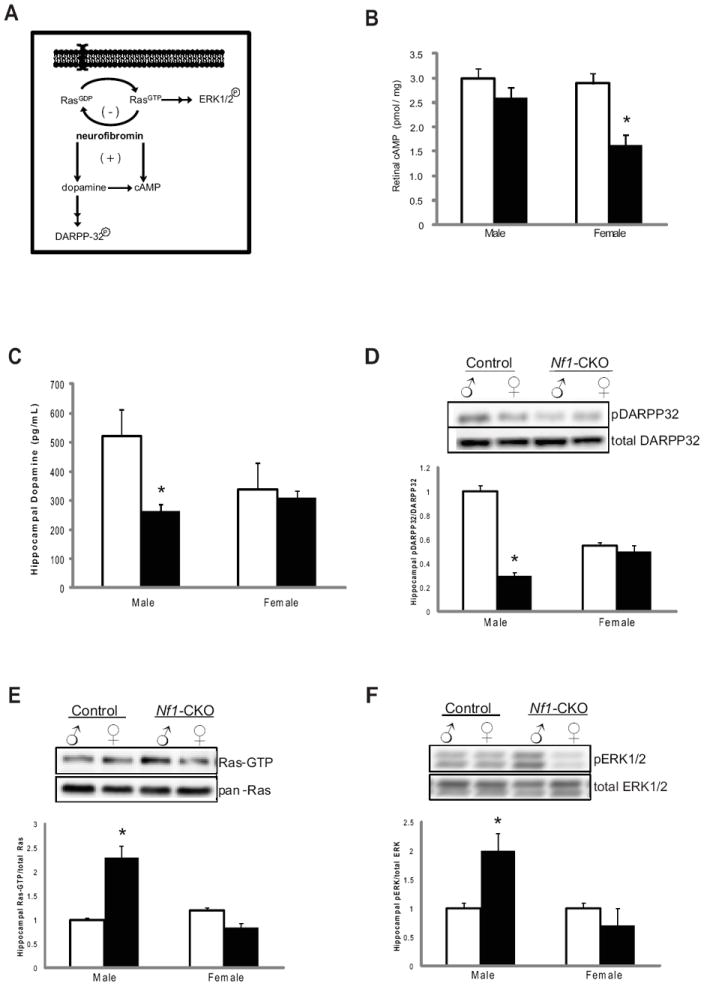
(A-B) During the first (A) and second (B) probe trials, control mice and female Nf1-CKO mice spent significantly more time in the target quadrant than all the other quadrants. In contrast, male Nf1-CKO mice showed no spatial preference and spent equal time in all quadrants. (C-D) Time spent in the target quadrant during the first (C) and second (D) probe trials was reduced by 25% and 23%, respectively in male Nf1-CKO mice. (E-F) Male Nf1-CKO mice entered the target 1.5-fold less frequently than controls in both the first (E) and second (F) probe trials. *p<0.05, **p<0.01.
Sex-dependent differences in neurofibromin function underlie the sexual dimorphic deficits in Nf1-CKO mice
Neurofibromin regulates several downstream signaling pathways within the nervous system10,19-22 (Fig 4A). As such, previous studies have demonstrated that reduced RGC axon lengths and survival results from impaired neurofibromin generation of cyclic AMP (cAMP), and treatment with agents that restore cAMP levels ameliorate the neurite length and survival defects in vitro and attenuate the optic glioma-associated RGC apoptosis in vivo18. Additionally, learning/memory deficits in Nf1-CKO mice reflect impairments in neurofibromin regulation of both hippocampal Ras activity and dopamine levels, such that either Ras inhibition (e.g., Lovastatin21 or dopamine elevation (e.g., methylphenidate23) corrects these learning/memory impairments in Nf1 mutant mice (Fig 4A).
Figure 4. Sexually dimorphic regulation of neurofibromin signaling pathways.
