Abstract
Aim
Previous studies have examined the association between quantitative computed tomography (CT) measures of cerebral edema and patient outcomes. It has been reported that a calculated gray matter to white matter attenuation ratio (GWR) of < 1.2 indicates a near 100% non-survivable injury post-cardiac arrest. The objective of the current study was to validate whether a GWR < 1.2 reliably indicates poor survival post-cardiac arrest. We also sought to determine the inter-rater variability among reviewers, and examine the utility of a novel GWR measurement to facilitate easier practical use.
Methods
We performed a retrospective analysis of post-cardiac arrest patients admitted to a single center from 2008 to 2012. Inclusion criteria were age ≥ 18 years, non-traumatic arrest, and available CT imaging within 24 hours after ROSC. Three independent physician reviewers from different specialties measured CT attenuation of pre-specified gray and white matter areas for GWR calculations.
Results
Out of 171 consecutive patients, 90 met the study inclusion criteria. Thirteen patients were excluded for technical reasons and/or significant additional pathology, leaving 77 head CT scans for evaluation. Median age was 66 years and 63% were male. In-hospital mortality was 65% and 70% of patients received therapeutic hypothermia. For the validation measurement, the intra-class correlation coefficient was 0.70. In our dataset, a GWR below 1.2 did not accurately predict mortality or poor neurological outcome (sensitivity 0.56–0.62 and specificity 0.63–0.81). A score below 1.1 predicted a near 100% mortality but was not a sensitive metric (sensitivity 0.14–0.20 and specificity 0.96–1.00). Similar results were found for the exploratory model.
Conclusion
A GWR < 1.2 on CT imaging within 24 hours after cardiac arrest was moderately specific for poor neurologic outcome and mortality. Based on our data, a threshold GWR < 1.1 may be a safer cut-off to identify patients with low chance of survival and good neurological outcome. Intra-class correlation among reviewers was moderately good.
Keywords: Cardiac Arrest, Post-Cardiac Arrest, CT, Computed Tomography, Cerebral Edema, Prognostication
1. Introduction
Patients who achieve return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest (OHCA) face significant morbidity and mortality, despite advances in post-arrest care.1 One of the key contributors to poor outcome is neurologic injury, which may be the cause of death in up to two-thirds of patients who initially experience ROSC.2
The ability to provide neurologic prognostication early in a post-arrest patient’s hospital course would be helpful in guiding therapy, targeting novel investigative interventions for high-risk populations, counseling patients’ families, and effectively utilizing health care resources. A variety of studies have examined the value of the physical exam and neurologic scoring systems in predicting outcomes in comatose post-cardiac arrest patients both with and without the application of therapeutic hypothermia. Poor neurological outcomes may be predicted with Glasgow Coma Scale scores and the absence of various neurologic reflexes and motor function at 24 and 72 hours post-arrest. Unfortunately, no early exam findings have been able to reliably predict or distinguish those patients who will go on to have favorable neurological outcome, leaving clinicians with little predictive help early in the post-arrest period.3–7
Diffuse cerebral edema is common after cardiac arrest, and may contribute to a large portion of deaths in this population.2,8–10 On computed tomography (CT), diffuse cerebral edema manifests with effacement of the cerebral sulci and gyri, as well as loss of the normal differentiation between gray and white matter. However, these findings are observer-dependent and notoriously difficult to detect in the early stages. A more quantitative approach utilizes CT attenuation measurements. By measuring the relative attenuations of gray and white matter throughout various regions of the brain, the gray/white matter ratio (GWR) can be calculated.11–16
Previous studies have examined the correlation between GWR measurements and clinical outcomes. Yanagawa et al. found that patients without recovery of consciousness had lower GWRs in the putamen and cerebral cortex than those patients with recovery of consciousness.12 A more recent study by Metter et al. concluded that a low GWR (< 1.2) post-arrest is predictable of a nearly 100% non-survivable injury, both with and without the use of therapeutic hypothermia.13 Others have reported a specificity of 100% with a cut-off of GWR 1.1411, 1.1614 or 1.1815.
The purpose of this investigation was to confirm the utility of GWR measurements on head CT for predicting the likelihood of poor outcomes post-cardiac arrest. Specifically, we sought to validate that a GWR less than 1.2 reliably predicts the likelihood of poor chance of survival and good neurological outcome post-cardiac arrest. Given that both radiologists and non-radiologist clinicians may use this tool, we also aimed to determine the inter-rater reliability among reviewers from different specialties. Finally, given the complexity of the current model for GWR calculation, we tested a novel, simpler set of GWR measurements to compare with the established model.
