Abstract
One of the key questions in understanding human morality is how central are emotions in influencing our decisions and in our moral judgments. Theoretical work has proposed that empathy could play an important role in guiding our tendencies to behave altruistically or selfishly. Neurosciences suggest that one of the core elements of empathic behavior in human and nonhuman primates is the capacity to internally mimic the behavior of others, through the activation of shared motor representations. Part of the neural circuits involves parietal and premotor cortical regions (mirror system), in conjunction with other areas, such as the insula and the anterior cingulate cortex. Together with this embodied neural mechanism, there is a cognitive route in which individuals can evaluate the social situation without necessary sharing the emotional state of others. For example, several brain areas of the prefrontal cortex track the effects of one’s own behavior and of the value of one’s own actions in social contexts. It is here proposed that, moral cognition could emerge as the consequence of the activity of emotional processing brain networks, probably involving mirror mechanisms, and of brain regions that, through abstract-inferential processing, evaluate the social context and the value of actions in terms of abstract representations.
A comparative-based approach to the neurobiology of social relations and decision-making may explain how complex mental faculties, such as moral judgments, have their foundations in brain networks endowed with functions related to emotional and abstract-evaluation processing of goods. It is proposed that in primate evolution these brain circuits have been coopted in the social domain to integrate mechanisms of self-reward, estimation of negative outcomes, with emotional engagement.
Keywords: Mirror neurons, neuroeconomics, mimicry, embodiment, orbitofrontal cortex
1. Introduction
Morality has been for centuries at the center of philosophical debates mainly because it is considered one of the building blocks of human societies. Indeed, the topic has fueled strong controversies in philosophy and science since it has profound implications on our understanding of the inner forces that drive and guide behaviors. Within these debates key questions have been addressed in the attempt to understand what characterizes our nature from that of other animals, and to which extent we can consider ourselves as unique: is humans’ inner nature good or bad? Can human behavior escape the instincts guiding other animals and selfish genes? Can morality emerge by emancipation from emotions, or are emotions central to our decision processes? Are human altruistic tendencies rational or do they derive from the capacity to share emotions with others? Some philosophers believe that we reason ourselves to moral principles, that emotions are secondary, that morality is all a product of the ratio, whereas others, such as David Hume give moral sentiments (passions & emotions) a central place.
Although it is outside the scopes of this paper to summarize different positions, it is worth noting that reflections on these issues have often suffered from a top-down perspective. In fact very rarely disciplines within the life sciences are given the opportunity to contribute to the debate primarily because of the lack of a proper evolutionary theorization, and secondly because it was only possible to infer, but not directly examine, internal processes guiding human behaviors.
The idea that human morality cannot escape the rules of evolutionary processes was first proposed by Darwin in the Descent of Man (1871): “Besides love and sympathy, animals exhibit other qualities connected with the social instincts, which in us would be called moral…. All animals living in a body, which defend themselves or attack their enemies in concert, must indeed be in some degree faithful to one another; and those that follow the leader must be in some degree be obedient…”. According to Darwin, moral decisions are strongly influenced by emotional processes, and in social animals these ‘social instincts’ are central for the feeling of pleasure when helping others and of unease when harming others.
The work on nonhuman primates has been of great value in challenging the dualistic view of human morality. Several studies have shown that monkeys and apes are capable of reciprocity, are sensitive to others’ distress, and can be altruistic without expecting an equal value as return (de Waal, 2008). The sensitivity to others’ emotions indicate that monkeys and apes are capable to empathize with others probably through some basic mechanisms of mirroring or embodied simulation (as described below) which allow individuals to directly access to others’ experience (de Waal, 2008; Palagi et al., 2009). The social nature of our species and of our relatives seems to have been inevitably rooted into mechanisms that facilitate the sharing of emotional experiences. The natural tendency to empathize with own group members might translate into behaviors that are indicative of evolved sophisticated altruistic tendencies in highly social species (de Waal, 2012).
Thus, studies on cooperation, consolation, sharing emotions and goods, are shedding light into the inner world of our relatives, and suggest that emotions and empathy not only likely play a major role in the decision-making processes, but are core elements necessary for the development of a moral cognition.
2. Empathy
Empathy is not only the capacity to share and understand others’ feeling and emotions, but it is becoming evident that it is a multilayered phenomenon in which emotions and cognitive processes are simultaneously at work (de Waal, 2008; Bernhardt & Singer, 2012). Instead of searching for a unified theory of empathy, several researchers have attempted to dissect it in its core elements and to understand its basic mechanisms in terms of neural underpinnings and cognitive processes.