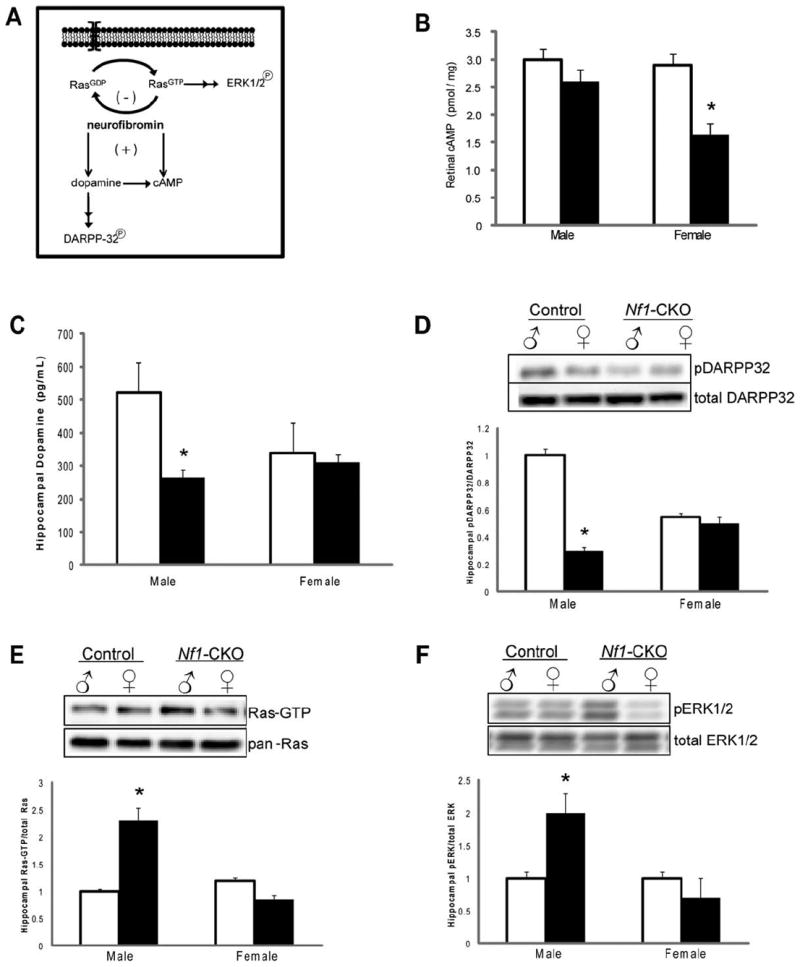
(A) Established molecular pathways regulated by the NF1 gene product, neurofibromin. (B) Retinal cyclic adenosine monophosphate (cAMP) levels were reduced by 47% in female Nf1-CKO mice relative to female controls. No change in retinal cAMP levels was observed in male Nf1-CKO mice relative to male controls. (C) Hippocampal dopamine levels were reduced 2-fold in male Nf1-CKO mice compared to male controls. No differences were observed in female Nf1-CKO mice relative to female controls. (D) DARPP32 phosphorylation was reduced in the hippocampus of Nf1-CKO male, but not female, mice. (E, F) Densitometric quantification reveals increased active (guanosine triphosphate [GTP]-bound) Ras (Ras-GTP levels relative to total Ras; E) and ERK (phospho-ERK1/2 relative to total ERK1/2; F) activation in male, but not female, Nf1-CKO hippocampi relative to controls. *p <0.05.
We sought to determine whether sex-dependent differences in neurofibromin regulation of cAMP, Ras and dopamine homeostasis account for the sexually-dimorphic abnormalities in learning and optic glioma-associated vision loss. First, we show that only female Nf1-CKO mice had lower retinal cAMP levels relative to controls (Fig 4B). Second, only Nf1-CKO male mice had reduced hippocampal dopamine levels and DARPP32 phosphorylation compared to controls (Fig 4C, D). Third, only male Nf1-CKO mice exhibited increased hippocampal Ras activation and ERK1/2 phosphorylation (activation) relative to controls (Fig 4E, F).
Discussion
While NF1 is a monogenetic disorder, the specific clinical manifestations and outcomes are not determined solely by the germline NF1 gene mutation. However, the individual factors that contribute to patient outcome are multi-factorial and often difficult to establish in human clinical studies. For this reason, Nf1 GEM strains provide experimentally-controlled platforms to assess potential risk factors. Using this approach, modifier genes have been identified in rodents that influence susceptibility to astrocytoma and malignant peripheral nerve sheath tumor (MPNST) development7,8. Although these autosomal genomic modifiers reflect differences between inbred mouse strains, we now establish for the first time that sex differentially impacts on NF1-associated neurologic dysfunction.
