Abstract
Importance
The established chronic kidney disease (CKD) progression endpoint, end-stage renal disease (ESRD) or doubling of serum creatinine (corresponding to a change in estimated glomerular filtration rate (eGFR) of −57% or greater) is a late event, limiting feasibility of nephrology clinical trials.
Objective
To characterize the association of decline in eGFR with subsequent progression to ESRD, with implications for using lesser declines in eGFR as potential alternative endpoints for CKD progression. Since most people with CKD die before reaching ESRD, we also investigated mortality risk.
Data Sources
Individual meta-analysis of up to 1.7 million participants with 12,344 ESRD events and 223,944 deaths from 35 cohorts.
Study Selection
Cohorts in the CKD Prognosis Consortium with a repeated measure of serum creatinine over 1-3 years and outcome data.
Data Extraction and Synthesis
Transfer of individual participant data or standardized analysis of outputs for random effects meta-analysis took place between July 2012 and September 2013 with baseline eGFRs during 1975-2012.
Main Outcomes and Measures
ESRD (initiation of dialysis or transplantation) or all-cause mortality risk related to percent change in eGFR over 2 years adjusted for potential confounders and first eGFR.
Results
The adjusted hazard ratios (HR) of ESRD and mortality were exponentially higher with larger eGFR decline. Among participants with baseline eGFR <60 ml/min/1.73m2, the adjusted HRs for ESRD were 32.1 (95% CI 22.3-46.3) and 5.4 (4.5-6.4) for −57% and −30% eGFR changes, respectively. However, changes of −30% or greater were much more common than changes of −57% (6.9% (6.4-7.4%) vs. 0.79% (0.52-1.06%) in the whole consortium). This association was strong and consistent across length of baseline (1 or 3 years), baseline eGFR, age, diabetes status, or albuminuria. Average adjusted 10-year risk of ESRD for eGFR changes of −57%, −40%, −30% and 0% were 99% (95-100%), 83% (71-93%), 64% (52-77%), vs. 18% (15-22%) respectively at baseline eGFR of 35 ml/min/1.73m2. Corresponding mortality risks were 77% (71-82%), 60% (56-63%), 50% (47-52%), vs. 32% (31-33%), showing a similar but weaker pattern.
Conclusions and Relevance
Declines in eGFR smaller than doubling of serum creatinine occur more commonly and are strongly and consistently associated with the risk of ESRD and mortality, supporting consideration of lesser declines in eGFR, such as 30% reduction over 2 years, as an alternative endpoint for CKD progression.
Background
Chronic kidney disease (CKD) is a world-wide public health problem, with increasing prevalence, poor outcomes and high cost.1 Yet, despite the availability of simple laboratory tests to identify people with earlier stages of CKD, there are fewer clinical trials for kidney disease than for other common diseases.2 One contributing reason may be that the established endpoint used to document CKD progression, namely, a doubling of serum creatinine from baseline (corresponding to a 57% reduction in estimated glomerular filtration rate (eGFR)), is a late event, requiring long follow-up periods and large sample sizes.2-4 Improved methods for GFR estimation may allow for use of smaller reductions in eGFR than doubling of serum creatinine as alternative endpoints to assess CKD progression.4,5 Evaluation of such alternative endpoints should include their enumeration and quantification of their relationship to future progression to end-stage renal disease (ESRD) across a wide range of settings. Standardized definitions of CKD progression outcomes would also benefit observational studies and clinical practice.
One-year change in estimated GFR was strongly related to risk of ESRD in the Alberta Kidney Disease Network (AKDN).6 Other studies focused on mortality and cardiovascular disease since these outcomes occur more commonly than ESRD and showed a strong relationship with various definitions for CKD progression.7-11 A systematic evaluation across studies using a uniform analytic approach is needed to provide a more rigorous basis for determining the prognosis associated with specific declines in eGFR. Clinical trials with doubling of serum creatinine or ESRD as an end-point typically have follow-up of approximately 5 years. The goal for alternative kidney endpoints is to enable clinical trials of shorter duration, with 2-years thought to be useful for observing a meaningful change in eGFR, but the prognostic implications of shorter and longer periods for observing eGFR change need to be quantified as well. A rigorous evaluation of estimates of CKD progression is also important to inform observational studies and clinical practice where various measures of CKD progression have been used.7
We examined the prognostic contribution of change in eGFR over 1, 2 and 3 years to subsequent ESRD and mortality in a large international consortium to test its strength and consistency across subgroups defined by baseline kidney function and comorbid conditions and provide the evidence base for evaluating the usefulness of potential alternative endpoints for CKD progression.
Methods
Study selection criteria
Details of the Chronic Kidney Disease Prognosis Consortium (CKD-PC) are described elsewhere and in eAppendices 1-2.12-16 Briefly, CKD-PC consists of 50 cohorts with at least 1,000 participants (not applied to cohorts predominantly enrolling persons with CKD [CKD cohorts]) with data on serum creatinine and albuminuria and 50 or more events of outcomes of interest (either mortality or kidney outcome).12-16 All cohorts with appropriate data opted into this study, which included 22 cohorts (4 general population cohorts, 5 high-risk cohorts in terms of cardiovascular risk, and 13 CKD cohorts) with a repeated measure of serum creatinine during an elapsed period of 0.5-3.5 years to determine the relationship of change in eGFR on subsequent ESRD; 35 cohorts (15 general population cohorts, 7 high-risk cohorts in terms of cardiovascular risk, and 13 CKD cohorts) included mortality outcomes. Each meta-analysis for the present study was restricted to cohorts with at least 10 events and participants aged ≥18 years. Data transfer and analysis took place between July 2012 and September 2013 with subsequent updates. This study was approved by the institutional review board for use of de-identified data at the Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland, USA).
Procedures
eGFR was calculated using the CKD-EPI 2009 creatinine equation.4,16 In cohorts where the creatinine measurement was not standardized to isotope dilution mass spectrometry (IDMS), creatinine concentrations were reduced by 5%, the established calibration factor, and drift over time was corrected when possible.17
We tested two indices of change in eGFR during a baseline period, percent change and slope (annual change in eGFR). Percent change in eGFR was calculated as follows: (last eGFR – first eGFR)/(first eGFR) * 100%. The slope was determined as an annual change estimated from a least-square regression model using all eGFR measurements in the baseline period. As the implications for the magnitude of change in eGFR may vary depending on the time for the change, we defined three baseline periods (1, 2, and 3 years) to determine the change in eGFR and repeated the analysis for each baseline period. The length of the baseline periods correspond to the median length of follow-up in a trial which would use change in eGFR as an endpoint, also corresponding to periods over which clinicians would want to determine if CKD has progressed. For each baseline period, a 0.5 year of margin before and after the end of the period was allowed for determining the last available eGFR to calculate the change (e.g., eGFR between 0.5 and 1.5 years after the first available eGFR could be used for the 1 year baseline period analysis), but the eGFR closest to the end of the period of interest was selected for each participant. Given that doubling of serum creatinine, the established kidney endpoint, corresponds to a −57% change in eGFR with the CKD-EPI equation (for serum creatinine ≥0.9 mg/dl in men and ≥0.7 mg/dl in women), our primary data presentation was based on percent change in eGFR. All covariates were assessed at the time of first eGFR (eAppendix 2 shows details for specific cohorts).