Several scholars agree that at the basis of empathic responses among several animal species, including humans, there is an emotional response that is shared between two or more individuals, named emotional contagion (Preston & de Waal, 2002; de Vignemont & Singer, 2006). This phenomenon is probably based on an action-perception mechanism and is widespread among primates. Recent work has shown that in humans, apes, and monkeys, yawning is highly contagious (Campbell et al., 2009; Norscia & Palagi, 2011; Demuru & Palagi, 2012; Palagi et al., 2009; Paukner & Anderson, 2006), and its frequency correlates with the quality of the relationship between individuals, suggesting that there is a link between contagious behaviors and interpersonal emotional connection.
These findings also suggest that one of the core elements of empathic behavior is the capacity to mimic the behavior of others. This unconscious and automatic phenomenon likely relies on brain mechanisms that facilitate the activation of shared motor representations, which may promote the emergence of a sense of familiarity and emotional connectedness between individuals (Palagi et al., 2009). Other behavioral phenomena involving body mimicry are common during affiliative social interactions. In a recent study, we demonstrated in gelada baboons (Theropithecus gelada) the presence of rapid facial mimicry during play (Mancini et al., 2013)(see Figure 1). Similar behaviors have also been described in apes (Davila-Ross et al., 2007). More interestingly, the speed and frequency of response were higher among individuals with strong bonds, such as mothers and their infants (Mancini et al., 2013). In general, work in humans and nonhuman primates converges in describing a close relationship between emotional contagion, mimicry, and social closeness.
Figure 1.
An example of rapid facial mimicry of full play face in two juvenile gelada baboons (photo by the author).
The capacity to mimic others’ behaviors and emotions seems to stem from an ancient evolutionary capacity that is already present very early in primate development. For example, human, ape, and monkey neonates are capable of imitating facial gestures displayed by a human model (Meltzoff & Moore, 1977; Bard, 2007; Ferrari et al., 2006). This capacity probably evolved to tune an infant’s behavior to that of the mother, thus facilitating the mother-infant relationship and imitative exchanges (Ferrari et al., 2006; Paukner et al., 2012). It is therefore not surprising that, according to several authors, the building blocks of empathy may be found in the early mother-infant relationship (de Waal, 2008; Decety, 2011). The early postnatal period of human and nonhuman primates is, in fact, characterized by intense affective communication between newborns and adults. Such communication involves a constellation of specific behavioral features that include close, sympathetic maternal imitation and elaboration of infant facial communicative and expressive signals (Stern, 1985; Trevarthen & Aitken, 2001), which are unique in anthropoid species.
Despite infants’ immature brains and limited cognitive skills, they demonstrate active interest in their social world, and show a surprising ability to discriminate adult communicative expressions and to imitate. These complex social exchanges are indicative of the brain’s precocious capacity to be tuned with social stimuli and to decode mothers’ emotional behaviors through action-perception mechanisms which internally simulate others’ emotional states and behaviors (embodied simulation – Gallese, 2001).
Human morality, therefore, can be seen as an expansion of the primate capacity to care for others, which is wired in humans’ social brain from its emergence in early ontogeny. The embodiment of interpersonal relations, in particular, has been the privileged route through which our brain can connect with others’ brains. These considerations seem to echo in Darwin’s observation that “The feeling of pleasure from society is probably an extension of the parental or filial affections, since the social instinct seems to be developed by the young remaining for a long time with their parents.” (Darwin, 1871).