2. Materials and Methods
Design and patient population
We retrospectively evaluated a cohort of patients who suffered OHCA with ROSC and were admitted to Beth Israel Deaconess Medical Center, Boston, USA between January 2008 and June 2012. The inclusion criteria were 1) age ≥ 18 years, 2) OHCA with sustained ROSC (defined as the presence of palpable pulses for > 20 minutes) 3) comatose after ROSC 4) non-traumatic arrest, and 5) a head CT scan within 24 hours of ROSC. Patients were included in the study regardless of whether they had undergone treatment with therapeutic hypothermia.
All OHCA patients who achieved ROSC were identified prospectively by multiple screening methods, including request for a post-cardiac arrest team consult, automatic computer-generated alerts based on the chief complaint or admitting diagnosis, as well as comprehensive daily screening of Emergency Department and Intensive Care Unit patients. These patients were subsequently prospectively entered into a post-cardiac arrest database. This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center and received a waiver of informed consent for medical record review.
CT measurements
CT examinations were performed on 16- and 64-row multidetector scanners (GE Healthcare Medical Systems, Milwaukee, WI, USA) in axial mode, with reconstruction of images at 5-mm intervals in soft tissue kernel.
Three physicians (from radiology, emergency medicine, and internal medicine respectively) served as reviewers. The reviewers underwent a self-directed training process with regard to CT landmark identification prior to the start of the study. Physicians were given an illustrated set of slides indicating the areas of interest in the brain for measurement (caudate, putamen, corpus callosum, thalamus, posterior limb of internal capsule, centrum semiovale, and high convexity). These slides were available for further reference during the measurement process.
Following imaging review, patients with CT scans with technical artifacts (streak artifact, patient motion, and/or excessive noise) and/or significant additional pathology (intracranial hemorrhage, major territorial infarction, prior surgery, and/or intracranial devices) were excluded. For the remaining patients, reviewers used a picture archiving and communication system (PACS) radiology workstation (Centricity Enterprise, GE) to measure CT attenuation (μ) as reported in Hounsfield units (HU) in pre-specified areas of gray and white matter, using regions of interest within a selected size range (10–15 mm2). The Hounsfield scale is a linear scale in which water is defined as 0 HU and air at −1000 HU.
Measurements were recorded using a standardized data sheet. Single measurements of the corpus callosum and average measurements for lateralized structures were used for calculation. The average GWR was calculated as shown in the supplementary material (Figure A)
An abbreviated exploratory protocol was also used, with larger regions of interest (30 mm2 for caudate, 40 mm2 for medial cortex, and 250 mm2 for centrum semiovale) in representative areas of gray and white matter. The average GWR was calculated as shown in the supplementary material (Figure B).
Reviewers performed measurements independently, and were blinded to patient clinical outcomes.
Other measurements and outcomes
Data, including demographics, past medical history and other baseline characteristics, were collected on all patients, as well as details about the arrest, initial vital signs, and laboratory results. Based on a retrospective review we categorized patients into three groups based on the clinical radiologist report of cerebral edema on the CT scan: no edema, mild edema, and severe/diffuse edema. Information on in-hospital mortality and neurological outcome was collected prior to initiation of the current study. Neurological prognostication at the study facility is determined by the treating physician in accordance with a consulting neurologist and is usually deferred until at least 72 hours after the arrest. Neurological prognostication is based on serial clinical exams as well as ancillary studies such as CT, magnetic resonance imaging and electroencephalography. The Cerebral Performance Category (CPC) score was used to access neurological status at discharge.17 The categories are as follows CPC 1: conscious, alert, able to work, CPC 2: conscious, sufficient cerebral function for independent activities of daily life, CPC 3: conscious, dependent on others for daily support, CPC 4: any degree of coma without the presence of all brain death criteria, CPC 5: brain death. A good neurological outcome was defined as a CPC score of 1 – 2 and poor neurological outcome as a CPC score of 3 – 5.