We defined diabetes as fasting glucose ≥7.0 mmol/L (126 mg/dl), non-fasting glucose ≥11.1 mmol/L (200 mg/dl), hemoglobin A1c ≥6.5%, use of glucose lowering drugs, or self-reported diabetes. Participants with a history of myocardial infarction, coronary revascularization, heart failure, or stroke were considered to have a history of cardiovascular disease (CVD). As albuminuria was not necessarily measured prior to the first available eGFR in several cohorts, adjustment for albuminuria when available was conducted only in sensitivity analyses. While the urine albumin-to-creatinine ratio was our primary measure of albuminuria, we also included studies with urine albumin excretion rate, urine protein-to-creatinine ratio (PCR), or quantitative dipstick protein.18
The primary outcome of interest was ESRD after the baseline period. We defined ESRD as initiation of renal replacement therapy or death due to kidney disease other than acute kidney injury. ESRD cases before baseline period were excluded from the relevant analyses. Since the majority of patients with CKD die without reaching ESRD, we repeated the analysis for all-cause mortality as well as cardiovascular mortality and non-cardiovascular mortality. Cardiovascular mortality was defined as death due to myocardial infarction, heart failure, stroke, or sudden cardiac death.
Statistical analysis
We applied a two-stage analytic approach, whereby each study was first analyzed separately, followed by a random-effects meta-analysis. The analysis overview and analytic notes for individual studies are described in eAppendix 2. We imputed missing values of covariates but not the main exposure, change in eGFR, using cohort-specific mean values (see Appendix 2 for details). We quantified heterogeneity with the I2 statistic and χ2 test12 and explored sources of heterogeneity with random-effects meta-regression analysis. Since the absolute risk of ESRD and the implication of change in eGFR vary substantially depending on baseline eGFR, analyses were done stratified by first eGFR with lower eGFR defined as less than 60 ml/min/1.73m2 and higher eGFR defined as ≥60 ml/min/1.73m2. Analyses were performed using Stata/SE 12 software (www.stata.com). We considered 2-sided P-values < 0.05 statistically significant.
We modeled the adjusted hazard ratios (HRs) of ESRD and mortality after the end of the baseline period as a spline function of percent change in eGFR with the aforementioned covariates. In each study, we fit piece-wise linear splines for percent change in eGFR (knots were placed at −57%, −25%, −10%, 10%, 25%) and 0% change as a reference point. Cox models were adjusted for age, sex, race/ethnicity (blacks vs. non-blacks), systolic blood pressure, total cholesterol, diabetes, history of CVD, and first eGFR. Potential effect modifiers with change in eGFR were assessed by incorporating interaction terms.
We illustrate the opposite effects of decreasing risk and increasing prevalence of smaller percent changes in eGFR using an approximation of the percent population attributable risk (%PAR) calculated from the prevalence of percent change in eGFR, using the overall population distributions as a fixed standard, and its adjusted HR and 95% confidence interval. It is best to view the calculated %PAR as an approximation of the overall percentage of ESRD or mortality risk explained, rather than a truly preventable fraction, since change in eGFR is not fully reversible.19 At the range of eGFR change with lower risk compared to the reference point, 0% change, %PAR has a negative value corresponding to reduced, rather than excess, risk in the population explained by this eGFR change.
We translated meta-analyzed adjusted HRs for percent change in eGFR to absolute risk of ESRD or mortality at 1, 3, 5, and 10 years after the baseline period using the weighted average baseline risk. One-year baseline risk in each cohort was calculated for the following combination of covariates: 60 year old, non-black, male, no change in eGFR, a first eGFR of 50 ml/min/1.73m2, a systolic blood pressure of 130 mm Hg, a total cholesterol of 5 mmol/L, no history of diabetes or CVD. Risk was scaled for longer follow-up and pooled across cohorts using a weighted average (eAppendix 2, implications of lower and higher baseline risk were also calculated). We applied the adjusted sub-HRs from competing risk models accounting for death as a competing endpoint.20
Results
Study characteristics
Twenty two cohorts provided data on change in eGFR for a baseline period of 1-year in 1,530,648 participants who had 12,344 ESRD events during a mean follow-up period of 3.1 years subsequently. For baseline periods of 2-and 3-years, the studies included 1,341,193 participants with 8,532 subsequent ESRD events and 1,080,274 with 5,159 subsequent ESRD events respectively (Table 1). Mortality analysis included 35 cohorts (1,757,886 participants with 223,944 deaths from 27 cohorts for 1-year, 1,589,257 participants with 158,603 deaths from 32 cohorts for 2-year, and 1,259,477 participants with 102,491 deaths from 34 cohorts for 3-year). Baseline assessments in each cohort took place between 1975-2012, with generally longer follow-up in older cohorts than newer cohorts. The results focus on the 2-year baseline period with details for the 1-year and 3-year period provided in supplementary appendices. Participating cohorts spanned a wide spectrum of sample size and baseline characteristics (Table 1 and 2 and eTable 1). The cohort averages for lower and higher eGFR strata were: first eGFR 48 and 92 ml/min/1.73m2, age 74 and 51 years, female sex 20% and 51%, black race 7% and 1%, diabetes 38% and 14% and history of CVD 35% and 6%, respectively.