3. The neuroscience of empathy: the embodied channel
Although the embodied channel (i.e., the use of shared representations to directly experience and interpret others’ behavior) will be emphasized in the present review, it is important to note that empathy also involves cognitive components. In theorizing that empathy is a multilayered phenomenon, several scholars consider the embodied channel central for automatically reproducing others’ affective states. However, other layers are built upon and integrated with this core, both from an ontogenetic and evolutionary point of view, which include psychological constructs and cognitive efforts. These latter components are responsible for the capacity of empathizing through perspective taking and mentalizing, putting oneself in others’ shoes. From a neurobiological standpoint, action-perception mechanisms have been widely investigated in the last few decades and recent advances in neuroscience were particularly stimulated by the discovery of mirror neurons (MN), neurons in the premotor and parietal cortices (see figure 2) that fire both when a specific action is observed and when the same action is performed by an individual (di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 1996; Ferrari et al., 2003; Fogassi et al. 2005; see Rizzolatti & Craighero, 2004 for a review). The fact that MN have been found in cortical areas involved in motor control, has led to the proposal that others’ actions can be translated into a motor code exploiting the inner knowledge, in terms of cortical motor representations of the individual. This translation allows an individual to map others’ actions/emotions onto the internal motor representation of that action/emotion. There are several advantages of this action-perception mechanism, compared to other models: A. It is a parsimonious mechanism to automatically exploit the internal motor knowledge of the individual in order to recognize others’ behavior; B. It can explain several phenomena of imitation and of motor social facilitation such as emotional contagion; and C. it requires no effort or complex cognitive skills, and is therefore suitable for organisms at an early stage of development, thus supporting several behavioral processes.
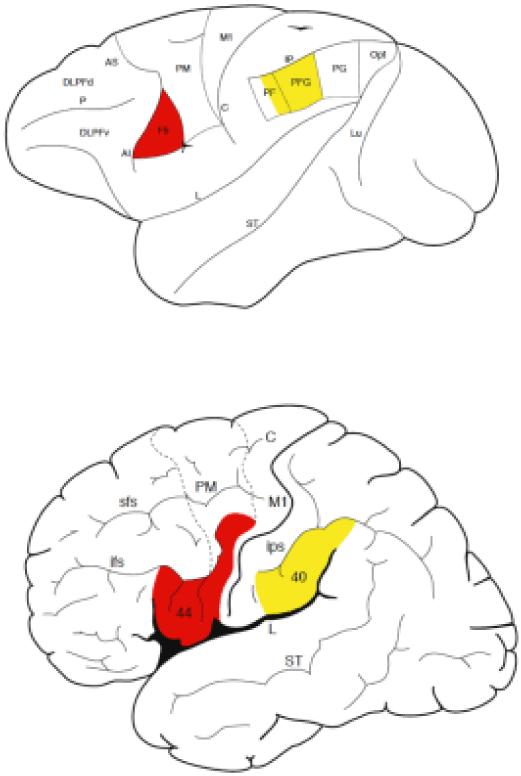
Figure 2.
Lateral view of the macaque monkey (top) and human (bottom) cerebral cortex with classical anatomical subdivision showing the areas in which mirror neurons have been found in the monkey and that in the hypothesized homolog areas of humans which: the ventral premotor cortex (red color: F5 in the monkey; lower part of the precentral gyrus, area 6, in human), part of the human inferior frontal gyrus (posterior part of area 44, and anterior part of 44 and area 45), and the inferior parietal lobule (PFG and part of PF in the monkey and area 40 in humans).
The properties of MN make them also a plausible candidate neural mechanism for empathy and social competence: 1) MN fire during observation of facial gestures; 2) the observation of emotional facial gestures also activates areas that are part of the emotional system and that control visceral responses; and 3) the mirror system is involved in imitation.
4. A neural simulation to empathize with others
Although MN were first described as a class of visuomotor neurons discharging for hand actions, more recently, an additional class of MN have been described, which fire for actions performed with the mouth (Ferrari et al., 2003). More interestingly, a small percentage of MN responded while the monkey observed affiliative communicative gestures (i.e., lipsmacking). Similarly, fMRI studies in humans have demonstrated that during the observation and imitation of emotional facial expressions areas of the MN system are activated (Carr et al., 2003; van der Gaag et al., 2007; Montgomery & Haxby, 2008; Lenzi et al., 2009). In addition to MN areas, the insular cortex and the anterior cingular cortex are activated during the observation of emotional facial expressions (Wicker et al., 2003; Carr et al., 2003; van der Gaag et al., 2007), thus suggesting that other brain structures, linked to emotions, possess mirror properties.
These brain regions are known to be involved in visceral responses typically associated with emotions. In recent electrical brain stimulation studies in the macaque monkey (Caruana et al., 2011; Jezzini et al., 2012) it was shown that stimulation of different regions of the insula elicits facial expressions, such as lipsmacking and disgust, suggesting that this brain region could be an important relay point for integrating motor programs and the vegetative responses associated with them.
