Abstract
Background
Increased susceptibility of older populations to secondary bacterial pneumonia-like infections following influenza infection has been well documented.1 Recent evidence in mouse models suggests that this increased risk from secondary bacterial infection occurs through a desensitization of the innate immune response.2 This recent finding, however, does not account for potential differences in immune responsiveness due to age.
Materials and methods
To address this parameter, we used three age groups (aged, adult, and young mice) to evaluate the role of age in influenza-mediated vulnerability to secondary bacterial challenge with Pseudomonas aeruginosa. All mice were evaluated for multiple parameters including: (i) survival; (ii) lung bacterial load; (iii) total lung protein content; (iv) immune cell infiltration; (v) cytokine/chemokine expression; and (vi) toll-like receptor (TLR) RNA expression profiles.
Results
Prior challenge with influenza contributed to aberrant cytokine/chemokine profiles and increased lung cellular infiltrate in response to secondary bacterial infection across all age groups, supporting a critical role for influenza infection in the alteration of immune responses to other pathogens. Also similar to human influenza, these changes were exacerbated by age in mice as demonstrated by increased bacterial load, mortality, and total lung protein content (an indicator of lung damage) after P. aeruginosa challenge.
Conclusions
These data support a potential role for virus-mediated and age-mediated alteration of innate immune effectors in the pathogenesis of influenza and the increased susceptibility of influenza virus infected mice to secondary bacterial infection. The understanding of the complex interaction of host and pathogen – and the role of age – in human influenza is critical in the development of novel therapeutics and improved vaccine approaches for influenza. Our results support further examination of influenza-mediated alterations in innate immune responses in aged and non-aged animals to allow elucidation of the molecular mechanisms of influenza pathogenesis in humans.
Keywords: Aging, bacteria, innate immunity, secondary infection, secondary infection, toll-like receptors
Introduction
There is considerable evidence in the clinical literature to support the role of influenza infections with an enhanced risk for secondary bacterial pneumonias.3–5 Given the increased pneumonia-related morbidity and mortality in both the young and elderly populations, there is rationale for gaining a deeper understanding as to the systemic changes in the pulmonary microenvironment. Although there are some recent reports that account for some of the molecular mechanisms at work in this disease process,2 there is a paucity of experimental evidence that considers the potential effects of age. Developmental changes in the immune system that occur in the aged environment have been well documented with regard to senescence of the adaptive immunity, global changes in myeloid cell function, and the establishment of a general pro-inflammatory state.6,7 The aim of this work was to provide evidence for the contribution of the aged immune environment to the pathology of influenza mediated secondary bacterial infections.
Materials and methods
Animals used in this study were housed under conditions approved by Tulane University’s Institutional Animal Use and Care Committee. Female Balb/C mice used in these studies were divided into three age groups: aged (18 months old), adult (6 months old), and young (2 months old). Each age group was subdivided into two groups: influenza infected and naïve (control). Mice were infected by the intranasal route with 4 × 105 PFU of mouse-adapted Influenza A/PR/8/34. Clinical disease was measured by body weight changes over a 6 week period post influenza challenge, and recovery was determined as return to pre-infection weight. All mice were subsequently challenged intransally with 1 × 107 CFU Pseudomonas aeruginosa strain PAO1.