Table 1.
Participating cohorts by baseline eGFR
| End-stage renal disease | All-cause mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Participants N |
Serum creatinine, Median (IQR) |
Events (n) |
Follow- up Mean (SD), years |
Events (n) |
Follow- up Mean (SD), years |
||||
|
| |||||||||
| Baseline eGFR | Baseline eGFR | Baseline eGFR | Baseline eGFR | Baseline eGFR | Baseline eGFR | ||||
|
<60 ml/min/1.73m2 |
60+ ml/min/1.73m2 |
<60 ml/min/1.73m2 |
60+ ml/min/1.73m2 |
<60 ml/min/1.73m2 |
60+ ml/min/1.73m2 |
||||
|
| |||||||||
| Meta-Analysis | |||||||||
|
| |||||||||
| Baseline, 1-y |
466,068
(458,965) * |
1,291,818
(1,071,683) * |
2(2-3) | 11,214 | 1,130 |
3.1
(2.3) |
144,558 | 79,386 |
4.4
(3.6) |
|
| |||||||||
| Baseline, 2-y |
363,143
(356,813) * |
1,226,114
(984,380) * |
3(3-5) | 7,523 | 1,009 |
2.4
(2.2) |
97,795 | 60,808 |
3.7
(3.6) |
|
| |||||||||
| Baseline, 3-y |
235,560
(230,178) * |
1,023,917
(850,096) * |
5 (4-5) | 4,058 | 1,101 |
2.0
(2.9) |
55,135 | 47,356 |
3.2
(4.0) |
|
| |||||||||
| Participating cohorts’ data for the 2 year baseline period | |||||||||
|
| |||||||||
| AASK | 744 | 169 | 7 (6-7) | 243 | 8 | 6 (3) | 112 | 24 | 6 (3) |
| ADVANCE | 1,542 | 8,457 | 4 (4-4) | 16 | 21 | 3 (0.5) | 150 | 407 | 3 (0.5) |
| Aichi | 14 | 1,798 | 2(2-3) | 1 | 15 | 7 (2) | |||
| AKDN | 35,617 | 257,597 | 3 (3-4) | 206 | 63 | 2 (1) | 3,878 | 5,779 | 2 (1) |
| ARIC | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| BC CKD | 7,986 | 656 | 10 (8-14) | 1,178 | 53 | 2 (1) | 1,730 | 67 | 3 (1) |
| CARE | 580 | 3,101 | 3 (3-3) | 62 | 150 | 3 (1) | |||
| CCF | 17,102 | 31 | 6 (4-9) | 290 | 1 | 1 (1) | 1,746 | 3 | 1 (1) |
| CHS | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| CIRCS | 175 | 4,286 | 3 (2-3) | 53 | 564 | 17 (4) | |||
| CRIB | 189 | 1 | 2 (2-2) | 63 | 0 | 4 (2) | 45 | 0 | 5 (2) |
| Framingham | 46 | 652 | 2 (2-2) | 17 | 54 | 6 (1) | |||
| Geisinger | 14,850 | 20 | 6 (4-9) | 257 | 0 | 3 (2) | 2,287 | 4 | 3 (2) |
| GLOMMS 1 | 645 | 2 | 11 (7-20) | 58 | 0 | 3 (1) | 274 | 1 | 3 (1) |
| IPHS | 2,147 | 60,319 | 3 (3-3) | 983 | 10,019 | 12 (3) | |||
| KP Hawaii | 5,468 | 15,140 | 5 (4-8) | 134 | 19 | 1 (0.7) | 364 | 329 | 1 (1) |
| KPNW | 320 | 202 | 7(4-12) | 21 | 10 | 4 (2) | 167 | 73 | 5 (2) |
| KSHS | 217 | 62,810 | 3 (3-5) | 5 | 169 | 3 (1) | |||
| Maccabi | 27,616 | 577,024 | 8(7-9) | 724 | 177 | 3 (1) | 6,199 | 14,042 | 4 (1) |
| MASTERPLAN | 513 | 66 | 8(7-9) | 111 | 3 | 4 (1) | 79 | 4 | 4 (1) |
| MDRD | 591 | 27 | 8 (7-8) | 431 | 13 | 7 (5) | 270 | 5 | 13 (4) |
| MESA | n/a | n/a | n/a | n/a | n/a | n/a | |||
| MRFIT | 185 | 11,342 | 3 (3-3) | 30 | 239 | 21 (6) | 86 | 3,900 | 23 (8) |
| NephroTest | 465 | 88 | 3 (2-3) | 92 | 3 | 3 (2) | 61 | 1 | 4 (2) |
| NZDCS | 1,913 | 7,093 | 3 (3-5) | 152 | 100 | 6 (2) | 728 | 1,081 | 6 (2) |
| Ohasama | 38 | 1,039 | 3 (3-3) | 6 | 59 | 7 (1) | |||
| Pima | 12 | 1,594 | 2 (2-2) | 6 | 101 | 12 (8) | 10 | 343 | 13 (8) |
| PREVEND | 406 | 4,334 | 2 (2-2) | 34 | 98 | 4 (1) | |||
| Rancho Bernardo | 33 | 174 | 2 (2-2) | 9 | 17 | 7 (1) | |||
| RENAAL | 1,083 | 118 | 10 (9-10) | 195 | 5 | 1 (1) | 147 | 7 | 1 (1) |
| Severance | 140 | 6,105 | 2 (2, 3) | 6 | 119 | 12 (2) | |||
| Sunnybrook | 1,484 | 1,173 | 7 (5-11) | 168 | 18 | 3 (2) | 437 | 90 | 5 (3) |
| Taiwan MJ | 2,247 | 96,533 | 2(2-3) | 362 | 1,676 | 7 (4) | |||
| VA CKD | 238,488 | 103,580 | 5 (4-7) | 3,148 | 175 | 3 (1) | 77,337 | 21,552 | 3 (1) |
| ZODIAC | 287 | 583 | 3 (3-3) | 150 | 156 | 7 (3) | |||
n/a: Not available for 2y baseline period but those studies are included in other baseline periods. eGFR: estimated glomerular filtration rate. IQR: interquartile range. SD: standard deviation.
Blank cells indicate the cohort did not have data on end-stage renal disease. Total numbers for cohorts with both end-stage renal disease and all-cause mortality as outcomes use data from the end-stage renal disease analysis.
Total number for end-stage renal disease analyses.
Table 2.
Baseline characteristics for participating cohorts in the 2-y baseline period analysis
| eGFR < 60 | eGFR ≥60 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Age | % Female |
% Black |
Baseline eGFR |
Total Chol |
SBP | % DM |
% CVD |
% Alb |
% Smoke |
Age | % Female |
% Black |
Baseline eGFR |
Total Chol |
SBP | % DM |
% CVD |
% Alb |
% Smoke |
| AASK | 54 (11) | 39 | 100 | 42 (11) | 5 (1) | 150 (24) | 0 | 53 | 64 | 44 | 55 (10) | 38 | 100 | 67 (6) | 5 (1) | 150 (24) | 0 | 45 | 38 | 41 |
| ADVANCE | 69 (6) | 54 | 0 | 51 (8) | 5 (1) | 147 (23) | 100 | 31 | 39 | 9 | 66 (6) | 40 | 0 | 83 (13) | 5 (1) | 144 (21) | 100 | 24 | 28 | 16 |
| Aichi | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 49 (6) | 18 | 0 | 92 (14) | 5 (1) | 126 (15) | 0.3 | 0.06 | 4.2 | 31 |
| AKDN | 73 (11) | 60 | 0 | 48 (10) | n/a | n/a | 18 | 17 | 12 | n/a | 54 (15) | 59 | 0 | 89 (16) | n/a | n/a | 7 | 4 | 4 | n/a |
| BC CKD | 70 (13) | 46 | 0 | 33 (10) | 5 (1) | 134 (22) | 42 | 4 | 69 | 6 | 56 (15) | 45 | 1 | 78 (16) | 5 (2) | 136 (23) | 52 | 1 | 67 | 6 |
| CARE | 66 (7) | 21 | 2 | 52 (7) | 5 (0) | 134 (20) | 18 | 100 | 19 | 8 | 58 (9) | 12 | 3 | 80 (13) | 5 (0) | 128 (18) | 13 | 100 | 11 | 17 |
| CCF | 72 (11) | 55 | 12 | 47 (10) | 5 (1) | 131 (19) | 26 | 22 | 27 | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| CIRCS | 63 (6) | 70 | 0 | 54 (6) | 5 (1) | 135 (19) | 6 | 4 | 7 | 14 | 54 (9) | 66 | 0 | 84 (12) | 5 (1) | 130 (17) | 5 | 1 | 2 | 23 |
| CRIB | 61 (15) | 34 | 6 | 28 (9) | 6 (1) | 150 (23) | 16 | 44 | 81 | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Framingham | 70 (6) | 52 | 0 | 51 (8) | 5 (1) | 139 (16) | 20 | 13 | n/a | 13 | 59 (9) | 50 | 0 | 89 (18) | 5 (1) | 127 (18) | 9 | 5 | 12 | 15 |