In children with NF1-associated optic glioma, we demonstrate that girls are more likely to require treatment as a result of visual decline. The increase in visual loss secondary to optic glioma in girls was not attributable to differences in patient age or tumor location, and did not reflect an increased prevalence of these tumors in girls with NF1. Based on these intriguing clinical findings, we leveraged Nf1 mutant mice to show that sex strongly influences the impact of an inactivating germline Nf1 gene mutation on optic glioma-associated visual loss. In addition, we demonstrate that this sexual dimorphic effect exists at both the tissue (retina) and molecular (cAMP) level. As such, only female Nf1-CKO mice exhibit reduced visual acuity due to reduced RGC survival and cAMP generation. Coupled with previous experiments demonstrating that cAMP elevation (Rolipram treatment) nearly completely ameliorated the optic glioma-induced retinal apoptosis in vivo18, these new observations underscore the need to consider sex when interpreting Nf1 preclinical GEM results as well as evaluating completed and future NF1 optic glioma therapeutic clinical studies.
While previous reports have revealed sex-related differences in cognition and behavior in both mice24 and in people with other neurologic conditions, including autism25 and reading disability26, there is currently a paucity of clinical data that specifically examined the impact of sex on cognitive function in children with NF1. In the two clinical studies evaluating sex as a potential risk factor, specific learning deficits were more prevalent in boys5,14. Using Nf1-CKO mice, we demonstrate that learning/memory deficits were only observed in males. This sex-specific abnormality results from selective impairments in both hippocampal dopamine and Ras signaling, and is consistent with studies that show that restoration of dopamine levels23 or inhibition of Ras hyperactivation21 reverse the learning/memory deficits in Nf1 mutant mice. While sexually dimorphic changes in Ras regulation have not been previously reported, embryonic midbrain dopaminergic cell number is determined by sex27,28. Current studies are focused on examining the relationship between dopamine homeostasis and Ras signaling in hippocampal neurons.
Previous reports have revealed that neurofibromin regulates different downstream signaling effectors (e.g., cAMP, dopamine, RAS/mTOR) in a both cell type- and brain region-specific manner19-22; however, the molecular mechanisms responsible for these sex-dependent effects are currently unknown. One possibility is potential differential sex hormonal influences, which will require castration/ovariectomy and hormonal manipulations to evaluate. Another complementary approach would entail the use of a novel complement of genetically-engineered mouse strains (four core genotype model), which allows for a direct assessment of sex chromosome contributions independent of gonadal sex29,30.
It is also plausible that sex interacts with a germline Nf1 gene mutation through epigenetic mechanisms, such as differential methylation or imprinting31,32 to produce global changes in gene expression33,34. In preliminary gene expression profiling experiments using Nf1+/- and wild-type embryonic mouse brains stratified by sex, the female Nf1+/- brain transcriptome clustered separately from the three other conditions (wild-type male and female brains, male Nf1+/- brains); however, no specific causative genes were identified (data not shown). Additional studies are planned to explore the etiology for these sexually-dimorphic effects.
Collectively, these observations support a model in which the clinical heterogeneity seen in individuals with NF1 likely results from the interplay between genomic determinants (e.g., sex) and neurofibromin function in specific tissues. Further elucidation of the underlying mechanisms may yield new predictive markers of patient outcome or unique targets for future therapeutic drug design.
Supplementary Material
Supplementary Figure 1. Sex does not influence performance of Nf1-CKO mice in cued or place trials of Morris Water Maze. In the cued trial of the Morris Water Maze, both male and female Nf1-CKO and control (CTL) mice exhibit similar escape latencies (A), path lengths (C), and swimming speeds (E). Place trial performance in the Morris water maze was also not affected by genotype or sex (B, D, F).
Acknowledgments
We thank Sara Conyers, Carla Yuede, and Joshua Dearborn in the Animal Behavior Core for technical assistance. K.D.A. was supported by a National Cancer Center Diversity Supplement Award. This work was funded by grants from the National Cancer Institute (to D.H.G.), Department of Defense (to D.H.G. and D.F.W.), and National Institute of Child Health and Human Development Center (P30 HD062171; D.F.W.). K.D.A., J.A.B., and S.M.G. performed the experiments and analysis. K.D.A. and D.H.G. wrote and edited the manuscript with comments from D.F.W. and J.B.R.
Footnotes
Potential conflicts of interests. The authors declare no potential conflicts of interest.
