Abstract
Objectives
Age is generally an inverse predictor of health literacy. However, the role of cognitive dysfunction among older adults in this relationship is not understood.
Methods
We conducted a cross-sectional survey of 446 adult patients in a large urban academic level one trauma center, assessing health literacy and cognitive dysfunction.
Results
Removing older patients (60 years of age and older) who screened positive for cognitive dysfunction attenuated the relationship between age and health literacy (r= -0.16, p=0.001 vs. r= -0.35, p<0.0001). Older patients screening positive for cognitive dysfunction had significantly lower health literacy than older patients screening negative and patients less than 60 years; health literacy scores did not generally differ significantly between the latter groups.
Conclusions
Much of the relationship between age and health literacy was driven by cognitive dysfunction among a subset of older adults.
Practice implications
Our findings suggest that older patients with cognitive dysfunction have the greatest need for health literacy interventions.
Keywords: health literacy, age, patient intervention, cognitive dysfunction, cognitive status
1. Introduction
Health literacy, or the degree to which individuals have the capacity to obtain, communicate, process, and understand basic health information and services needed to make appropriate health decisions [1], is an important predictor of health outcomes. Limited health literacy has been linked with higher incidence of chronic illness, poorer self-reported health, lower utilization of preventive health services, higher rates of hospitalization, and higher health care costs [2-9]. Limited health literacy has been shown to be associated with sociodemographic characteristics including age, race/ethnicity, and socioeconomic status [10]; understanding the mechanisms underlying these associations is critical to developing educational and intervention strategies that are effective across patient subgroups.
In many prior studies, age has been found to be a significant inverse predictor of health literacy, both in general populations [10-14] and among older individuals [15,16]. In one study, the prevalence of limited health literacy was 15.6% among Medicare enrollees aged 65-69 and 58.0% among those 85 years of age or older [11]. However, the role of cognitive dysfunction (i.e., mild cognitive impairment, delirium, and various stages of dementia) [17] in this relationship is not well understood. Cognitive status has been shown to be a significant predictor of health literacy among older adults [18,19]. In a few prior studies conducted with older adults, cognitive status accounted for some of the variance in health literacy when examining the relationship between age and health literacy [11,16]. Among 314 community-dwelling adults with chronic heart failure, education and cognitive ability both explained some of the age differences in health literacy [14]. However, despite prior research showing a relationship between cognitive status and health literacy, cognitive status has generally been treated as an exclusion criterion [20,21] or has not been explicitly considered [5,10] in most health literacy studies. Therefore, the role of cognitive dysfunction in the relationship between age and health literacy needs to be examined further among adult patient populations.
To address this issue, we investigated the relationship between age and health literacy among adult patients seeking care for a variety of health issues in an emergency department setting and examined how cognitive dysfunction among older adults affected this relationship. The emergency department has not been a focus of research on the relationships between age, cognitive status, and health literacy, despite the importance of this setting for the care of older adults [22]. We administered multiple health literacy and numeracy assessments and a brief screen for cognitive dysfunction to a diverse sample of patients. Based on prior research, we hypothesized that age would be inversely related to health literacy, and that this association would be attenuated by excluding older patients who screened positive for cognitive dysfunction. We also examined the performance of standard health literacy assessments among patients stratified by age and cognitive dysfunction. These issues are of importance to the care of older patients because cognitive dysfunction is prevalent in patient populations, but often remains undetected [23-25]. Understanding how age and cognitive dysfunction are related to health literacy is therefore critical to providing quality care and developing effective health literacy interventions for older patients.
2. Methods
2.1. Participants
We conducted a cross-sectional survey of a convenience sample of 446 patients 18 years of age or older seeking care in the Barnes-Jewish Hospital Emergency Department in St. Louis, MO, an urban academic level one trauma center with over 95,000 annual visits. Exclusion criteria were: altered mental status, aphasia, mental handicap, previously diagnosed dementia, acute psychiatric illness, insurmountable communication barrier, non-English speaking, high patient distress as judged by the attending physician, sexual assault, or corrected visual acuity worse than 20/100. Trained research assistants approached eligible patients from March 2011 to February 2012. 590 patients were approached; 132 refused (22.4%), 12 (2.0%) were ineligible, and 446 (75.6%) agreed to participate. Age, race and gender did not differ significantly between patients who agreed to participate, those who refused, and the overall patient population.
2.2. Data collection
After informed consent, research assistants administered a series of assessments of health literacy, numeracy, and cognitive dysfunction, as described below. The study was approved by the hospital Institutional Review Board.
2.3. Measures
2.3.1. Health literacy and numeracy
Trained data collectors assessed health literacy with three objective measures and three self-report screener items. Numeracy was specifically assessed with four additional items.