| Geisinger | 70 (10) | 59 | 1 | 52 (8) | 5 (1) | 131 (19) | 31 | 15 | 44 | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| GLOMMS 1 | 70 (13) | 50 | 0 | 33 (7) | n/a | n/a | 61 | 48 | 72 | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| IPHS | 70 (6) | 68 | 0 | 54 (6) | 5 (1) | 139 (17) | 9 | 16 | 9 | 9 | 59 (10) | 68 | 0 | 87 (12) | 5 (1) | 133 (18) | 5 | 5 | 2 | 7 |
| KP Hawaii | 71 (11) | 53 | 0 | 47 (10) | 5 (1) | 137 (22) | 52 | 35 | 66 | 7 | 58 (13) | 49 | 0 | 86 (16) | 5 (1) | 135 (20) | 67 | 16 | 41 | 13 |
| KPNW | 71 (10) | 48 | 2 | 47 (11) | n/a | 142 (23) | 40 | 24 | 8 | 13 | 67 (10) | 55 | 5 | 73 (10) | n/a | 142 (22) | 46 | 20 | 11 | 11 |
| KSHS | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 37 (7) | 34 | 0 | 91 (11) | 5 (1) | 114 (14) | 2 | 1 | 1 | 30 |
| Maccabi | 72 (11) | 58 | 0 | 50 (9) | 5 (1) | 134 (19) | 30 | 9 | 40 | 1 | 47 (15) | 59 | 0 | 94 (19) | 5 (1) | 124 (17) | 11 | 1 | 17 | 2 |
| MASTERPLAN | 61 (12) | 31 | 0 | 36 (11) | 5 (1) | 136 (20) | 24 | 30 | 37 | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| MDRD | 52 (12) | 38 | 7 | 35 (11) | 6 (1) | 132 (18) | 4 | 13 | 83 | 10 | 47 (12) | 63 | 11 | 65 (4) | 6 (1) | 129 (20) | 7 | 7 | n/a | n/a |
| MRFIT | 52 (5) | 0 | 5 | 55 (5) | 6 (1) | 130 (17) | 10 | 3 | 13 | 34 | 47 (6) | 0 | 7 | 89 (13) | 6 (1) | 128 (14) | 5 | 1 | 3 | 58 |
| NephroTest | 60 (14) | 32 | 10 | 37 (12) | 5 (1) | 137 (20) | 24 | 19 | 96 | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| NZDCS | 71 (9) | 57 | 0 | 48 (10) | 5 (1) | 142 (21) | 100 | 2 | 14 | 8 | 59 (13) | 49 | 0 | 86 (16) | 5 (1) | 138 (19) | 100 | 1 | 7 | 16 |
| Ohasama | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 63 (8) | 67 | 0 | 84 (11) | 5 (1) | 129 (17) | 8 | 2 | 5 | 16 |
| Pima | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 32 (14) | 63 | 0 | 122 (15) | 4 (1) | 118 (17) | 27 | n/a | 18 | 28 |
| PREVEND | 67 (9) | 53 | 0 | 53 (7) | 5 (1) | 137 (21) | 16 | 16 | 27 | 22 | 52 (11) | 49 | 1 | 83 (13) | 5 (1) | 125 (18) | 8 | 5 | 8 | 32 |
| Rancho Bernardo | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 69 (5) | 52 | 0 | 78 (11) | 5 (1) | 132 (17) | 16 | 10 | 8 | 8 |
| RENAAL | 61 (7) | 38 | 13 | 40 (11) | 6 (1) | 152 (19) | 100 | 44 | 100 | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Severance | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 44 (9) | 38 | 0 | 90 (15) | 5 (1) | 120 (18) | 1 | 0.4 | 4 | 32 |
| Sunnybrook | 69 (14) | 40 | 0 | 38 (12) | n/a | n/a | 37 | 48 | 81 | 5 | 52 (16) | 46 | 0 | 91 (20) | n/a | n/a | 26 | 23 | 79 | 5 |
| Taiwan | 63 (10) | 40 | 0 | 52 (8) | 5 (1) | 139 (24) | 9 | 9 | 12 | 21 | 40 (12) | 50 | 0 | 96 (15) | 5 (1) | 119 (18) | 2 | 2 | 1 | 22 |
| VA CKD | 75 (9) | 3 | 8 | 48 (9) | 4 (1) | n/a | 43 | 45 | 41 | n/a | 69 (10) | 3 | 11 | 71 (13) | 4 (1) | n/a | 52 | 38 | 72 | n/a |
| ZODIAC | 74 (8) | 72 | 0 | 50 (8) | 6 (1) | 159 (24) | 100 | 43 | 43 | 13 | 64 (11) | 49 | 0 | 77 (12) | 6 (1) | 155 (25) | 100 | 28 | 32 | 23 |
|
| ||||||||||||||||||||
| Total | 74 (10) | 20 | 7 | 48 (10) | 4 (1) | 135 (20) | 38 | 35 | 38 | 6 | 51 (16) | 51 | 1 | 92 (18) | 5 (1) | 125 (18) | 14 | 6 | 17 | 8 |
Mean (SD). n/a: Not available for relevant baseline period but included in other baseline period(s).eGFR: estimated glomerular filtration rate. Chol: cholesterol. SBP: systolic blood pressure. DM: diabetes mellitus. CVD: history of cardiovascular disease. Alb: albuminuria, proportion of participants with urine albumin-to-creatinine ratio ≥30 mg/g or urine protein-to-creatinine ratio ≥50 mg/g or dipstick protein ≥1+. Smoke: current cigarette smoking.
Appendices 1 and 2 provide further information regarding each cohort.
ESRD risk according to change in eGFR
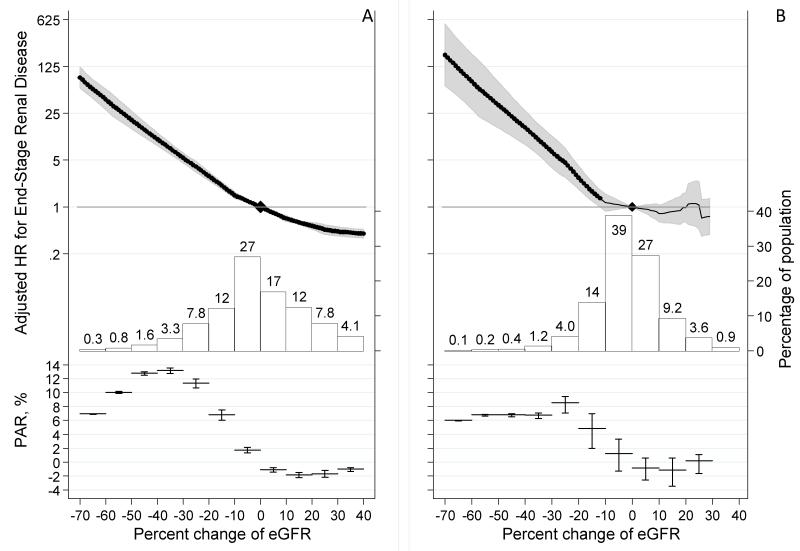
Overall, change in eGFR over 2 years had a median (5th–95th percentile) of −1% (−26% to +26%), with a distribution skewed towards negative values indicating more prevalent eGFR decline (Figure 1). The prevalence of −57% change in GFR was much lower than for lesser changes. Fifty-two percent of ESRD cases had a −30% change in eGFR over 2 years whereas only 16% of ESRD cases reached −57% eGFR change in this time frame. Subsequent risk of ESRD showed exponentially higher adjusted HRs at greater negative percent changes in eGFR and lower HRs at greater positive percent changes in eGFR compared to no change in eGFR, with similar associations for lower and higher eGFR strata (Figure 1 panels A and B). A −57% change in eGFR was associated with an adjusted HRs of ESRD of 32.1 (95% CI 22.3-46.3) and 57.2 (21.9-149.1) at lower and higher eGFR respectively. A −30% change in eGFR was associated with an adjusted HR of ESRD of 5.4 (95% CI 4.5-6.4) and 6.7 (3.9-11.5) at lower and higher eGFR, respectively. Sensitivity analyses assessing the percentage change in eGFR over shorter (1 year) and longer (3 year) baseline periods yielded similar associations with ESRD in both the lower and higher GFR strata (Table 3 and eFigures 1-2). Further adjustment for albuminuria yielded similar results (eFigure 3), as did multiple imputation of missing data (eAppendix 2).
Figure 1.
Adjusted hazard ratio of end-stage renal disease associated with percent change in eGFR during a 2-year baseline period in eGFR<60 ml/min/1.73m2 (A) and eGFR≥60 ml/min/1.73m2 (B) and a histogram of the percent change in eGFR as well as approximate percent population attributable risk of end-stage renal disease. Values trimmed at <−70% change (0.22% and 0.055% of the study population in eGFR <60 and ≥60 ml/min/1.73m2, respectively) and >40% change (5.9% and 0.51% of the population in eGFR <60 and ≥60 ml/min/1.73m2, respectively).
Table 3.