Twenty-four hours post-Pseudomonas challenge, BAL with sterile PBS was performed on all mice in all groups. Total RNA from the cellular fraction was pooled from three experimental animals from each group. TLR mRNA was detected by qRT-PCR, where expression levels were determined as relative to β-actin mRNA levels. cDNA was synthesized from total cellular RNA from BAL samples using iScript cDNA synthesis kit (Biorad). PCR reactions were composed of 0·1 μg cDNA forward and reverse primers according to optimized conditions and 12·5 μl of 2 × Syber Green icycler supermix (Biorad), in a total volume of 25 μl and were run using a Biorad iCycler utilizing melting point determination. Primers and concentrations used in this study included: MUS_TLR2F: TGCTTTCCTGCTGGAGATTT-600 nm, MUS_TLR2R: TGTAACGCAACAGCTTCAGG-900 nm, MUS_TLR3F: ATATGCGCTTCAATCCGTTC-300 nm, MUS_TLR3R: CAGGAGCATACTGGTGCTGA-600 nm, MUS_TLR4F: GGCAGCAGGTGGAATTGTAT-600 nm, MUS_TLR4R: AGGCCCCAGAGTTTTGTTCT-900 nm, MUS_TLR5F: CTGGGGACCCAGTATGCTAA-600 nm, MUS_TLR5R: ACAGCCGAAGTTCCAAGAGA-900 nm, MUS_TLR7F: GGAGCTCTGTCCTTGAGTGG-900 nm, MUS_TLR7R: CAAGGCATGTCCTAGGTGGT-600 nm, MUS_ B-ACTINF: AGCCATGTACGTAGCCATCC-600 nm, MUS_B-ACTINR: CTCTCAGCTGTGGTGGTGAA-900 nm. As a measure of protein leakage into the alveolar space, total protein content in each BAL was measured by BCA assay of each supernatant fraction according to manufacturer’s instructions (Pierce). Cytokine and chemokines levels were measured by multiplexed bead array (Bioplex, BioRad). Immune cell characterization of BAL was estimated by flow cytometry. Lymphocyte populations were gated by forward versus side scatter and characterized as B cells (F4/80−, CD19+) or T cells (CD11b−, CD4+). The myeloid population that is composed of macrophages, neutrophils, dendritic cells, and natural killer cells was enumerated by gating all but those found in the lymphocyte gate using forward versus side scatter plots. Flow cytometry data was analyzed using FloJo software (TreeStar). Statistical analysis, where appropriate, was performed using a two-way analysis of variance (age versus influenza infection status) supported by Bonferonni’s correction for multiple comparisons.8
Results and discussion
A recent finding by Didierlaurent, et al.,2 described an influenza mediated desensitization of TLR function as a primary contributor to an increase in bacterial burden when challenged after resolution of the primary influenza infection. This finding, however, was obtained using animals that were 6–8 weeks of age, where our study included two cohorts of older mice (6 months and 18 months). Using whole protein content of the BAL as an estimate of protein leakage into the lumen of the lung, we found elevated protein content in aged mice as compared to young and adult mice. In aged mice, a slightly lower total lung protein when comparing influenza infected to protein in the BAL from influenza naïve mice challenged with P. aeruginosa (Table 1).
Table 1.
Summary of experimental findings
| Endpoint | Age | Control
|
Influenza
|
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | ||
| BAL total protein (μg/ml) | Young | 2·02E+03 | 1·8E+02 | 15 | 1·99E+03 | 8·0E+01 | 14 |
| Adult | 2·04E+03 | 1·4E+02 | 4 | 1·81E+03 | 7·4E+01 | 6 | |
| Aged | 3·14E+03 | 1·6E+02 | 6 | 2·49E+03 | 3·1E+02 | 9 | |
| Pseudomonas aeruginosa bacterial load | Young | 4·17E+06 | 6·6E+06 | 15 | 2·57E+06 | 3·8E+06 | 13 |
| Adult | 1·51E+06 | 1·4E+06 | 4 | 3·40E+07 | 4·7E+07 | 2 | |
| Aged | 1·76E+07 | 1·2E+07 | 6 | 4·24E+07 | 4·8E+07 | 8 | |
| Cytokine/chemokines (pg/ml) | |||||||
| GM-CSF | Young | 9·31E+02 | 7·6E+02 | 4 | 7·18E+02 | 2·3E+02 | 4 |