The first of the objective measures of health literacy was the abbreviated Short Test of Functional Health Literacy in Adults [S-TOFHLA], which is comprised of two reading comprehension passages [26]. The S-TOFHLA uses a modified Cloze procedure in which every 5th to 7th word is removed and the correct answer is selected based on the surrounding context [6]; patients receive up to seven minutes for completion. Number of correct answers is categorized as adequate (23-36), marginal (17-22), or inadequate (0-16) health literacy. The Rapid Estimate of Adult Literacy in Medicine – Revised [REALM-R] is a word recognition test in which patients are asked to read aloud eight medical and health-related terms [27,28]. REALM-R scores are based on number of words pronounced correctly and dichotomized as adequate (7-8) or inadequate (0-6) health literacy. The Newest Vital Sign [NVS] is a six-item measure based on interpretation of information in a food nutrition label, which requires reading comprehension and numeracy skills [7,29]. NVS scores are based on number of correct answers: 0-1 is a high likelihood of limited health literacy, 2-3 possibility of limited health literacy and 4-6 adequate health literacy [7].
We also administered three single item literacy screeners [SILS], which assess patients’ self-reported health literacy skills in different domains: how often they have someone help them read hospital materials; how confident they are filling out medical forms; and how often they have difficulty understanding written information about a medical condition [30]. Items are answered on a five-point Likert scale. The SILS have been validated against the S-TOFHLA and REALM [30].
We assessed numeracy with three general items [31] and an item from an expanded health numeracy scale [32] used in a national survey (i.e., “Which of the following numbers represents the biggest risk of getting a disease? 1 in 10, 1 in 100, 1 in 1000) [33]. The number of correct items was summed; possible numeracy scores ranged from 0 to 4.
2.3.2. Cognitive dysfunction
We assessed occult cognitive dysfunction with the Brief Alzheimer Screen (BAS), a brief instrument developed to screen patients 60 years of age and older for Alzheimer's Disease (AD) in clinical settings [34]. The BAS was developed by identifying Mini-Mental State Examination (MMSE) tasks [35,36] and a category fluency task that best discriminate mild AD patients from normal older adults [34]. The BAS has four tasks that assess the domains of orientation, registration-recall, verbal fluency, and attention (i.e., knowing the correct date; three-item recall; spelling “WORLD” backwards; number of animals named in 30 seconds). We selected the BAS because our prior work has shown it to be an accurate cognitive dysfunction screening instrument for use in the ED [24] and because complex global cognitive assessment batteries have been shown not to be feasible or acceptable in this setting [37]. Older patients (60 years of age or older) with a BAS score of 26 or less were considered to screen positive for cognitive dysfunction [24].
2.3.3. Patient characteristics
We assessed patients’ age, gender, education, race/ethnicity, insurance status, having a personal doctor and current number of prescribed medications.
2.4. Data analysis
For analyses, we excluded 11 patients missing S-TOFHLA scores. Descriptive statistics were examined for all variables. We examined bivariate associations between age and S-TOFHLA score using Spearman correlation coefficients due to the non-normal distributions. We compared performance on BAS items by S-TOFHLA score using chi-squared and ANOVA tests. Because our interest was in examining cognitive dysfunction among older adults, we stratified at age 60. We chose this age for two reasons. The BAS has been validated as a screen for adults aged 60 or older [34]. In addition, although definitions of “older adult” vary, the World Health Organization has defined the older population as starting at age 60 [38]. We therefore stratified patients into three groups: patients 60 years of age or older who had a positive screen for cognitive dysfunction; patients 60 years of age or older who had a negative screen; and younger patients less than age 60. We examined differences between these patient strata on S-TOFHLA, REALM-R, NVS, SILS, and numeracy scores using chi-squared and ANOVA tests. We investigated bivariate relationships between scores on the S-TOFHLA and the other assessments within patient strata with Spearman correlation coefficients. Finally, we examined the area under the receiver operating characteristic curve (AUROC) for each health literacy and numeracy assessment against the S-TOFHLA as a criterion standard for the overall sample and within each patient stratum. Data were analyzed using SAS/STAT® Software Version 9.3 for Windows (Cary, NC). Statistical significance was assessed as p<0.05.
3. Results
About half (56%) of participating patients were female (Table 1); 67% self-identified as Black. Only 32% had education beyond high school. About 27% were uninsured; 62% reported having a personal doctor. Patients were taking 3.8 prescription drugs, on average. Of the 79 patients who were 60 years of age or older, 36 (46%) screened positive for cognitive dysfunction.
Table 1.