How could a mirror mechanism mediate embodiment of others’ emotions? When we display an emotional facial expression we typically have a distinct feeling. When we see somebody else producing the same facial expression, the activation of MN in our brain also evokes the feeling associated with the act of smiling through embodied simulation (Gallese, 2003; Gallese et al., 2003; Niedenthal et al., 2010). Thus, we feel what others feel.
Mirror neurons can make us empathize with others in two possible ways. In one case, mirror neurons can simulate the observed action and, by communicating with emotional brain centers, trigger activity in those brain centers to evoke the corresponding feeling. In the other case, activity in mirror neurons alone would suffice to evoke the feeling.
Studies on the imitation and observation of emotional facial expressions demonstrate that a large-scale neural network—composed of the ventral premotor cortex, the inferior frontal gyrus (two putative mirror neuron areas), and the anterior insula and the amygdala—is active during facial expression observation and even more so during facial expression imitation (Carr et al., 2003). Brain-behavior correlations have also demonstrated that activity in this network of areas correlates with measures of empathy and interpersonal skills (Pfeifer et al., 2008). These results seem to suggest that a mirror mechanism could simulate facial expressions, which may evoke simulated activity in limbic structures.
Several studies have investigated the neuroscience of empathy by exploring brain activity while participants witness pain suffered by another person (Morrison & Downing, 2007; Cheng et al., 2007; Singer et al., 2004, 2006; Lamm et al., 2010). In a couple of studies, while undergoing fMRI scans, participants received a painful stimulation or to perceived pain in another person (delivered through mild electrical stimulation). In both conditions, there were overlapping regions of the anterior cingulate cortex and the anterior insula were active (Singer et al., 2004, 2006). Similar results were obtained when participants observed images of body parts receiving painful stimulation (Morrison & Downing, 2007; Cheng et al., 2007; Lamm et al., 2007).
Together, these data are compatible with the neural simulation hypothesis, in which an individual can empathize with another person through a process of inner imitation (Iacoboni, 2009). This concept echoes with Theodor Lipps’ theoretical account of empathy or Einfühlung (literally it means “feeling into”), in which the capacity to empathize relies on a mechanism of projecting the self into the other. The psychological process of projection Lipps proposed was based on imitating the inner part of the emotion (“innere Nachahmung” – inner imitation).
The behavioral phenomena ranging from facial mimicry to emotional contagion, and the neurobiological underpinnings described here, reflect automatic responses that do not require complex voluntary control and cognitive efforts. They also demonstrate the close link between the external manifestations of facial/body imitation with the inner imitation of the feeling associated with the emotion.
5. Evaluating goods and others’ actions
Work on empathy in humans and nonhuman primates is measuring the degree of sensitivity to others’ emotional states. However, work on empathy has also shown that together with the embodied channels there is a cognitive route in which individuals can evaluate the social situation without necessary sharing the emotional state of others. This is especially useful when, for example, individuals make decisions about how to respond in a situation in which their own behavior could be risky for their own survival (e.g., supporting a companion in a physical conflict with a competitor). In these cases, the empathic mirroring response would be useful for understanding the emotions of others, but the appropriate response should take into account important aspects of the context, with an accurate evaluation of costs and benefits as consequences of one’s decision.
Moreover, while engaging in cognitive perspective taking, a person may understand the affective state of another person based on personal previous knowledge, but without sharing the emotion. Accordingly, one can apply to others the same rules that are applied to oneself. This egocentric perspective is very important in social species because, over the course of development, individuals learn how to behave in different social contexts, and what to expect from other individuals when experiencing the same situation. This principle is probably at the basis of important complex behaviors, such as reciprocity and cooperation (de Waal, 2008).
From a neurobiological perspective, we should expect that when making decisions about how to interact with others in social situations (i.e., supporting a conspecific, sharing food) the brain must integrate several pieces of information regarding previous experiences, including the risks and advantages of an action, and it should assign values to others’ behaviors or to their own responses (Forbes & Grafman, 2010).
Neuroscientists have only recently explored these issues. In particular, in the last few years neuroscientists have investigated this topic in the monkey brain with clear implications for the field of neuroeconomics as well as for the current theme on morality.