Prevalence and adjusted hazard ratio of end-stage renal disease associated with percent decline in eGFR during baseline periods of one to three years duration by level of kidney function
| Percent decline in eGFR during the 2-year baseline period | |||||||
|---|---|---|---|---|---|---|---|
| −57% | −40% | −30% | −25% | −20% | 0% (Stable) | ||
|
eGFR<60
ml/min/ 1.73m2 |
|||||||
| Baseline, 1-y | Adjusted HR | 21.5 (16.1, 28.8) | 7.4 (6.1, 8.9) | 4.0 (3.4, 4.6) | 3.0 (2.6, 3.4) | 2.4 (2.2, 2.7) | ref |
| Cumulative Prevalence, % | 0.43 (0.30, 0.57) | 1.7 (1.4, 1.9) | 4.2 (3.9, 4.6) | 6.4 (6.1, 6.8) | 10 (10, 11) | 54 (54, 55) | |
| Cumulative PAR, % | 4.3 (4.2, 4.3) | 15 (14, 15) | 25 (24, 25) | 30 (29, 31) | 35 (33, 36) | 46 (43, 48) | |
| Baseline, 2-y | Adjusted HR | 32.1 (22.3, 46.3) | 10.2 (8.2, 12.7) | 5.4 (4.5, 6.4) | 4.0 (3.3, 4.8) | 2.9 (2.5, 3.3) | ref |
| Cumulative Prevalence, % | 0.79 (0.52, 1.06) | 3.2 (2.8, 3.7) | 6.9 (6.4, 7.4) | 10 (10, 11) | 15 (14, 15) | 54 (53, 54) | |
| Cumulative PAR, % | 10 (10, 10) | 31 (31, 32) | 44 (43, 45) | 51 (49, 52) | 55 (54, 57) | 63 (60, 65) | |
| Baseline, 3-y | Adjusted HR | 36.8 (27.3, 49.7) | 10.4 (8.0, 13.4) | 5.0 (3.9, 6.4) | 3.2 (2.4, 4.2) | 2.5 (2.1, 3.1) | ref |
| Cumulative Prevalence, % | 1.3 (0.9, 1.7) | 4.8 (4.3, 5.4) | 9.5 (8.9, 10.2) | 13 (13, 14) | 18 (18, 19) | 53 (52, 54) | |
| Cumulative PAR, % | 17 (17, 17) | 40 (40, 41) | 52 (51, 53) | 56 (55, 57) | 60 (58, 61) | 65 (62, 67) | |
|
eGFR≥60
ml/min/ 1.73m2 |
|||||||
| Baseline, 1-y | Adjusted HR | 48.4(19.0, 123.0) | 13.1 (7.9, 21.6) | 5.5 (3.6, 8.4) | 3.7 (2.5, 5.5) | 2.5 (1.8, 3.3) | ref |
| Cumulative Prevalence, % | 0.13 (0.05, 0.21) | 0.56 (0.42, 0.70) | 1.8 (1.6, 2.0) | 3.4 (3.2, 3.7) | 6.8 (6.5, 7.0) | 64 (63, 64) | |
| Cumulative PAR, % | 4.9 (4.7, 4.9) | 13 (12, 13) | 20 (19, 21) | 25 (23, 26) | 30 (27, 31) | 37 (31, 42) | |
| Baseline, 2-y | Adjusted HR | 57.2 (21.9, 149.1) | 15.3 (8.5, 27.2) | 6.7 (3.9, 11.5) | 4.6 (2.8, 7.6) | 2.7 (1.8, 4.1) | ref |
| Cumulative Prevalence, % | 0.18 (0.07, 0.29) | 0.85 (0.62, 1.09) | 2.2 (2.0, 2.5) | 4.0 (3.7, 4.3) | 6.8 (6.5, 7.1) | 62 (62, 62) | |
| Cumulative PAR, % | 8.5 (8.4, 8.6) | 21 (20, 21) | 28 (27, 29) | 32 (30, 34) | 35 (33, 37) | 41 (33, 47) | |
| Baseline, 3-y | Adjusted HR | 60.6 (19.0, 193.0) | 15.7 (7.4, 33.4) | 7.0 (3.9, 12.7) | 4.6 (2.6, 7.9) | 2.9 (2.0, 4.2) | ref |
| Cumulative Prevalence, % | 0.24 (0.07, 0.41) | 1.1 (0.8, 1.4) | 2.7 (2.4, 3.0) | 4.6 (4.3, 4.9) | 8.5 (8.1, 8.8) | 67 (66, 67) | |
| Cumulative PAR, % | 6.3 (6.2, 6.3) | 13 (13, 14) | 20 (19, 21) | 23 (22, 24) | 27 (25, 29) | 34 (28, 38) | |
Cumulative indicates an eGFR decline of this level or greater. In parentheses are the 95% confidence intervals based on the whole eligible study sample in the Consortium as a standard population.
eGFR: estimated glomerular filtration rate. HR: hazard ratio. PAR: population attributable risk.
The %PAR of ESRD was positive for those with decline in eGFR and negative for those with a rise in eGFR compared to those with a stable eGFR (Figure 1). The %PAR showed that lower risk associated with smaller reductions in eGFR were offset by a higher prevalence leading the %PAR to peak around −40% to −30% and −30% to −20% for the lower and higher eGFR strata, respectively. In the lower eGFR stratum with a 2-year baseline the cumulative prevalence for eGFR changes of −57% or greater vs. −30% or greater was 0.79% (0.52-1.06%) and 6.9% (6.4-7.4%), respectively (Table 3). As a result, the cumulative %PAR rose markedly from 10% to 44%, respectively. Thus, of the 63% of ESRD attributable to eGFR decline (below 0%), 16% can be attributed to the participants with eGFR change of −57% or greater compared to 70% with eGFR change of −30% or greater. Similar results were observed in the higher eGFR stratum. As expected, the cumulative prevalence of any given decline in eGFR and the cumulative %PAR were lower during shorter baseline periods and higher during longer baseline periods.
The strength of associations of percent eGFR decline with ESRD was consistent at lower eGFR (19 studies) and higher eGFR (9 studies) (eFigure 4). Variation in HRs across studies was not related to variation in study characteristics. For example, the adjusted HR of ESRD associated with a −30% change in eGFR during 2-years was unrelated to baseline eGFR, prevalence of diabetes and median albuminuria (eFigures 5-6), despite each being a strong risk factor for ESRD. Meta-regression of these factors as well as mean follow-up time and age across three different baseline periods and two eGFR strata showed no pattern (only 4 of 30 combinations with p-values<0.05, eFigures 5-10).
The average absolute risk of ESRD was very strongly related to the first eGFR as well as to the length of follow-up and the change in eGFR (Table 4 and eTables 2-3). For example, at a baseline eGFR of 35 ml/min/1.73m2 the average 10-year risks of ESRD after a 2-year baseline period during which eGFR changed by −57%, −30% and 0% were 99% (95% CI, 95-100%), 64% (95% CI, 52-77%),, and 18% (95% CI, 15-22%), respectively, adjusted for covariates and competing mortality risk. While greater decline in eGFR was always associated with a higher subsequent risk of ESRD, the absolute and attributable risk varied markedly across patient characteristics, as well as across cohorts even after adjustment for covariates (eFigures 11-12 and eTables 2-3).
Table 4.
Risk of end-stage renal disease by percent decline in eGFR during a 2-year baseline period, first eGFR and subsequent follow-up time.

|
Baseline risk is calculated for: 0% eGFR decline, eGFR 50 ml/min/1.73m2, age 60 y, male, non-black, systolic blood pressure 130, cholesterol 5 mmol/L, no diabetes, no history of cardiovascular disease.
eGFR: estimated glomerular filtration rate.