Characteristics of patient sample, stratified by age and cognitive dysfunction.
| Overall | Younger patients (age <60) | Patients age 60+ Negative screen for cognitive dysfunction | Patients age 60+ Positive screen for cognitive dysfunction | |
|---|---|---|---|---|
| N=435 | N=356 | N=43 | N=36 | |
| Patient characteristic | M(SD) or N(%) | M(SD) or N(%) | M(SD) or N(%) | M(SD) or N(%) |
| Age | 45.3 (15.7) | 40.3 (12.4) | 66.4 (6.4) | 69.5 (7.6) |
| Femalea | 241 (55.5%) | 200 (56.3) | 27 (62.8%) | 14 (38.9%) |
| Education | ||||
| Less than high school | 77 (17.7%) | 59 (16.6%) | 3 (7.0%) | 15 (41.7%) |
| High school diploma/GED | 218 (50.1%) | 180 (50.6%) | 21 (48.8%) | 17 (47.2%) |
| Some college or higher | 140 (32.2%) | 117 (32.9%) | 19 (44.2%) | 4 (11.1%) |
| Race/ethnicitya | ||||
| White | 133 (30.6%) | 98 (27.5%) | 23 (53.5%) | 12 (33.3%) |
| Black | 292 (67.1%) | 249 (69.9%) | 19 (44.2%) | 24 (66.7%) |
| Other | 10 (2.3%) | 9 (2.5%) | 1 (2.3%) | 0 (0.0%) |
| Insuranceb | ||||
| Public | 164 (37.8%) | 120 (33.8%) | 24 (55.8%) | 20 (55.6%) |
| Private | 155 (35.7%) | 126 (35.5%) | 15 (34.9%) | 14 (38.9%) |
| Uninsured | 115 (26.5%) | 109 (30.7%) | 4 (9.3%) | 2 (5.6%) |
| Have personal doctor | 268 (61.6%) | 205 (57.6%) | 35 (81.4%) | 28 (77.8%) |
| Current prescriptions | 3.8 (4.5) | 3.3 (4.1) | 5.6 (4.5) | 7.2 (6.2) |
Variable missing three values.
Variable missing one value.
3.1. Relationships between age, health literacy, and cognitive dysfunction
In the full sample, age had a moderate, inverse association with S-TOFHLA score (r= -0.35, p<0.0001; data not shown). When older patients who screened positive for cognitive dysfunction were removed, this correlation was still significant but attenuated (r= -0.16, p=0.001). Among the sample of older patients, scores on three BAS tasks differed significantly by health literacy (Table 2); there were significant positive associations between S-TOFHLA score and performance on 3-item recall (p=0.004), animal naming (p<0.0001) and spelling “WORLD” backwards (p<0.0001). However, orientation (i.e., knowing correct date) was not significantly associated with health literacy.
Table 2.
Scores for older patients (age 60+) on each Brief Alzheimer's Screen item, stratified by cognitive dysfunction.
| Abbreviated S-TOFHLA score | ||||
|---|---|---|---|---|
| Inadequate N=27 | Marginal N=13 | Adequate N=39 | ||
| BAS items | M (SD) or N (%) | M (SD) or N (%) | M (SD) or N (%) | p-value |
| Total BAS score | 16.7 (7.0) | 26.2 (5.8) | 30.1 (4.7) | <0.001 |
| Know correct date | 23 (85.2%) | 12 (92.3%) | 38 (97.4%) | 0.18 |
| # of 3 items recalled | 1.6 (1.0) | 2.1 (1.0) | 2.4 (0.9) | 0.004 |
| # of animals in 30 seconds | 7.5 (3.0) | 10.9 (2.7) | 12.5 (3.4) | <0.001 |
| # of letters in WORLD spelled backwards | 1.5 (1.8) | 4.1 (1.5) | 4.9 (0.5) | <0.001 |
As shown in Table 3, 24% of patients had limited health literacy based on the S-TOFHLA, 49% based on REALM-R, and 65% based on NVS. Patients generally had high self-reported health literacy skills based on the SILS, as shown by mean scores of 4.0 or higher for these items. Numeracy skills were fairly limited (M=1.4, SD=1.0). Scores on all assessments of health literacy and numeracy differed significantly across patient strata defined by age and cognitive dysfunction. We therefore examined pair-wise differences in mean scores for the health literacy and numeracy assessments across the strata. Among older patients screening positive for cognitive dysfunction, mean S-TOFHLA and NVS scores were both significantly lower than among older patients screening negative (p-values<0.0001) and younger patients (p-values<0.0001). Similarly, mean scores on all three SILS were significantly lower among older patients screening positive for cognitive dysfunction than among older patients screening negative (p-values<0.0001) and younger patients (p-values<0.0001). However, mean scores did not differ significantly between older patients screening negative for cognitive dysfunction and younger patients for the S-TOFHLA, NVS, or SILS items. Mean REALM-R scores were also significantly lower among older patients screening positive for cognitive dysfunction than among older patients screening negative (p<0.0001) and younger patients (p=0.001). However, mean REALM-R scores were significantly higher among older patients screening negative for cognitive dysfunction than among younger patients (p=0.0004). Mean numeracy score did not differ significantly between strata.
Table 3.