| Adult | 3·59E+02 | 3·3E+02 | 4 | 1·26E+02 | 1·9E+02 | 4 | |
| Aged | 2·58E+03 | 7·3E+02 | 4 | 2·18E+03 | 8·8E+02 | 4 | |
| TNF-α | Young | 2·05E+03 | 7·2E+02 | 4 | 2·17E+03 | 8·5E+02 | 4 |
| Adult | 1·57E+03 | 1·0E+03 | 4 | 1·16E+03 | 8·0E+02 | 4 | |
| Aged | 5·66E+03 | 2·3E+03 | 4 | 5·34E+03 | 3·1E+03 | 4 | |
| IFN-γ | Young | 1·00E–05 | 9·5E–13 | 4 | 9·08E–01 | 2·1E+00 | 4 |
| Adult | 1·25E+00 | 2·8E+00 | 4 | 1·01E+00 | 2·5E+00 | 4 | |
| Aged | 1·42E+01 | 1·7E+01 | 4 | 4·53E+01 | 4·7E+01 | 4 | |
| IL-5 | Young | 3·96E+01 | 9·7E+00 | 4 | 4·04E+01 | 1·8E+01 | 4 |
| Adult | 1·04E+01 | 6·3E+00 | 4 | 2·01E+01 | 1·3E+01 | 4 | |
| Aged | 8·02E+01 | 5·7E+01 | 4 | 9·31E+01 | 5·5E+01 | 4 | |
| IL-10 | Young | 8·76E+01 | 7·2E+01 | 4 | 6·25E+01 | 2·5E+01 | 4 |
| Adult | 4·74E+01 | 4·3E+01 | 4 | 3·29E+01 | 4·7E+01 | 4 | |
| Aged | 3·54E+02 | 1·0E+02 | 4 | 1·60E+02 | 7·1E+01 | 4 | |
| Inflammatory cell (% of BAL cells) | |||||||
| B cells | Young | 8·85E–01 | 3·7E–01 | 2 | 3·61E+00 | 3·7E+00 | 2 |
| Adult | 1·09E+00 | 0·0E+00 | 1 | 2·17E+00 | 2·1E+00 | 2 | |
| Aged | 7·68E+00 | 9·1E–01 | 2 | 4·31E+00 | 2·3E+00 | 2 | |
| CD4+ T cells | Young | 1·25E+01 | 3·5E–01 | 2 | 1·57E+01 | 6·4E–01 | 2 |
| Adult | 2·95E+00 | 1·8E+00 | 2 | 1·05E+01 | 9·7E+00 | 2 | |
| Aged | 1·06E+01 | 9·2E–01 | 2 | 1·27E+01 | 4·5E+00 | 2 | |
| Myeoid | Young | 9·20E+01 | 1·4E+00 | 2 | 8·95E+01 | 7·1E–01 | 2 |
| Adult | 9·15E+01 | 2·1E+00 | 2 | 6·85E+01 | 7·8E+00 | 2 | |
| Aged | 8·60E+01 | 2·8E+00 | 2 | 8·00E+01 | 5·7E+00 | 2 | |
| TLR mRNA expression (relative expression to β-actin) | |||||||
| TLR2 | Young | 4·23E+01 | 3 | 2·57E+01 | 3 | ||
| Adult | 1·68E+01 | 3 | 2·11E+00 | 3 | |||
| Aged | 1·23E+02 | 3 | 8·60E+01 | 3 | |||
| TLR3 | Young | 1·63E+03 | 3 | 2·23E+04 | 3 | ||
| Adult | 1·14E+04 | 3 | 8·04E+03 | 3 | |||
| Aged | 1·21E+04 | 3 | 1·86E+03 | 3 | |||
| TLR4 | Young | 1·70E+02 | 3 | 1·69E+02 | 3 | ||
| Adult | 9·40E+01 | 3 | 1·72E+01 | 3 | |||
| Aged | 2·57E+02 | 3 | 1·30E+02 | 3 | |||
| TLR5 | Young | 2·39E+03 | 3 | 3·55E+02 | 3 | ||
| Adult | 5·24E+03 | 3 | 1·75E+03 | 3 | |||
| Aged | 4·71E+04 | 3 | 6·23E+03 | 3 | |||
| TLR7 | Young | 3·53E+02 | 3 | 1·23E+02 | 3 | ||
| Adult | 2·00E+02 | 3 | 8·04E+01 | 3 | |||
| Aged | 9·83E+02 | 3 | 3·75E+02 | 3 | |||
Supporting previously published studies showing a generalized pro-inflammatory cytokine environment in the aged immune system, we provide evidence for significantly elevated cytokines/chemokines (TNFα, GM-CSF, IL-5, and IFNγ) in aged mice (P < 0·0001). Previously reported2 elevations in TNF-α expression in influenza infected animals measured at earlier time points post challenge suggest an earlier temporal expression of this cytokine not observed in our later post-challenge evaluation. There is also a significant difference in animals that have resolved influenza infection where a decreased amount of GM-CSF (P = 0·0465) and an increase in IFNγ (P = 0·0323) was detected. The decrease in GM-CSF correlates well with a previous report that GM-CSF is less prevalent in influenza resolved animals (Table 1).2
We also report a noticeable change in the immune cell populations with respect to B-cells, CD4+ T-cells, and the myeloid cell populations. There is a trend of increased prevalence in CD4 T-cells in the post-influenza environment across all ages. B-cell numbers also trend toward increase in influenza treated animals in young and adult animals; however, there is a noticeable decrease in the B-cells in aged animals. Across all age groups, there is a general decrease in frequency of cells that would normally make up the myeloid cellular fraction of the BAL (macrophages, neutrophils, dendritic cells, and natural killer cells) (Table 1).