For example, there are areas in the prefrontal cortex that code that value of food received (i.e., juice) and of the physical efforts exerted in order to obtain the food. Some studies have examined the activity of the orbitofrontal cortex (OFC) while the monkey examines different choices requiring an evaluation of food quantity, food type, probability of receiving food, and time delay in the delivery of the food (Padoa-Schioppa, 2011). Surprisingly, different populations of neurons in this area display a discharge that is modulated by all of these factors, suggesting that, in the monkey, the OFC encodes the subjective value of goods, defined in terms of behavioral trade-offs. This same area is also critical in evaluating the rewarding or aversive nature of a stimulus (Morrison and Salzman;, 2009), and in comparing the value of different objects.
Similarly, in humans, several brain imaging studies have shown that the OFC and the medial prefrontal cortex (mPFC) are involved in evaluating money amounts, food types, time delays, and probabilities of receiving aversive stimulation (Padoa-Schioppa, 2009; Padoa-Schioppa & Cai, 2011 for a review). Consistent with these findings, lesions to OFC and mPFC result in significant impairments to value-based decision-making (Machado & Bachevalier, 2007; Simmons et al., 2010).
More interestingly, these prefrontal regions seem to maintain an abstract representation of goods that can also be extended to the social domain. For example, in a recent fMRI study, participants made evaluations of moral judgments requiring hypothetical decisions on how to behave in situations in which the sacrifice of one’s own life could save the lives of others (Shenhav & Greene, 2010). The ventral medial prefrontal cortex and the ventral striatum were active while participants made these moral judgments. The role of the prefrontal cortex in evaluating actions within a social domain has also been the target of recent neurophysiological studies in the macaque monkey (Azzi et al., 2012; Chang et al., 2013), in which a monkey performed the task facing other monkeys. In one of these studies (Azzi et al., 2012) each monkey performed the task on alternating days. The task consisted of making an eye gaze fixation and the releasing of a handle, which resulted in receiving a reward. There were two different conditions: in the nonsocial blocks of trials, only the active monkey received the reward, but in the social blocks of trials, both the active and the passive monkeys received the reward. They found that the firing of neurons in the OFC was modulated by the condition, with increased/decreased firing when the reward was social (given to all monkeys), compared to just the active individual. Moreover, the monkeys preferred to deliver joint food with specific partners and were less likely to share rewards when facing more dominant monkeys. OFC neurons track social preferences by enhancing their firing when joint reward is obtained in sessions in which they faced the preferred partner.
Neurophysiological experiments, including those in social situations, show that, in primates, specific brain areas track the effects of one’s own behavior and of the value of one’s own actions in social contexts. These cerebral networks probably evolved to encode an abstract representation of goods/values, in value-based decision-making, and have been coopted in the social domain to mediate the process of self-reward and negative outcomes, motivation, and emotional connections with others.
6. Conclusions
How we can make sense of the neurobiological and behavioral literature on empathy and morality? Neuroscientists have shown that primate brains can empathize with others through a direct body route that involve structures of the MN system, as well as regions that are strictly connected with the visceral response of the organism, while having first-person experiences of others’ emotions. Though it is seems likely that this automatic, basic response, shared with several other species, may be the core mechanism supporting empathy, it has also been demonstrated that, built on this core, empathy could have multidimensional layers which require the activity of cognitive perspective-taking mechanisms (Preston & de Waal, 2002; Singer & Hein, 2012; Bzdok et al., 2012). These two systems for empathy have also been demonstrated in patients with brain lesions affecting the inferior frontal gyrus (IFG - part of the MN system) or the ventromedial prefrontal cortex (Shamay-Tsoory et al., 2009). Patients with lesions in the IFG showed impairments in emotional empathy and emotion recognition; while patients with lesions the ventromedial prefrontal cortex (BA 11 and 10) showed deficits in the scores of cognitive empathy.
Though there is a neurological dissociation between these forms of empathy, it is also evident that moral judgments require that the ‘emotional/embodied’ brain is preserved because it contributes and affects an individual’s capacity to represent intentions and actions’ values. This is evident by the studies in psychopathic populations, who show impairments in empathic skills, as well as in their capacity to evaluate moral behaviors (Hare, 2003). Interestingly, individuals with autism spectrum disorders also show impairments in embodied empathy (Baron-Cohen, 2010; Williams et al., 2006), deficits in the MN system (Hadjikhani et., 2007; Dapretto et al., 2006), and difficulties in moral judgments (Gleichgerrcht et al., 2012; Zalla et al., 2011). Conversely, the experience of shared emotions with others in normal individuals, has been shown to reduce aggressive actions towards other people and to promote affiliative behaviors (Eisenberg, 2000).