Health literacy and numeracy among patient sample, stratified by age and cognitive dysfunction.
| Overall | Younger patients (age <60) | Patients age 60+ Negative screen for cognitive dysfunction | Patients age 60+ Positive screen for cognitive dysfunction | ||
|---|---|---|---|---|---|
| N=435 | N=356 | N=43 | N=36 | p-value** | |
| Measure | M (SD) or N (%) | M (SD) or N (%) | M (SD) or N (%) | M (SD) or N (%) | |
| Abbreviated S-TOFHLA* | <0.001 | ||||
| Inadequate | 57 (13.1%) | 30 (8.4%) | 3 (7.0%) | 24 (66.7%) | |
| Marginal | 47 (10.8%) | 34 (9.5%) | 7 (16.3%) | 6 (16.7%) | |
| Adequate | 331 (76.1%) | 292 (82.0%) | 33 (76.8%) | 6 (16.7%) | |
| Mean score | 28.8 (9.0) | 30.2 (7.4) | 29.8 (8.6) | 13.7 (10.0) | |
| NVS† | <0.001 | ||||
| High likelihood | 127 (29.7%) | 91 (25.8%) | 6 (15.9%) | 30 (83.3%) | |
| Possible | 150 (35.1%) | 131 (37.1%) | 14 (35.9%) | 5 (13.9%) | |
| Adequate | 151 (35.3%) | 131 (37.1%) | 19 (48.7%) | 1 (2.8%) | |
| Mean score | 2.8 (1.9) | 2.9 (1.9) | 3.6 (1.9) | 0.9 (1.2) | |
| REALM-R‡ | <0.001 | ||||
| Limited | 210 (48.5%) | 175 (49.3%) | 9 (21.4%) | 26 (72.2%) | |
| Adequate | 223 (51.5%) | 180 (50.7%) | 33 (78.6%) | 10 (27.8%) | |
| Mean score | 5.4 (2.8) | 5.4 (2.8) | 7.1 (1.6) | 3.7 (3.1) | |
| Help reading SILS§ | 4.3 (1.0) | 4.3 (1.0) | 4.6 (0.7) | 3.7 (1.5) | <0.001 |
| Medical form SILS∥ | 4.0 (1.2) | 4.1 (1.1) | 4.2 (1.0) | 3.1 (1.5) | <0.001 |
| Ability to read SILS¶ | 4.5 (0.9) | 4.5 (0.8) | 4.7 (0.6) | 3.7 (1.3) | <0.001 |
| Numeracy score# | 1.4 (1.0) | 1.4 (1.0) | 1.7 (1.2) | 1.0 (0.8) | 0.016 |
Scores categorized as: inadequate functional health literacy (0-16), marginal functional health literacy (17-22), adequate functional health literacy (23-36)
Scores categorized as: high likelihood of limited literacy (0-1), possible likelihood of limited literacy (2-3), adequate literacy (4-6); N=428
Scores categorized as: limited health literacy (≤6), adequate health literacy (7+); N=433
“How often do you have someone help you read hospital materials?”
“How confident are you filling out medical forms by yourself?”
“How often do you have problems learning about your medical condition because of difficulty understanding written information?”
Range of numeracy scores: 0-4
p-value is for comparison between 3 groups stratified by age and cognitive dysfunction
Correlations between assessments of health literacy and numeracy differed substantially across strata (Table 4). The correlation between NVS score and S-TOFHLA score was weaker among older patients screening positive for cognitive dysfunction (r=0.25, ns) than among older patients screening negative (r=0.45, p<0.001) and younger patients (r=0.40, p<0.001). In contrast, the correlation between REALM-R score and S-TOFHLA score was weaker among older patients screening negative (r=0.28, ns) than among the other two strata. All SILS were most highly correlated with S-TOFHLA score among older patients screening positive for cognitive dysfunction. However, numeracy score was least correlated with S-TOFHLA score in this stratum.
Table 4.
Spearman correlation coefficients between scores on health literacy and the abbreviated S-TOFHLA, stratified by age and cognitive dysfunction.