Statistical Analysis
Descriptive statistics were used to describe the study population. Goodness of fit testing was used to analyze the normality of the data prior to statistical modeling. Data for continuous variables are presented as means with standard deviations or medians with inter-quartile ranges (IQR) depending on the distribution of the variable. Fisher’s exact tests were used to compare the probability of death or poor neurological outcome in patients above a given GWR cut off point compared to those below the same GWR cutoff point. Receiver operating characteristic (ROC) curves were analyzed to determine the optimal (highest specificity) threshold value of GWR to predict mortality and poor neurologic outcome. Performance of each predictive measure was expressed as area under the curve (AUC) for the ROC curves. Sensitivity, specificity and positive and negative predictive value at the optimal cut off point was calculated from the ROC curves. Inter-rater reliability among the three physicians on the GWR scores was tested using Shrout-Fleiss’ intra-class correlation coefficient. A p-value less than 0.05 was defined as statistically significant throughout our statistical analyses. All tests of the data were performed in SAS v. 9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
A total of 171 patients had ROSC after OHCA in the study period; of those 90 patients met the inclusion criteria. Thirteen patients were excluded after image viewing due to technical reasons and/or significant additional pathology, leaving 77 for the primary analysis (see Figure 1).
Figure 1. Patient Flowchart.
177 patients had an out-of-hospital cardiac arrest in the study period. After inclusion/exclusion criteria 77 were included in the main analysis.
ROSC: Return of spontaneous circulation, OHCA: Out-of-hospital cardiac arrest, CT: Computed tomography.
Table 1 identifies the baseline characteristics of the patient population. Median age was 66 years (IQR 53 – 77). The majority of patients (63%) were male and 70% of patients received therapeutic hypothermia. Median time from ROSC to head CT was 157 minutes (IQR: 89 – 247). In-hospital mortality was 65%. Of those who survived, 85% had a good neurological outcome. Table 1 also identifies baseline characteristics for the patients who meet all inclusion criteria except a CT scan within 24 hours after ROSC. The only significant difference between the groups was a slightly higher incidence of hypertension in the group without a CT scan.
Table 1.
| All patients (n = 77) | Comatose patients with no head CT (n = 75) | |
|---|---|---|
| Demographics | ||
| Sex (% male) | 64 | 73 |
| Age (years) | 66 (53 – 77) | 66 (52 –90) |
| Race (% white) | 79 | 85 |
| Co-morbidities (%) | ||
| Coronary artery disease | 32 | 28 |
| Congestive heart failure | 16 | 11 |
| COPD | 9 | 9 |
| Diabetes | 18 | 17 |
| Hypertensionc | 44 | 61 |
| Hyperlipidemia | 23 | 32 |
| Liver disease | 3 | 4 |
| Renal disease | 12 | 4 |
| Cancer | 10 | 4 |
| Arrest details | ||
| Witnessed (%) | 69 | 69 |
| Bystander CPR (%) | 65 | 62 |
| Initial rhythm (%shockable) | 44 | 49 |
| Downtime (minutes) | 20 (10 – 35) | 15 (10 –30) |
| Initial vital signs | ||
| Heart rate (minutes−1) | 98 (29.2) | 96 (24) |
| Systolic blood pressure (mm Hg) | 120 (94 –139) | 113 (97 – 135) |
| Diastolic blood pressure (mm Hg) | 66 (50 –82) | 64 (55 – 77) |
| Respiratory rate (minutes−1) | 17 (15 – 20) | 17 (15 – 20) |
| Oxygen saturation (%) | 99 (96 – 100) | 100 (96 – 100) |
| Initial lactate | 5.2 (3.7 –7.3) | 6.3 (3.2 – 10.0) |
| APACHE II score | 25 (22 – 31) | 25 (20 – 31) |
| Therapeutic Hypothermia (%) | 70 | 67 |
All continuous variables are expressed as medians (inter quartile range) due to non-normality of the data except initial heart rate which is expressed as mean (standard deviation). Categorical data is expressed as frequencies.
COPD: Chronic Obstructive Pulmonary Disease, CPR: Cardiopulmonary Resuscitation, APACHE II: Acute Physiology and Chronic Health Evaluation II
p = 0.03 for comparison between the two groups
Validation component
Using the previously suggested cut-off GWR of 1.2, more patients were found to have poor outcomes in the terms of mortality and poor neurologic outcome at hospital discharge with a GWR < 1.2 as compared to a GWR > 1.2 (significant difference for reader 1 and 3, Table 2). However, as shown in Table 2 there was a significant amount of patients with GWR < 1.2 who survived (15–27% depending on the reader) or had good neurological outcome (15–19% depending on the reader). The value of GWR < 1.2 as a predictor of poor outcome for the different readers is outlined in Table 3.