Together, these empirical accounts suggest that under normal circumstances several autonomous brain networks are simultaneously at work and interacting during decision-making processes. Moral cognition emerges as the consequence of the activity of emotional processing brain networks, probably involving mirror mechanisms, and of brain regions that, through abstract-inferential processing, evaluate the social context and the value of actions in terms of abstract representations.
Several scholars agree on the fact that embodied representations of affect and perspective-taking processes are not mutually exclusive processes, but rather, are integrated (Keysers & Gazzola, 2007; Mitchell, 2005; Singer & Hein, 2012).
From an evolutionary perspective, the neurophysiological work on nonhuman primates demonstrates that complex mental faculties, such as moral judgments, have their foundations in brain structures endowed with functions related to emotional processing, in the case of emotional contagion, and to the assignment of values to goods through an abstract representations of such goods. Decision-making processes must compute a comparison of different values and guide the selection of a suitable action. These brain areas probably evolved because they were necessary for animals to make decisions about daily activities that were necessary for survival and reproduction, such as the type of food to eat, the amount of time devoted to search, and the cost of an activity. The complexity of social relations in primates (and probably other social species as well) demands a neural architecture that can support necessary cognitive and emotional responses, including empathy. Being cooperative and altruistic require the capacity to understand other emotions and needs, as well as their intentions. It also requires the capacity to evaluate and remember others’ actions to reciprocate positive rewarding behaviors, and to avoid potential risky situations. Neurophysiological findings suggest the same mechanisms involved in economic choice have been recruited for the evaluation of the action value.
A comparative-based approach to the neurobiology of empathy and morality may explain how moral decisions, including those of humans, have emerged through mechanisms shared in the primate lineage, and that the core elements are present both in the emotions and in the rationality. Such an approach is promising for drawing an integrated theory of the nature of human morality.
Acknowledgements
This research was supported by the NIH P01HD064653 grant. I would like to thank Elizabeth Simpson for her comments on an early version of the draft.
References
- Azzi JC, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc. Natl. Acad. Sci. USA. 2012;109:2126–2131. doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard KA. Neonatal imitation in chimpanzees (Pan troglodytes) tested with two paradigms. Anim. Cogn. 2007;10:233–42. doi: 10.1007/s10071-006-0062-3. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Empathizing, systemizing, and the extreme male brain theory of autism. Prog. Brain Res. 2010;186:167–175. doi: 10.1016/B978-0-444-53630-3.00011-7. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Ann. Rev. Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MW, Carter JD, Proctor D, Eisenberg ML, de Waal FB. Computer animations stimulate contagious yawning in chimpanzees. Proc. Biol. Sci. 2009;276:4255–4259. doi: 10.1098/rspb.2009.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. USA. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr. Biol. 2011;21:195–199. doi: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Chang SW, Gariépy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nature Neurosci. 2013;16:243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, Decety J. Expertise modulates the perception of pain in others. Curr. Biol. 2007;17:1708–1713. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Darwin C. The descent of man. Murray, London: 1871. [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila Ross M, Menzler S, Zimmermann E. Rapid facial mimicry in orangutan play. Biol. Lett. 2007;4:27–30. doi: 10.1098/rsbl.2007.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuru E, Palagi E. In bonobos yawn contagion is higher among kin and friends. PLoS One. 2011;7:e49613. doi: 10.1371/journal.pone.0049613. doi: 10.1371/journal.pone.0049613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. The neuroevolution of empathy. Ann. N .Y. Acad. Sci. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? TiCS. 2006;10:435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- de Waal FB. Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- de Waal FB. The antiquity of empathy. Science. 2012;336:874–876. doi: 10.1126/science.1220999. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Exp. Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Ann. Rev. Psychol. 2000;51:665–697. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Gallese Vittorio, Rizzolatti Giacomo, Fogassi Leonardo. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur. J. Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4:1501–1508. [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral jusdgement. Annu. Rev. Neurosci. 2010;33:229–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Gallese V. The “shared manifold” hypothesis: from mirror neurons to empathy. J. Conscious. Stud. 2001;8:33–50. [Google Scholar]