| Overall | Patients age <60 | Patients age 60+ Negative screen for cognitive dysfunction | Patients age 60+ Positive screen for cognitive dysfunction | |
|---|---|---|---|---|
| N=435 | N=356 | N=43 | N=36 | |
| NVS† | 0.48*** | 0.40*** | 0.45*** | 0.25 |
| REALM-R‡ | 0.37*** | 0.36*** | 0.28 | 0.49** |
| Help reading SILS | 0.21*** | 0.16*** | 0.03 | 0.40* |
| Medical form SILS | 0.35*** | 0.28*** | 0.21 | 0.51*** |
| Ability to read SILS | 0.48*** | 0.46*** | 0.32* | 0.52** |
| Numeracy | 0.30*** | 0.31*** | 0.27 | 0.02 |
p<0.05
p<0.01
p<0.001
N=428
N=433
3.2. Prediction of limited health literacy across patient strata
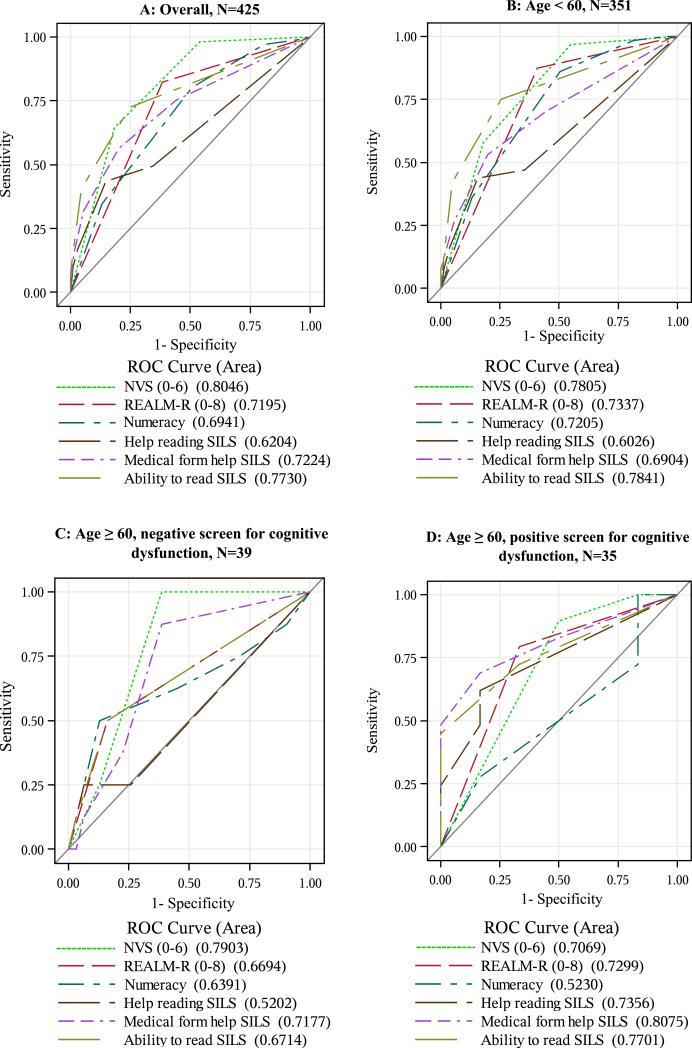
Performance of the three objective health literacy assessments (i.e., S-TOFHLA, REALM-R, NVS) in predicting limited health literacy also varied across strata (see Figure 1). For the sample overall, AUROC values were highest for NVS (0.80), and this was also the case among younger patients (0.78). However, NVS was not as predictive among older patients screening positive for cognitive dysfunction (0.71). Conversely, REALM-R was not as predictive among older patients screening negative for cognitive dysfunction (0.67) compared with older patients screening positive (0.73). The three SILS generally had higher AUROC values among older patients screening positive for cognitive dysfunction than among older patients screening negative or younger patients, with the exception of the ability to read SILS, which had the highest AUROC value among younger patients (0.78). Among older patients screening positive for cognitive dysfunction, the medical forms SILS was the most predictive of all assessments (0.81). AUROC values for numeracy were lowest among older patients screening positive for cognitive dysfunction (0.52) compared with the other strata.
Figure 1.
Receiver operating characteristics curves for health literacy measures against STOFHLA as the criterion standard, stratified by age and health literacy.
4. Discussion and conclusion
4.1. Discussion
We examined how cognitive dysfunction among older patients affected the relationship between age and health literacy in an emergency department population. Overall, age was moderately and inversely correlated with health literacy; this relationship was substantially attenuated when older adults screening positive for cognitive dysfunction were removed. Older patients screening positive for cognitive dysfunction had significantly lower scores on all health literacy assessments than either older patients screening negative or younger patients. Interestingly, for health literacy assessments other than REALM-R, scores did not differ significantly between older patients screening negative for cognitive dysfunction and younger patients. For REALM-R, older patients screening negative had the highest health literacy scores.
The mechanisms underlying the relationship between age and health literacy [10-16] are not well understood. Prior research has found a general decline in cognitive functioning in older adults [39,40], with age-related decreases in executive functioning, verbal fluency, verbal memory, and cognitive speed [40]. Older individuals are more likely to have a dementing illness that could affect reading ability [11], and even mild cognitive impairment may affect literacy tasks [16]. Fluid and crystallized cognitive abilities have been demonstrated to attenuate the relationship between health literacy and performance of health care tasks among older adults [41]. In one study, memory and verbal fluency, cognitive abilities commonly impaired in older adults, were strongly associated with health literacy among independently living older adults [19]. In another large study of community-dwelling elderly persons, the relationship between age and health literacy was attenuated after adjusting for MMSE score [16]. Some researchers have therefore suggested that age-related declines in cognitive processes may drive decreases in health literacy with age [14,16,18], even without dementing illness [16]. However, we found that older adults screening negative for cognitive dysfunction did not have significantly lower health literacy than younger adults, and that the magnitude of the association between age and health literacy was decreased when older adults screening positive for cognitive dysfunction were excluded. These findings suggest that much of the apparent relationship between age and health literacy in our patient population is driven by cognitive dysfunction among a subset of older adults, with less contribution from other age-related changes in cognitive abilities.