Analysis of change in eGFR using slope rather than percent change showed strong association with ESRD risk as well (eFigures 13-15). In addition, longer follow-up narrowed the distribution of slopes and strengthened the association with ESRD.
Mortality risk according to change in eGFR
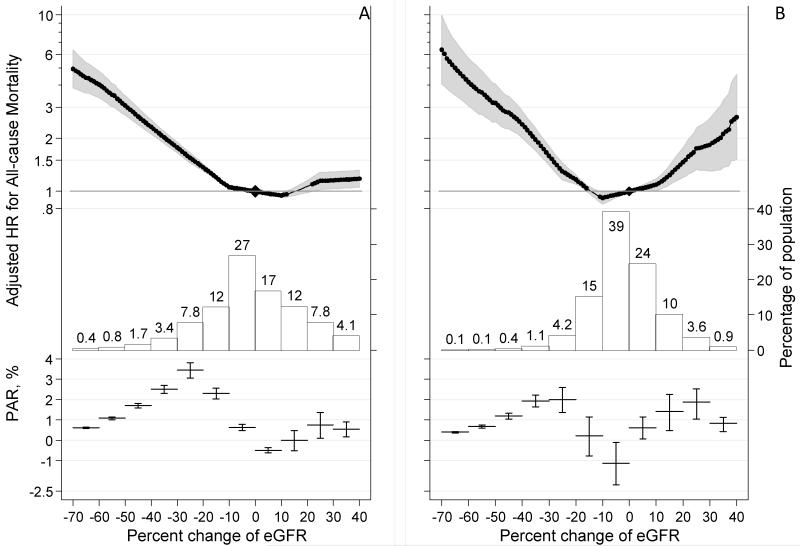
In cohorts with mortality data, again, approximately seven-fold more individuals had an eGFR change of −30% or greater compared to a −57% change or greater (cumulative prevalence of 7.1% (95% CI 6.6-7.7%) vs. 0.97% (0.70-1.25%); Figure 2A and Table 5). Compared to those with stable eGFR (eGFR change of 0%), the adjusted HR of all-cause mortality was higher with greater eGFR decline but was largely flat in the range of minimal decline (−10% change or less) or rise (Figure 2A). For example, the adjusted HR was 1.8 (95% CI: 1.6-1.9) for −30% change, 2.3 (2.1-2.5) for −40% change, and 3.7 (3.2-4.4) for a −57% change. In terms of %PAR, a higher prevalence of smaller changes in eGFR surpassed the corresponding lower relative risk, with a peak of %PAR around −30 to −20% change (Figure 2A). Largely similar associations were observed among individuals with higher baseline eGFR, but a higher risk associated with a rise in eGFR (positive change in eGFR) was evident (Figure 2B). Of note, the prevalence of decline in eGFR was consistently less in those with higher baseline eGFR, and a −57% change in eGFR was very rare (~0.2%). A 30% decline in eGFR was consistently associated with higher subsequent all-cause mortality risk across cohorts for both lower and higher baseline eGFR (eFigure 16), although the absolute mortality risk varied markedly across cohorts even after accounting for covariates (eFigures 17 and 18). We observed consistent results for cardiovascular mortality and non-cardiovascular mortality (eFigures 19 and 20).
Figure 2.
Adjusted hazard ratio of all-cause mortality by percent change in eGFR, its distribution, and corresponding percent population attributable risk during a 2-year baseline period in eGFR<60 ml/min/1.73m2 (A) and eGFR≥60 ml/min/1.73m2 (B). Values trimmed at <−70% change (0.30% and 0.050% of the study population in eGFR <60 and ≥60 ml/min/1.73m2, respectively) and >40% change (5.8% and 0.46% of the population in eGFR <60 and ≥60 ml/min/1.73m2, respectively).
Table 5.
Prevalence and adjusted hazard ratio of all-cause mortality associated with percent decline in eGFR during baseline periods of one to three years duration by level of kidney function
| Percent decline in eGFR during the 2-year baseline period | |||||||
|---|---|---|---|---|---|---|---|
| −57% | −40% | −30% | −25% | −20% | 0% (Stable) | ||
|
eGFR<60
ml/min/ 1.73m2 |
|||||||
| Baseline, 1-y | Adjusted HR | 3.8 (3.3, 4.4) | 2.4 (2.2, 2.6) | 1.9 (1.7, 2.0) | 1.6 (1.5, 1.8) | 1.4 (1.4, 1.5) | ref |
| Cumulative Prevalence, % | 0.48 (0.34, 0.62) | 1.8 (1.5, 2.0) | 4.3 (4.0, 4.7) | 6.5 (6.1, 6.9) | 11 (10, 11) | 54 (54, 55) | |
| Cumulative PAR, % | 0.48 (0.45, 0.50) | 1.8 (1.7, 1.9) | 3.7 (3.4, 3.9) | 4.9 (4.5, 5.2) | 6.5 (5.9, 7.1) | 9.8 (8.6, 11) | |
| Baseline, 2-y | Adjusted HR | 3.7 (3.2, 4.4) | 2.3 (2.1, 2.5) | 1.8 (1.6, 1.9) | 1.5 (1.4, 1.6) | 1.4 (1.3, 1.4) | ref |
| Cumulative Prevalence, % | 0.97 (0.70, 1.25) | 3.5 (3.1, 3.9) | 7.1 (6.6, 7.7) | 11 (10, 11) | 15 (14, 16) | 54 (53, 55) | |
| Cumulative PAR, % | 0.96 (0.89, 1.01) | 3.7 (3.4, 3.9) | 6.2 (5.8, 6.6) | 7.9 (7.3, 8.5) | 9.6 (8.7, 10.4) | 12 (11, 14) | |
| Baseline, 3-y | Adjusted HR | 3.3 (2.7, 3.9) | 2.2 (2.0, 2.4) | 1.8 (1.6, 1.9) | 1.5 (1.4, 1.7) | 1.4 (1.3, 1.4) | ref |
| Cumulative Prevalence, % | 1.7 (1.3, 2.1) | 5.3 (4.7, 5.9) | 10 (9, 11) | 14 (13, 15) | 19 (18, 20) | 54 (53, 55) | |
| Cumulative PAR, % | 1.5 (1.4, 1.6) | 5.1 (4.7, 5.5) | 8.2 (7.5, 8.9) | 10 (9, 11) | 12 (11, 13) | 14 (13, 16) | |
|
eGFR≥60
ml/min/ 1.73m2 |
|||||||
| Baseline, 1-y | Adjusted HR | 3.6 (2.5, 5.0) | 2.1 (1.8, 2.5) | 1.6 (1.4, 1.7) | 1.3 (1.2, 1.4) | 1.2 (1.1, 1.2) | ref |
| Cumulative Prevalence, % | 0.12 (0.04, 0.19) | 0.48 (0.36, 0.61) | 1.6 (1.4, 1.8) | 3.1 (2.9, 3.4) | 6.5 (6.2, 6.8) | 64 (64, 65) | |
| Cumulative PAR, % | 0.38 (0.33, 0.42) | 1.4 (1.2, 1.6) | 2.9 (2.5, 3.3) | 3.7 (3.1, 4.3) | 4.6 (3.8, 5.4) | 4.8 (2.5, 6.9) | |
| Baseline, 2-y | Adjusted HR | 3.8 (2.8, 5.2) | 2.4 (2.0, 2.9) | 1.6 (1.4, 1.8) | 1.3 (1.2, 1.5) | 1.2 (1.1, 1.2) | ref |
| Cumulative Prevalence, % | 0.16 (0.06, 0.26) | 0.73 (0.51, 0.94) | 2.0 (1.7, 2.2) | 3.7 (3.4, 4.0) | 6.7 (6.4, 7.0) | 64 (63, 64) | |
| Cumulative PAR, % | 0.63 (0.56, 0.68) | 2.6 (2.2, 2.8) | 4.5 (3.8, 5.0) | 5.7 (4.6, 6.5) | 6.4 (5.1, 7.5) | 5.3 (2.0, 8.3) | |
| Baseline, 3-y | Adjusted HR | 4.8 (3.7, 6.1) | 2.3 (2.0, 2.6) | 1.5 (1.3, 1.6) | 1.2 (1.1, 1.3) | 1.1 (1.1, 1.1) | ref |
| Cumulative Prevalence, % | 0.22 (0.06, 0.38) | 0.98 (0.71, 1.26) | 2.6 (2.3, 2.9) | 4.7 (4.3, 5.0) | 8.8 (8.4, 9.2) | 68 (67, 68) | |
| Cumulative PAR, % | 0.89 (0.83, 0.93) | 3.3 (3.0, 3.5) | 5.1 (4.5, 5.6) | 6.0 (5.0, 6.8) | 6.7 (5.4, 7.8) | 5.7 (2.0, 9.1) | |
Cumulative indicates an eGFR decline of this level or greater. In parentheses are the 95% confidence intervals based on the whole eligible study sample in the Consortium as a standard population.