- Gallese V. The manifold nature of interpersonal relations: The quest for a common mechanism. Phil. Trans. Royal Soc. London B. 2003;358:517–528. doi: 10.1098/rstb.2002.1234. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Ferrari PF, Umiltà MA. The mirro matching system: a shared manifold of intersubjectivity. Behav. Brain Sci. 2003;25:35–36. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum. Brain Mapp. 2007;28:441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. The hare psychopathy checklist - revised. Multi-Health Systems; Toronto: 2003. [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annu. Rev. Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn. Sci. 2007;11:194–196. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G. Functional organization of the insula and inner perisylvian regions. Proc. Natl. Acad. Sci. USA. 2012;109:10077–10082. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2010;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. 2007;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Lenzi GL, Ammaniti M. Attachment models affect brain responses in areas related to emotions and empathy in nulliparous women. Hum. Brain Mapp. 2009;34:1399–1414. doi: 10.1002/hbm.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur. J. Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Mancini G, Ferrari PF, Palagi E. Rapid facial mimicry in geladas. Sci. Rep. 2013;3:1527. doi: 10.1038/srep01527. doi: 10.1038/srep01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198(4312):75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. The false dichotomy between simulation and theory-theory: the argument’s error. Trends Cogn. Sci. 2005:363–364. doi: 10.1016/j.tics.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Montgomery KJ, Haxby JV. Mirror neuron system differentially activated by facial expressions and social hand gestures: a functional magnetic resonance imaging study. J. Cog. Neurosci. 2008;20:1866–1877. doi: 10.1162/jocn.2008.20127. [DOI] [PubMed] [Google Scholar]
- Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007;37:642–651. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J. Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal PM, Mermillod M, Maringer M, Hess U. The Simulation of Smiles (SIMS) model: Embodied simulation and the meaning of facial expression. Behav. Brain Sci. 2010;33:417–433. doi: 10.1017/S0140525X10000865. [DOI] [PubMed] [Google Scholar]
- Norscia I, Palagi E. Yawn contagion and empathy in Homo sapiens. PLoS ONE. 2011;6:e28472. doi: 10.1371/journal.pone.0028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Range-adapting representation of economic value in the orbitofrontal cortex. J. Neurosci. 2009;29:14004–14014. doi: 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Neurobiology of economic choice: a good-based model. Annu. Rev. Neurosci. 2011;32:333–359. doi: 10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Cai X. The orbitofrontal cortex and the computation of subjective value: consolidated concepts and new perspectives. Ann. N. Y. Acad. Sci. 2011;1239:130–137. doi: 10.1111/j.1749-6632.2011.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagi E, Leone A, Mancini G, Ferrari PF. Contagious yawning in gelada baboons as a possible expression of empathy. Proc. Natl. Acad. Sci. USA. 2009;106:19262–19267. doi: 10.1073/pnas.0910891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A, Anderson JR. Video-induced yawning in stumptail macaques (Macaca arctoides) Biol. Lett. 2006;2:36–38. doi: 10.1098/rsbl.2005.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A, Ferrari PF, Suomi SJ. A comparison of neonatal imitation abilities in human and macaque infants. In: Banaji MR, Gelman SA, editors. Navigating the Social World: A Developmental Perspective. Oxford University Press; Oxford: 2012. [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others’ emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39:2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Price JL. Definition of orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann. N. Y. Acad. Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Greene JD. Moral judgments recruit domain-general valuation mechanisms to integrate representations of probability and magnitude. Neuron. 2010;67:667–677. doi: 10.1016/j.neuron.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Simmons JM, Minamimoto T, Murray EA, Richmond BJ. Selective ablations reveal that orbital and lateral prefrontal cortex play different roles in estimating predicted reward value. J. Neurosci. 2010;30:15878–15887. doi: 10.1523/JNEUROSCI.1802-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Hein G. Human empathy through the lens of psychology and social neuroscience. In: de Waal FB, Ferrari PF, editors. The Primate Mind. Harvard University Press; Cambridge: 2012. pp. 158–174. [Google Scholar]
- Stern D. The interpersonal world of the infant: A view from psychoanalysis and developmental psychology. Basic Books; New York: 1985. [Google Scholar]
- Trevarthen C, Aitken KJ. Infant intersubjectivity: research, theory, and clinical applications. J. Child Psychol.Psychiatry. 2001;42:3–48. [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc. Neurosci. 2007;2:179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Zalla T, Barlassina L, Buon M, Leboyer M. Moral judgment in adults with autism spectrum disorders. Cognition. 2011;121:115–126. doi: 10.1016/j.cognition.2011.06.004. [DOI] [PubMed] [Google Scholar]