Our results therefore add to the evolving understanding of the complex, bidirectional relationships between age, cognitive abilities, health literacy, and health outcomes [19], relationships with important consequences for health. Health literacy and cognition both independently predict mortality among older community-dwelling adults [42], with a nearly twofold increase in mortality among older patients with limited literacy compared with those with adequate literacy [43]. While differences in cognitive abilities may explain some of the effect of age on health literacy [14], limited literacy may also lead to declines in cognitive abilities with age [44]. Early cognitive deficits may cause poor reading comprehension [18], and poor health status over the life course may contribute to limited health literacy [43]. Our research adds to the literature by examining these issues an emergency department population. Despite the importance of this setting for the care of older adults [22] and the unique features of the emergency department for patient care [45], research has not examined the associations between age, health literacy, and cognitive dysfunction among older adults in this population. Our findings showed that three cognitive domains (i.e., registration-recall, verbal fluency, attention) were strongly related to health literacy among older patients, and this was not limited to cognitive tasks most directly related to literacy (i.e., spelling “world” backward) [18]. Longitudinal research is needed to examine the directionality of these relationships over the life course.
In addition, the effects of cognitive ability and cognitive dysfunction on measurement of health literacy need to be explored. Age and cognitive abilities may affect various health literacy assessments differently; word recognition tasks may be less affected by current cognitive functioning than reading comprehension tasks [18,44]. In a study conducted with older adults, TOFHLA and NVS were more associated with fluid abilities (i.e., abilities to learn and apply new information) and REALM was associated more with crystallized abilities (i.e., background knowledge) [41]. In one study with older diabetes patients, the S-TOFHLA reading comprehension component was correlated with age, but the NVS and REALM-SF were not [46]. Our results indicate that the BAS assesses cognitive domains relevant to measurement of health literacy.
In our prior study, we examined feasibility and diagnostic accuracy of health literacy assessments, finding that NVS and REALM-R were most discriminant compared with the STOFHLA [47]. Here we found that the diagnostic accuracy of health literacy and numeracy assessments in predicting limited health literacy was substantially different across patient strata defined by age and cognitive dysfunction. The NVS and numeracy assessments were not as predictive among older patients screening positive for cognitive dysfunction, while REALM-R and SILS were more predictive in this stratum. Wolf et al. (2012) recently suggested that health literacy reflects individual differences across a broad set of cognitive skills [41]. Our findings add that cognitive dysfunction among older adults has a different effect on the measurement of numeracy and on health literacy assessments with a numeracy component than on reading ability.
The impact of health literacy on measurement of cognitive functioning and cognitive dysfunction also needs to be investigated. No cognitive screening instruments, such as the BAS, have been validated among patients with limited health literacy specifically. Prior research has demonstrated that MMSE scores are significantly related to health literacy among older adults [18,48], but it is unknown whether this is due to literacy-related measurement error or to actual differences in cognitive functioning. Measures of cognitive function that have been validated amongst adults with limited health literacy are critically needed to advance this area of inquiry.
This study has a number of limitations that should be considered. This cross-sectional study was not able to examine direction of causation for observed associations and was conducted at a single institution with primarily English-speaking patients. Our measure of cognitive dysfunction is intended as a screen for clinical settings, not as a comprehensive assessment of cognitive function [34]. More comprehensive measures, such as the MMSE [35,36], are less feasible in busy clinical settings [24,34], leading to our selection of the BAS for this study. It is important to note that the BAS is an inaccurate screening instrument for mild cognitive impairment for which instruments like the Montreal Cognitive Assessment (MoCA) [49] are preferable. However, more comprehensive measures, such as the MoCA [49] and MMSE [35,36], are less feasible in busy clinical settings [24,34] and have not been validated for ED use [50]. Because screeners may miss more subtle cognitive impairments that may affect health literacy, it will be important to validate additional measures of cognitive abilities for use in future ED research. This study did not conduct any formal assessment for delirium [17,51]. Future research could explore these findings among a sample with a higher number of older adults or could define “older adult” differently than in this study. While our interest was in examining cognitive dysfunction among older adults, additional studies could examine how changes in cognitive status along the age continuum affects the relationship between age and health literacy. Current measures of health literacy do not capture the entire construct [52,53]. We used the STOFHLA as a criterion standard because it is associated with health outcomes and has been used to validate other health literacy measures [5,7,30,52,53], but it does not capture some domains of health literacy.