eGFR: estimated glomerular filtration rate. HR: hazard ratio. PAR: population attributable risk.
With the larger eGFR declines, absolute all-cause mortality risk was consistently higher for all the levels of baseline eGFR across different subsequent follow-up time (Table 6 and eTables 4-5). For a baseline eGFR of 35 ml/min/1.73m2, the absolute all-cause mortality in 10 years was 32% (95% CI, 31-330%) if eGFR was stable, whereas the mortality risk was 50% (95% CI, 47-52%) for an eGFR change of −30%, 60% (95% CI, 56-63%) for −40% change, and 77% (95% CI, 71-82%) for −57% change. Similar patterns were observed for the CVD and non-CVD mortality risk (eTables 6 and 7).
Table 6.
Risk of all-cause mortality by percent decline in eGFR during a 2-year baseline period, first eGFR and subsequent follow-up time.

|
Baseline risk is calculated for: 0% eGFR decline, eGFR 50 ml/min/1.73m2, age 60 y, male, non-black, systolic blood pressure 130, cholesterol 5 mmol/L, no diabetes, no history of cardiovascular disease.
eGFR: estimated glomerular filtration rate.
Further adjustment for smoking and albuminuria, when available, did not alter the results substantially (eFigures 21 and 22). Consistent results, greater mortality risk at a greater percent decline in eGFR, were observed for analyses using baseline period of 1-year or 3-year (eFigures 23-28 and Table 5). Results were similar for the analysis using the 2-year slope of change in eGFR, although as anticipated, a given absolute decline in eGFR contributed to higher relative risk among those with lower vs. higher baseline eGFR (eFigures 29-31).
Discussion
In this international consortium meta-analysis of more than 1.7 million participants with 12,344 ESRD events and 223,944 deaths, we documented that smaller reductions in eGFR from baseline than doubling of serum creatinine were very strongly and consistently associated with subsequent risk of ESRD and captured a much higher proportion of the subsequent ESRD risk, providing a basis for their use as alternative outcomes for CKD progression. The HR of ESRD adjusted for first eGFR and other covariates was exponentially higher with greater declines in eGFR across a wide range of cohorts, GFR levels, and other patient characteristics. eGFR changes of −57% and −30% were associated with greater than 30 and 5-fold adjusted HRs of ESRD, respectively, but the prevalence of the latter was nearly tenfold higher than the former and consequently had a much higher %PAR (44% vs. 10%). Although weaker than ESRD risk, associations with mortality were qualitatively similar. These data provide a basis for understanding the trade-off between higher risk and lower prevalence in choosing a larger or smaller percent change in eGFR as an outcome when studying CKD progression. While HRs were consistent across studies, absolute risks varied dramatically by baseline eGFR, participant characteristics and different cohorts, but were substantially higher than the life-time risk for comparably aged unselected populations.21
Doubling of serum creatinine has been accepted by the Food and Drug Administration as a surrogate endpoint for CKD progression in clinical trials since 1993.3 Adoption of lesser eGFR decline as an alternative endpoint for CKD progression has the potential to shorten duration of follow up, reduce costs and increase efficiency of clinical trials. Consistency of effects over time suggests applicability for shorter as well as for longer trials, which is relevant for diseases that are progressing more rapidly or slowly, respectively. The strong and consistent ESRD risk associations that we demonstrated here are a necessary, but not sufficient condition for a surrogate endpoint in clinical trials. Several other types of data are useful, and preliminary results support our suggestion of a 30% to 40% decline in eGFR as an outcome for clinical trials in CKD.22-24 First, evaluation of outcomes other than ESRD, such as cardiovascular disease and death, is important since they often occur more frequently and may precede ESRD. Our analyses show a 50% mortality risk in 10 years with 30% change in eGFR compared to 32% with stable eGFR and similar demographic and clinical characteristics, if baseline eGFR is 35 ml/min/1.73m2. Second, evaluation of clinical trials is necessary to assess whether the effect of the treatment on the surrogate is consistently associated with the effect of treatment on the clinical endpoint. Attenuation of the hazard ratio of the treatment effect for lesser eGFR declines compared to the hazard ratio for the clinical endpoint can outweigh the benefit of an increased number of endpoint events. 11,22 Third, since the number and type of trials in CKD are limited, particularly with respect to length of follow-up and number of ESRD events reached, simulation studies are necessary to assess a wide range of potential scenarios to evaluate the utility, robustness and power of lesser eGFR declines.23 In particular, simulation studies can address the impact of short-term (acute) effects on kidney function in the same or opposite direction from the long term (chronic) effect of a treatment, such as lower blood pressure or renin-angiotensin system inhibition. In principle, acute treatment effects on eGFR will be more important for endpoints defined by a smaller percent declines in eGFR. An understanding of acute treatment effects on eGFR should be a part of any clinical trial which relies on an eGFR change as an alternative to ESRD. Difficulty in ruling out small acute treatment effects provide a rationale for favoring a larger decline in clinical trials (e.g. −30% or −40% change) than in observational studies and clinical practice where guidelines define a certain drop in eGFR as a drop in GFR category accompanied by a 25% or greater drop in eGFR from baseline.7 Finally, absolute risk of ESRD is important to consider. Our results indicate that eGFR decline starting at severely reduced eGFR is associated with very high rates of ESRD during the subsequent 1-5 years. However, eGFR decline starting at moderately reduced or normal eGFR is associated with a markedly lower risk with ESRD occurring after 10 or more years. There is also marked variation across studies suggesting caution in the translation of the level of eGFR decline to exact risk of ESRD.
To our knowledge, only one study investigated the association of eGFR change with ESRD risk,6 but our results regarding change in kidney function and mortality risk are consistent with several previous reports.8-10,25-28 Most of them investigated annualized rate of change,9,26-28 but a few reported that ≥~20-25% decline in eGFR over 1-3 years conferred mortality risk.8,10,25 A time to event endpoint based on percent change in eGFR calculated from only two measurements of serum creatinine at baseline and follow-up is simpler and easier to implement in clinical trials than an end point defined on the rate of decline in eGFR. Similarly, percent change may be used as a clinical outcome in cohort studies and/or clinical care. Nevertheless, we comprehensively studied both percent and absolute change of eGFR over three different baseline periods and adjusted for baseline eGFR and covariates uniformly across cohorts. The consistent results across a wide range of cohorts in various settings/regions support the generalizability of our findings.