Table 2.
Validation GWR groups (cut-off 1.2) and outcomesa
| Survival | p-value | Good neurological outcome | p-value | ||
|---|---|---|---|---|---|
| Reader 1 | GWR < 1.2 | 5/33 (15) | 0.002 | 5/33 (15) | 0.023 |
| GWR ≥ 1.2 | 22/44 (50) | 18/44 (41) | |||
| Reader 2 | GWR < 1.2 | 10/37 (27) | 0.232 | 7/37 (19) | 0.050 |
| GWR ≥ 1.2 | 17/40 (43) | 16/40 (40) | |||
| Reader 3 | GWR < 1.2 | 8/39 (21) | 0.009 | 6/39 (15) | 0.006 |
| GWR ≥ 1.2 | 19/38 (50) | 17/38 (45) | |||
Values are fraction of patients with good outcome within each GWR group with percent in parenthesis.
Table 3.
Validation GWR < 1.2 as a predictor of mortality and poor neurological outcome
| Mortality | Poor neurological outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | |
| Reader 1 | 0.56 | 0.81 | 0.85 | 0.50 | 0.52 | 0.78 | 0.85 | 0.41 |
| Reader 2 | 0.54 | 0.63 | 0.73 | 0.43 | 0.56 | 0.70 | 0.81 | 0.40 |
| Reader 3 | 0.62 | 0.70 | 0.79 | 0.50 | 0.61 | 0.74 | 0.85 | 0.45 |
Receiver operating curves (ROC) were created and area under the curve (AUC) was calculated for GWR predicting mortality (supplementary materials, Figure C) and poor neurological outcome (supplementary materials, Figure D). The highest possible cut-off where essentially no patients with a GWR below that cut-off survived was 1.1. Reviewers 2 and 3 had no patients with GWR < 1.1 that survived, and for reviewer 1 only one patient with a GWR < 1.1 survived. Tables 4 and 5 outlines the outcomes with a GWR measurement cut-off of 1.1, illustrating a higher specificity than for cut-off GWR of 1.2.
Table 4.
Validation GWR groups (cut-off 1.1) and outcomesa
| Survival | p-value | Good neurological outcome | p-value | ||
|---|---|---|---|---|---|
| Reader 1 | GWR < 1.1 | 1/8 (13) | 0.248 | 1/8 (13) | 0.423 |
| GWR ≥ 1.1 | 26/69 (38) | 22/69 (31) | |||
| Reader 2 | GWR < 1.1 | 0/10 (0) | 0.012 | 0/10 (0) | 0.028 |
| GWR ≥ 1.1 | 27/67 (40) | 23/67 (34) | |||
| Reader 3 | GWR < 1.1 | 0/8 (0) | 0.045 | 0/10 (0) | 0.097 |
| GWR ≥ 1.1 | 27/69 (39) | 23/67 (33) | |||
Values are fraction of patients with good outcome within each GWR group with percent in parenthesis.
Table 5.
Validation GWR < 1.1 as a predictor of mortality and poor neurological outcome
| Mortality | Poor neurological outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | |
| Reader 1 | 0.14 | 0.96 | 0.88 | 0.38 | 0.13 | 0.96 | 0.88 | 0.32 |
| Reader 2 | 0.20 | 1.00 | 1.00 | 0.40 | 0.19 | 1.00 | 1.00 | 0.34 |
| Reader 3 | 0.16 | 1.00 | 1.00 | 0.39 | 0.15 | 1.00 | 1.00 | 0.33 |
We performed two subgroup analyses and calculated sensitivity and specificity for a cut-off of GWR 1.1 to predict poor outcome within the two groups. The first group consisted of all patients receiving therapeutic hypothermia (54 patients, supplementary material Table A) and in the second group we excluded patients who had asphyxiation as the cause of their arrest (four patients, results not shown). We found similar results in both subgroup analyses as compared to the main analysis.
Eleven patients were categorized by the clinical radiologist as having severe/diffuse edema on the CT scan. All of these patients died. Five out of six patients categorized as having mild edema died. Seven out of 8 (reader 1), 8/9 (reader 2) and 8/8 (reader 3) patients with a GWR < 1.1 were categorized as having severe/diffuse edema. The remaining one patient for reader 1 and 2 were categorized as having “no edema”.