References
- 1.Diggs-Andrews KA, Gutmann DH. Modeling cognitive dysfunction in neurofibromatosis-1. Trends Neurosci. 2013;36:237–247. doi: 10.1016/j.tins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114:788–792. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 3.Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125:63–66. doi: 10.1016/s0022-3476(94)70122-9. [DOI] [PubMed] [Google Scholar]
- 4.Sangster J, Shores EA, Watt S, North KN. The cognitive profile of preschool-aged children with neurofibromatosis type 1. Child Neuropsychol. 2011;17:1–16. doi: 10.1080/09297041003761993. [DOI] [PubMed] [Google Scholar]
- 5.Hyman SL, Shores A, North KN. The nature of frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 6.Hyman SL, Arthur Shores E, North KN. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Dev Med Child Neurol. 2006;48:973–977. doi: 10.1017/S0012162206002131. [DOI] [PubMed] [Google Scholar]
- 7.Amlin-Van Schaick J, Kim S, Broman KW, Reilly KM. Scram1 is a modifier of spinal cord resistance for astrocytoma on mouse Chr 5. Mamm Genome. 2012;23:277–285. doi: 10.1007/s00335-011-9380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walrath JC, Fox K, Truffer E, Gregory Alvord W, Quinones OA, Reilly KM. Chr 19(A/J) modifies tumor resistance in a sex- and parent-of-origin-specific manner. Mamm Genome. 2009;20:214–223. doi: 10.1007/s00335-009-9179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neurofibromatosis. Arch Neurol; Conference statement. National Institutes of Health Consensus Development Conference; 1988. pp. 575–578. [PubMed] [Google Scholar]
- 10.Brown JA, Emnett RJ, White CR, et al. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Hum Mol Genet. 2010;19:4515–4528. doi: 10.1093/hmg/ddq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Glioma Task Force. Ann Neurol. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 13.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14:790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coude FX, Mignot C, Lyonnet S, Munnich A. Academic impairment is the most frequent complication of neurofibromatosis type-1 (NF1) in children. Behav Genet. 2006;36:660–664. doi: 10.1007/s10519-005-9040-9. [DOI] [PubMed] [Google Scholar]
- 15.Bajenaru ML, Hernandez MR, Perry A, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. [PubMed] [Google Scholar]
- 16.Hegedus B, Hughes FW, Garbow JR, et al. Optic nerve dysfunction in a mouse model of neurofibromatosis-1 optic glioma. J Neuropathol Exp Neurol. 2009;68:542–551. doi: 10.1097/NEN.0b013e3181a3240b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KY, Ju WK, Hegedus B, Gutmann DH, Ellisman MH. Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma. Neuroscience. 2010;170:178–188. doi: 10.1016/j.neuroscience.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JA, Gianino SM, Gutmann DH. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. J Neurosci. 2010;30:5579–5589. doi: 10.1523/JNEUROSCI.3994-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DY, Yeh TH, Emnett RJ, et al. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24:2317–2329. doi: 10.1101/gad.1957110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JA, Diggs-Andrews KA, Gianino SM, Gutmann DH. Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in cAMP/PKA/ROCK-dependent manner. Mol Cell Neurosci. 2012;49:13–22. doi: 10.1016/j.mcn.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa RM, Federov NB, Kogan JH, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 22.Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nature Neurosci. 2002;5:95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- 23.Diggs-Andrews KA, Tokuda K, Izumi Y, Zorumski CF, Wozniak DF, Gutmann DH. Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice. Ann Neurol. 2013;73:309–315. doi: 10.1002/ana.23793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rial D, Xikota JC, Miozzo A, Cruz VE, Prediger RD, Walz R. Differential gender-related susceptibility to learning and memory deficits in mice submitted to neonatal freezing microgyria model. Brain Res Bull. 2009;79:177–181. doi: 10.1016/j.brainresbull.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- 26.Liederman J, Kantrowitz L, Flannery K. Male vulnerability to reading disability is not likely to be a myth: a call for new data. J Learn Dis. 2005;38:109–129. doi: 10.1177/00222194050380020201. [DOI] [PubMed] [Google Scholar]
- 27.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nature Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 29.Ehlen JC, Hesse S, Pinckney L, Paul KN. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS One. 2013;8:e62205. doi: 10.1371/journal.pone.0062205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker JM, Torresgrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinius B, Shi C, Hengshuo L, et al. Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse. BMC Genomics. 2010;11:614. doi: 10.1186/1471-2164-11-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin LC, Lewis DA, Sibille E. A human-mouse conserved sex bias in amygdala gene expression related to circadian clock and energy metabolism. Mol Brain. 2011;4:18. doi: 10.1186/1756-6606-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Schadt EE, Wang S, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Sex does not influence performance of Nf1-CKO mice in cued or place trials of Morris Water Maze. In the cued trial of the Morris Water Maze, both male and female Nf1-CKO and control (CTL) mice exhibit similar escape latencies (A), path lengths (C), and swimming speeds (E). Place trial performance in the Morris water maze was also not affected by genotype or sex (B, D, F).