As previously reported,25,27,29,30 we observed an increase in mortality risk with a rise in eGFR, particularly among individuals with higher first eGFR. We expanded the previous literature to include cause-specific death (CVD vs. non-CVD) and confirmed a similar relationship for both outcomes. This association may be a consequence of loss of muscle mass associated with chronic illness resulting in a decline in creatinine generation.28,31 It may also be a consequence of acute illness associated with resolving acute kidney injury. The role of hyperfiltration in poor prognosis among those with higher eGFR is yet to be elucidated.32
Despite its large size, broad scope and robustness of the findings across a large number of sensitivity analyses, this study has a number of limitations. Standardization of serum creatinine values may have varied across time and studies. Percent change in eGFR based on a single first and single last eGFR is less precise than alternative designs where multiple measures are available at each time point. Variation in design across cohorts introduces heterogeneity, but consistency across cohorts despite dramatic variation in design and populations increases our confidence in the results.
Adjustment for the first eGFR means that the groups being compared have already diverged markedly at the end of the baseline period when follow-up for ESRD begins. For example, at a first eGFR of 30 ml/min/1.73m2, percent changes of 57%,30% and 0% over a 2-year baseline correspond to a last eGFR during this period of 13, 21 and 30 ml/min/1.73m2, which provides insight to why greater eGFR declines are associated with greater subsequent ESRD and mortality risk. However, it also points out the distinction from clinical practice in which the last eGFR is known and the question of interest is often different -- whether previous progression adds information about risk above and beyond the last measurement.8,30 For clinical practice, the present analysis is useful for defining what level of change in the future can be considered important and what its consequences would be. However, once the change has occurred, the relative importance of the change vs. the last eGFR measure requires further analysis, and is beyond the scope of this paper.
Conclusions
This meta-analysis of a wide range of cohorts with a large number of ESRD events confirms the validity of doubling of serum creatinine, which corresponds to a 57% decline in eGFR and is associated with a greater than 30-fold higher risk of ESRD, as an endpoint for CKD progression. However, over a one to three year period, a doubling of serum creatinine was rare in most studies. A 30% decline in eGFR was approximately ten times more common and was associated with an approximately 5-fold increased risk of ESRD after adjustment for covariates including the first eGFR, providing an opportunity to define an alternative kidney endpoint for clinical trials, observational studies and clinical practice, with greater power over shorter periods of follow-up.
Supplementary Material
Acknowledgements
The CKD-PC Data Coordinating Center is funded in part by a program grant from the US National Kidney Foundation (NKF funding sources include AbbVie and Amgen) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100446-01). A variety of sources have supported enrollment and data collection including laboratory measurements, and follow-up in the collaborating cohorts of the CKD-PC. These funding sources include government agencies such as national institutes of health and medical research councils as well as foundations and industry sponsors listed in eAppendix 3 p 15-16. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication, other than planning for the NKF-FDA meeting.
Footnotes
Contributors: JC, KM, LAI, PEdJ, KI, BS, RTG, and ASL conceived of the study concept and design. JC, KM, YS, SHB and the CKD-PC investigators/collaborators listed below acquired the data. YS and the Data Coordinating Center members listed below analyzed the data. JC and KM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and all authors had final responsibility for the decision to submit for publication, informed by discussions with collaborators. All authors took part in the interpretation of the data. JC, TCT, KM, YS, SHB, RTG, and ASL drafted the manuscript, and all authors provided critical revisions of the manuscript for important intellectual content. All collaborators shared data and were given the opportunity to comment on the manuscript. JC obtained funding for CKD-PC and individual cohort and collaborator support is listed in eAppendix 3 p 15-16.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013 Jul 13;382(9887):158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Palmer SC, Sciancalepore M, Strippoli GF. Trial quality in nephrology: how are we measuring up? Am J Kidney Dis. 2011 Sep;58(3):335–337. doi: 10.1053/j.ajkd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993 Nov 11;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006 Jan;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 6.Turin TC, Coresh J, Tonelli M, et al. Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant. 2012 Oct;27(10):3835–3843. doi: 10.1093/ndt/gfs263. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013;3(1):1–150. doi: 10.1016/j.kisu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009 Dec;20(12):2617–2624. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009 Dec;20(12):2625–2630. doi: 10.1681/ASN.2009050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng TY, Wen SF, Astor BC, Tao XG, Samet JM, Wen CP. Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle-aged working population in Taiwan. Am J Kidney Dis. 2008 Dec;52(6):1051–1060. doi: 10.1053/j.ajkd.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Lambers Heerspink HJ, Weldegiorgis M, Inker LA, et al. Estimated GFR Decline as a Surrogate End Point for Kidney Failure: A Post Hoc Analysis From the Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) Study and Irbesartan Diabetic Nephropathy Trial (IDNT) Am J Kidney Dis. 2014;63(2):244–250. doi: 10.1053/j.ajkd.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011 Jun;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 14.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes in both general and high-risk populations. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011 Jul;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011 Jun;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012 May 9;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate with Standardized Serum Creatinine Values. Clin Chem. 2007 Apr 1;53(4):766–772. doi: 10.1373/clinchem.2006.077180. 2007. [DOI] [PubMed] [Google Scholar]
- 18.Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009 Jan;55(1):24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 19.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998 Jan;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grams ME, Coresh J, Segev DL, Kucirka LM, Tighiouart H, Sarnak MJ. Vascular disease, ESRD, and death: interpreting competing risk analyses. Clin J Am Soc Nephrol. 2012 Oct;7(10):1606–1614. doi: 10.2215/CJN.03460412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013 Aug;62(2):245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, Lambers Heerspink HJ, Mondal H, Coresh J, Greene T, Levey A. GFR Decline as an Endpoint for Clinical Trials in CKD – A Meta-Analysis of Treatment Effects from Randomized Trials: Report of an NKF-FDA Workshop [Abstract] J Am Soc Nephrol. 2013;24:12A. [Google Scholar]
- 23.Greene T, Teng C, Ying J, et al. Validity and Statistical Power of Alternative eGFR-Based Endpoints: A Report from an NKF FDA Workshop [Abstract] J Am Soc Nephrol. 2013;24:151A. [Google Scholar]
- 24.National Kidney Foundation [Accessed October 15, 2013];Research: GFR decline as an endpoint in clinical trials for CKD. 2013 http://www.kidney.org/professionals/research/research_info.cfm.
- 25.Turin TC, Coresh J, Tonelli M, et al. One-year change in kidney function is associated with an increased mortality risk. Am J Nephrol. 2012;36(1):41–49. doi: 10.1159/000339289. [DOI] [PubMed] [Google Scholar]
- 26.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008 Nov 10;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkins RM, Bucaloiu ID, Kirchner HL, Ashouian N, Hartle JE, Yahya T. GFR decline and mortality risk among patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011 Aug;6(8):1879–1886. doi: 10.2215/CJN.00470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Aly Z, Zeringue A, Fu J, et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol. 2010 Nov;21(11):1961–1969. doi: 10.1681/ASN.2009121210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita K, Ballew SH, Astor BC, et al. Cohort Profile: The Chronic Kidney Disease Prognosis Consortium. Int J Epidemiol. 2013 Dec 12;42:1660–1668. doi: 10.1093/ije/dys173. [DOI] [PubMed] [Google Scholar]
- 30.Turin TC, Coresh J, Tonelli M, et al. Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int. 2013 Apr;83(4):684–691. doi: 10.1038/ki.2012.443. [DOI] [PubMed] [Google Scholar]
- 31.Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009 Aug;90(2):407–414. doi: 10.3945/ajcn.2008.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonelli M, Klarenbach SW, Lloyd AM, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011 Dec;80(12):1306–1314. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.