Exploratory component
ROC curve’s for the exploratory GWR predicting mortality and poor neurological outcome are shown in supplementary materials (Figures E and F). A cut-off of GWR 1.1 also yielded the highest specificity for prediction of poor outcome in the exploratory model (supplementary materials, Tables B and C). While the exploratory measurements were faster and easier to perform than the validation measurements, the AUC’s were generally lower. However, the exploratory model with a cut-off of GWR 1.1 had similar high specificity as the validation model. We conducted similar subgroup analyses for the exploratory measurements as we did for the validation measurement. The results in the subgroups were not different from the main analysis (results not shown).
Inter-rater reliability
For the validation measurement, the intra-class correlation coefficient was 0.70 (95%CI: 0.60–0.78). For the exploratory model the intra-class correlation coefficient was 0.78 (95%CI: 0.69–0.84).
4. Discussion
In our study, we employed three independent reviewers to measure GWR’s on head CT scans in 77 post-cardiac arrest patients. In this validation study, a GWR below 1.2 was a moderately specific predictor of mortality and poor neurological outcome. In our data, we found that a GWR below 1.1 predicted a near 100% mortality (only one reviewer had a patient with a GWR below 1.1 who survived).
Cerebral edema can cause early changes that are evident on CT imaging within hours after a cardiac arrest. In the post-arrest period, quantitative CT measurements of edema have been studied to assist with prognostication of both mortality and neurologic outcome. Although studies have demonstrated an association of GWR ratio with patient outcomes a precise GWR threshold has not been established for use in routine clinical practice.13,14 Furthermore, in prior studies conducting similar measurements and ratios, the entire cohort of patients was evaluated by one reviewer with only a small percentage of that cohort secondarily reviewed by a second reviewer.13
Metter et al. reported a threshold of GWR < 1.2 as discriminatory for poor patient outcomes.13 When applied to our data, the criterion of GWR < 1.2 was moderately specific for poor outcomes across all three physician reviewers. There were patients in all reviewer groups with GWR < 1.2 who survived and had good neurological outcome (CPC score of 1–2). In our patient cohort, a GWR cutoff of 1.1 appeared to be a better metric for predicting poor patient outcomes. Only one reviewer had one patient with a GWR of < 1.1 who had a good outcome. Our findings are closer to those of a number of other studies reporting a specificity of 100% with a cut-off of GWR 1.1411, 1.1614 or 1.1815. Our findings, in conjunction with recent studies, underscore the notion that a specific cutoff value should not be used as a basis for withdrawal of care, but instead must be taken as one valuable piece of information in the entire clinical picture. Our exploratory metric, which utilized large regions of interest over representative areas of gray and white matter, showed similar high specificity. The exploratory model was both easier and faster to calculate and this model might prove more feasible in the clinical setting. However, further studies are needed to clarify this.
We found a moderate to strong correlation between the reviewers, with an interclass correlation of 0.70. The differences in assessment of GWR may reflect different educational backgrounds and training, particularly with regard to neuroanatomy and CT interpretation. This has important practical implications, given that all three reviewer specialties (radiology, emergency medicine, and internal medicine), would likely encounter these patients in the immediate post-arrest period. The radiologist’s measurements (reader 3) most accurately predicted mortality and morbidity illustrated by higher AUC values. This may indicate that radiologists may be best suited for making these measurements in the clinical environment.
Gross cerebral edema is sometimes noted by a radiologist as part of the reading of a head CT scan. As noted, we found that those with clinical readings of gross cerebral edema all died. However, not all patients with a report of gross cerebral edema had GWR < 1.1. These findings do raise the question of whether a gross analysis by a radiologist may be comparable or even superior to the quantitative use of GWR though this was not an a planned analysis for the current study. In addition, this does raise some concern that the report of gross cerebral edema in the clinical setting could have been acted upon and thus confound our overall assessment by creating a self-fulfilling prophecy.