4.2 Conclusion
Our findings, together with prior research, have important research and practice implications for older patients. We observed that the apparent inverse association between age and health literacy in an emergency department sample was driven at least in part by cognitive dysfunction among a subset of older patients, showing that it is critical to examine cognitive status when examining how age and health literacy are related. Interestingly, health literacy and numeracy skills generally did not differ significantly between patients less than age 60 and patients 60 years of age or older who screened negative for cognitive dysfunction. Future research is needed to tease apart the contributions of age-related changes in cognitive abilities and of cognitive dysfunction in the relationship between age and health literacy, perhaps by stratifying older patients into finer age categories. In addition, our findings highlight the importance of measurement issues in examining the relationships between age, cognitive status, and health literacy. Our findings showed that cognitive dysfunction affected different health literacy instruments differently; it will be important to investigate how cognitive dysfunction affects the assessment of the various component domains of health literacy. Additionally, there is a strong need for cognitive screening instruments validated for use with patients who have limited health literacy in order to advance this area of research.
4.3 Practice implications
Many researchers have emphasized the importance of targeting elderly patients with health literacy interventions [11,15,21,54]. However, our data suggest that older patients with occult cognitive dysfunction might have the greatest health literacy needs. Because subtle forms of cognitive impairment can be difficult to detect [55], it may be important to screen for cognitive status among older patients to reach this high risk group. Even in the absence of screening, it is critical to design health-related information in ways that are appropriate for both the health literacy skills and cognitive abilities of older patients [19]. While universal precautions that address the health literacy needs of all patients are needed [56], such interventions should take into account all cognitive demands faced by patients in the clinical setting rather than focusing only on literacy demands [41,42]. Development of such educational and intervention strategies is critical for improving the care of older patients.
Acknowledgements
We would like to thank the participating patients and team of data collectors. This work was supported by the Alvin J. Siteman Cancer Center, the Barnes Jewish Hospital Foundation and the Divisions of Public Health Sciences and Emergency Medicine at the Washington University School of Medicine.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.U.S. Department of Health and Human Services Healthy People 2020. 2011 http://www.healthypeople.gov/2020/about/default.aspx.
- 2.Baker DW, Parker RM, Williams MV, Pitkin K, Parikh N, Coates W, Imara M. The health care experiences of patients with low literacy. Arch Fam Med. 1996;5:329–334. doi: 10.1001/archfami.5.6.329. [DOI] [PubMed] [Google Scholar]
- 3.Berkman N, Pignone MP, DeWalt D, Sheridan S. Health literacy: Impact on health outcomes. Agency for Healthcare Research and Quality; Rockville, MD: 2004. [Google Scholar]
- 4.Gazmararian JA, Williams M, Peel J, Baker D. Health literacy and knowledge of chronic disease. Patient Educ Couns. 2003;51:267–275. doi: 10.1016/s0738-3991(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen-Bohlman L, Panzer AM, Kindig DA, editors. Health literacy: A prescription to end confusion. National Academies Press; Washington, DC: 2004. [PubMed] [Google Scholar]
- 6.Parker RM, Baker DW, Williams MV, Nurss JR. The Test of Functional Health Literacy in Adults: A new instrument for measuring patients' literacy skills. J Gen Intern Med. 1995;10:537–541. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- 7.Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA. Quick assessment of literacy in primary care: the Newest Vital Sign. Ann Fam Med. 2005;3:514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss BD, Hart G, McGee DL, D'Estelle S. Health status of illiterate adults: Relation between literacy and health status among persons with low literacy skills. J Am Board Fam Pract. 1992;5:257–264. [PubMed] [Google Scholar]
- 9.Weiss BD, Palmer R. Relationship between health care costs and very low literacy skills in a medically needy and indigent Medicaid population. J Am Board Fam Med. 2004;17:44–47. doi: 10.3122/jabfm.17.1.44. [DOI] [PubMed] [Google Scholar]
- 10.Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd R. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175–184. doi: 10.1111/j.1525-1497.2005.40245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazmararian JA, Baker DW, Williams MV, Parker RM, Scott TL, Green DC, Fehrenbach SN, Ren J, Koplan JP. Health literacy among Medicare enrollees in a managed care organization. JAMA. 1999;281:545–551. doi: 10.1001/jama.281.6.545. [DOI] [PubMed] [Google Scholar]
- 12.Williams MV, Parker RM, Baker DW, Coates W, Nurss J. Inadequate functional health literacy among patients at two public hospitals. JAMA. 1995;274:1677–1682. [PubMed] [Google Scholar]