Our study also shows, as cited by others, that toll-like receptor (TLR) gene expression in the post-influenza environment is decreased in cells found in the BAL2 after both influenza and Pseudomonas infection. Our data support the previous finding of a reduced expression of TLR mRNA in influenza-cleared mice when we measured TLR 2, 3, 5, and 7. Only TLR4 showed differences with respect to age with young mice showing little or no detectable change in TLR4 mRNA expression. Our results show an increase in the expression across all TLRs examined in the aged mice group (Table 1) irrespective of influenza infection status. These data support earlier studies performed with adult mice that showed reduced TLR mRNA expression in the post-influenza environment. This study also expands the current understanding of the potential role of age in influenza mediated bacterial infection-induced mortality.
The impact of these alterations in the immune microenvironment across age groups and infection status is highlighted by the ability of bacterially challenged animals to clear infection. Assessment of bacterial load in the lungs of P. aeruginosa challenged mice indicated a difference in young and adult mice if previously infected with influenza virus. In aged mice, both influenza challenged and influenza-naïve mice had higher bacterial loads and less variability when comparing within the age group, supporting the risk of age alone in susceptibility to bacterial pneumonia (Table 1, Figure 1).
Figure 1.
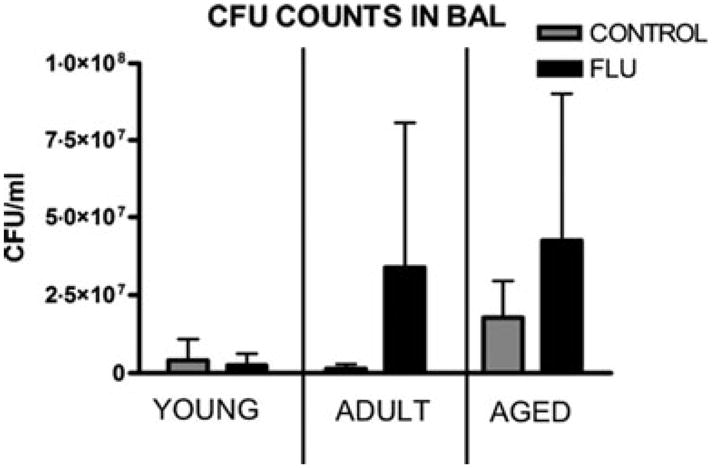
CFU Counts of Pseudomonas aeruginosa 24 h PI in BAL.
Taken together, these data support the potential role for both virus-mediated and age-mediated alteration of innate immune effectors in the pathogenesis of influenza and increased the susceptibility to secondary bacterial infection that results from influenza infection in mice. These findings highlight distinct differences in the immune environment between age groups and thus reveal necessity for further examination as to the mechanisms of immunity across age with respect to current infection status. Garnering a clearer understanding as to the complex interaction of host and pathogen with respect to age in influenza infections is central to the development of increased efficacy in vaccine and therapeutic strategies.
References
- 1.Castle SC, Uyemura K, Fulop T, et al. Host resistance and immune responses in advanced age. Clin Geriatr Med. 2007;23:463–479. doi: 10.1016/j.cger.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Didierlaurent A, Goulding J, Patel S, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE. 2009;4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Bacterial Coinfections in Lung Tissue Specimens from Fatal Cases of 2009 Pandemic Influenza A (H1N1) – United States, May–August 2009. MMWR. Sep 29, 2009. [Accessed 1 October 2009]. p. 58. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm58e0929a1.htm. [PubMed]
- 6.Franceschi C, Bonafè M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- 7.Salminen A, Huuskonen J, Ojala J, et al. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Neter J, Wasserman W, Kutner MH. Applied Linear Statistical Models. 3. Irwin: CRC press; 1990. pp. 741–744. [Google Scholar]