Our study has several limitations. First, given that only 77 out of a total of 177 patients with OHCA with ROSC were included in the study we cannot dismiss the possibility of some selection bias in terms of which patients received CT imaging early in the post-arrest period. Given the nature of our patient population, the results might not be fully generalizable to all post-arrest patients. Secondly, we decided to include patients with CT scans within the first 24 hours. It might be that the current measurement (GWR) is a better prognostic tool early after ROSC (e.g. within one hour) or later in the post-cardiac arrest course. Timed CT measurements and correlation with follow-up CT and/or magnetic resonance (MR) imaging may be a topic for future research. Since we utilized clinical CT scans for our analysis we cannot exclude that the CT findings might have affected the clinical decision making especially concerning neurological withdrawal of care. While none of the GWR measurements were available to the treating clinicians gross reports of cerebral edema were and this group had substantial overlap as described.
5. Conclusion
We found that head CT GWR cutoffs for predicting patient outcome after cardiac arrest may vary across medical centers. The previously reported GWR cutoff of 1.2 was moderately specific for predicting poor outcome in our patient population, while a GWR < 1.1 had better performance. This raises questions about the optimal GWR cutoff for prediction of poor patient outcomes. A larger, prospective multicenter trial would be useful for further investigation, and would be critical before this tool could be routinely implemented to inform clinical decisions about pursuing aggressive care early in the post-arrest period.
Supplementary Material
Figure A: Equations for the validation GWR calculations
GWR: Gray matter to white matter ratio, μ: computed tomography attenuation in Hounsfield units
Figure B: Equations for the exploratory GWR calculations
GWR: Gray matter to white matter ratio, μ: computed tomography attenuation in Hounsfield units
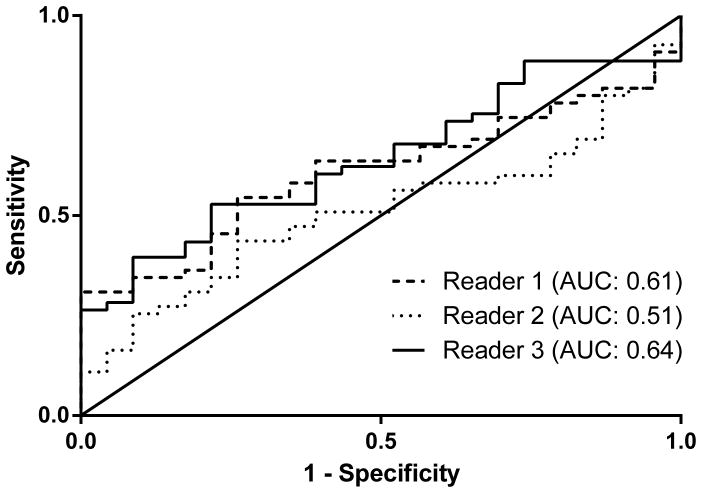
Figure C. ROC curve for prediction of mortality – validation measurement
ROC: Receiver operating characteristics, AUC: Area under the curve
Figure D. ROC curve for prediction of poor neurological outcome – validation measurement
ROC: Receiver operating characteristics, AUC: Area under the curve
Figure E. ROC curve for prediction of mortality – exploratory measurement
ROC: Receiver operating characteristics, AUC: Area under the curve
Figure F. ROC curve for prediction of poor neurological outcome – exploratory measurement
ROC: Receiver operating characteristics, AUC: Area under the curve
Acknowledgments
We thank Francesca Montillo for her editorial assistance in preparing the submitted manuscript. Dr. Donnino is supported by NHLBI (1K02HL107447-01A1). The project described was supported, in part, by Grant Number UL1 RR025758- Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
Footnotes
6. Conflict of Interest Statement
The authors declare that they have no conflict of interest to declare
7. Author’s contributions
CC, MNC and MWD were responsible for study conception and design. CC, MLH and SL did the GWR measurements. TG, JS and BS were responsible for the statistical analysis. CC drafted the initial manuscript. SP and LWA were involved with data interpretation, and critical review and edits of the manuscript. All authors took part in critical revision of the manuscript, interpreted the data, and provided intellectual content. All authors were responsible for drafting and revising the manuscript and read and approved the final submission.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cristal Cristia, Email: cristal.cristia@gmail.com.
Mai-Lan Ho, Email: mailanho@yahoo.com.
Sean Levy, Email: sdlevy@bidmc.harvard.edu.
Lars W. Andersen, Email: lwanders@bidmc.harvard.edu.
Sarah M. Perman, Email: sarah.perman@ucdenver.edu.
Tyler Giberson, Email: t.a.giberson@gmail.com.
Justin Salciccoli, Email: justin.salciccioli@gmail.com.
Brian Z. Saindon, Email: bsaindon@caregroup.org.
Michael N. Cocchi, Email: mcocchi@bidmc.harvard.edu.
Michael W. Donnino, Email: mdonnino@bidmc.harvard.edu.
References
- 1.Nolan JP, Laver SR, Welch CA, Harrison DA, Gupta V, Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC Case Mix Programme Database. Anaesthesia. 2007 Dec;62(12):1207–1216. doi: 10.1111/j.1365-2044.2007.05232.x. [DOI] [PubMed] [Google Scholar]
- 2.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive care medicine. 2004 Nov;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 3.Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA: the journal of the American Medical Association. 2004 Feb 18;291(7):870–879. doi: 10.1001/jama.291.7.870. [DOI] [PubMed] [Google Scholar]
- 4.Edgren E, Hedstrand U, Kelsey S, Sutton-Tyrrell K, Safar P. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet. 1994 Apr 30;343(8905):1055–1059. doi: 10.1016/s0140-6736(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 5.Schefold JC, Storm C, Kruger A, Ploner CJ, Hasper D. The Glasgow Coma Score is a predictor of good outcome in cardiac arrest patients treated with therapeutic hypothermia. Resuscitation. 2009 Jun;80(6):658–661. doi: 10.1016/j.resuscitation.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: A systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation. 2013 Jul 3; doi: 10.1016/j.resuscitation.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: A systematic review and meta-analysis. Part 1: Patients not treated with therapeutic hypothermia. Resuscitation. 2013 Jun 27; doi: 10.1016/j.resuscitation.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008 Dec 2;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 9.Naples R, Ellison E, Brady WJ. Cranial computed tomography in the resuscitated patient with cardiac arrest. The American journal of emergency medicine. 2009 Jan;27(1):63–67. doi: 10.1016/j.ajem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Inamasu J, Miyatake S, Suzuki M, et al. Early CT signs in out-of-hospital cardiac arrest survivors: Temporal profile and prognostic significance. Resuscitation. 2010 May;81(5):534–538. doi: 10.1016/j.resuscitation.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Choi SP, Park KN, Youn CS, Oh SH, Choi SM. Early brain computed tomography findings are associated with outcome in patients treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013;21:57. doi: 10.1186/1757-7241-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagawa Y, Un-no Y, Sakamoto T, Okada Y. Cerebral density on CT immediately after a successful resuscitation of cardiopulmonary arrest correlates with outcome. Resuscitation. 2005 Jan;64(1):97–101. doi: 10.1016/j.resuscitation.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Metter RB, Rittenberger JC, Guyette FX, Callaway CW. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation. 2011 Sep;82(9):1180–1185. doi: 10.1016/j.resuscitation.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheel M, Storm C, Gentsch A, et al. The prognostic value of gray-white-matter ratio in cardiac arrest patients treated with hypothermia. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013 Apr 8;21(1):23. doi: 10.1186/1757-7241-21-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torbey MT, Selim M, Knorr J, Bigelow C, Recht L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke; a journal of cerebral circulation. 2000 Sep;31(9):2163–2167. doi: 10.1161/01.str.31.9.2163. [DOI] [PubMed] [Google Scholar]
- 16.Choi SP, Park HK, Park KN, et al. The density ratio of grey to white matter on computed tomography as an early predictor of vegetative state or death after cardiac arrest. Emergency medicine journal: EMJ. 2008 Oct;25(10):666–669. doi: 10.1136/emj.2007.053306. [DOI] [PubMed] [Google Scholar]
- 17.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975 Mar 1;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A: Equations for the validation GWR calculations
GWR: Gray matter to white matter ratio, μ: computed tomography attenuation in Hounsfield units
Figure B: Equations for the exploratory GWR calculations
GWR: Gray matter to white matter ratio, μ: computed tomography attenuation in Hounsfield units
Figure C. ROC curve for prediction of mortality – validation measurement
ROC: Receiver operating characteristics, AUC: Area under the curve
Figure D. ROC curve for prediction of poor neurological outcome – validation measurement
ROC: Receiver operating characteristics, AUC: Area under the curve
Figure E. ROC curve for prediction of mortality – exploratory measurement
ROC: Receiver operating characteristics, AUC: Area under the curve
Figure F. ROC curve for prediction of poor neurological outcome – exploratory measurement
ROC: Receiver operating characteristics, AUC: Area under the curve