- 13.Kutner M, Greenberg E, Jin Y, Paulsen C, White S. The health literacy of America's adults: Results from the 2003 National Assessment of Adult Literacy. National Center for Education Statistics; Washington, DC: 2006. [Google Scholar]
- 14.Morrow D, Clark D, Tu W, Wu J, Weiner M, Steinley D, Murray MD. Correlates of health literacy in patients with chronic heart failure. The Gerontologist. 2006;46:669–676. doi: 10.1093/geront/46.5.669. [DOI] [PubMed] [Google Scholar]
- 15.Benson JG, Forman WB. Comprehension of written health care information in an affluent geriatric retirement community: use of the Test of Functional Health Literacy. Gerontology. 2002;48:93–97. doi: 10.1159/000048933. [DOI] [PubMed] [Google Scholar]
- 16.Baker DW, Gazmararian JA, Sudano J, Patterson M. The association between age and health literacy among elderly persons. J Gerontol. 2000;55B:S368–S374. doi: 10.1093/geronb/55.6.s368. [DOI] [PubMed] [Google Scholar]
- 17.Holsinger T, Deveau J, Boustani M, Williams J,JW. Does this patient have dementia? JAMA. 2007;297:2391–2404. doi: 10.1001/jama.297.21.2391. [DOI] [PubMed] [Google Scholar]
- 18.Baker DW, Gazmararian JA, Sudano J, Patterson M, Parker RM, Williams MV. Health literacy and performance on the Mini-Mental State Examination. Aging & Mental Health. 2002;6:22–29. doi: 10.1080/13607860120101121. [DOI] [PubMed] [Google Scholar]
- 19.Federman AD, Sano M, Wolf MS, Siu AL, Halm EA. Health literacy and cognitive performance in older adults. J Am Geriatr Soc. 2009;57:1475–1480. doi: 10.1111/j.1532-5415.2009.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker DW, Parker RM, Williams MV, Clark WS, Nurss JR. The relationship of patient reading ability to self-reported health and use of health services. Am J Public Health. 1997;87:1027–1030. doi: 10.2105/ajph.87.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13:791–798. doi: 10.1046/j.1525-1497.1998.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang U, Shah MN, Han JH, Carpenter CR, Siu AL, Adams JG. Transforming emergency care for older adults. Health Aff. 2013;32:1–7. doi: 10.1377/hlthaff.2013.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hustey F, Meldon S. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39:248–253. doi: 10.1067/mem.2002.122057. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter CR, Bassett ER, Fischer GM, Shirshekan J, Galvin JE, Morris JC. Four sensitive screening tools to detect cognitive dysfunction in geriatric emergency department patients: Brief Alzheimer's Screen, Short Blessed Test, Ottawa 3DY, and the Caregiver-completed AD8. Acad Emerg Med. 2011;18:374–384. doi: 10.1111/j.1553-2712.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han JZ, Zimmerman EE, Cutler N, Schnelle J, Morandi A, Dittus RS, Storrow AB, Ely EW. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16:193–200. doi: 10.1111/j.1553-2712.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss JR. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38:33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 27.Davis TC, Crouch MA, Long SW, Jackson RH, Bates P, George RB, Bairnsfather LE. Rapid assessment of literacy levels of adult primary care patients. Fam Med. 1991;23:433–435. [PubMed] [Google Scholar]
- 28.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, Crouch MA. Rapid Estimate of Adult Literacy in Medicine: A shortened screening instrument. Fam Med. 1993;25:391–395. [PubMed] [Google Scholar]
- 29.Osborn CY, Weiss BD, Davis TC, Skripkauskas S, Rodriguez C, Bass PF, Wolf MS. Measuring adult literacy in health care: Performance of the Newest Vital Sign. Am J Health Behav. 2007;31:S36–S46. doi: 10.5555/ajhb.2007.31.supp.S36. [DOI] [PubMed] [Google Scholar]
- 30.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, VanRyn M. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23:561–566. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127:966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute Health Information National Trends Survey. 2007 http://hints.cancer.gov/questions/index.jsp.
- 34.Mendiondo MS, Ashford JW, Kryscio RJ, Schmitt FA. Designing a Brief Alzheimer Screen (BAS). J Alzheimer's Dis. 2003;5:391–398. doi: 10.3233/jad-2003-5506. [DOI] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State Examination. doi: 10.1016/0022-3956(75)90026-6. http://www4.parinc.com/Products/Product.aspx?ProductID=MMSE. [DOI] [PubMed]
- 37.Carpenter CR. Does this patient have dementia? Ann Emerg Med. 2008;52:554–556. doi: 10.1016/j.annemergmed.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization [10/19/13];Definition of an older or elderly person. http://www.who.int/healthinfo/survey/ageingdefnolder/en/print.html.
- 39.Jolles J. Cognitive, emotional and behavioral dysfunctions in aging and dementia. In: Swaab DF, Fliers E, Mirmiran M, Van Gool WA, Van Haaren F, editors. Progress in Brain Research. Vol. 70. Elsevier Science Publishers; 1986. pp. 15–39. [DOI] [PubMed] [Google Scholar]
- 40.van Hooren SAH, Valentjin AM, Bosma H, Ponds RWHM, van Boxtel MPJ, Jolles J. Cognitive functioning in healthy older adults aged 64-81: a cohort study into the effects of age, sex, and education. Aging, Neuropsychology, and Cognition. 2007;14:40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- 41.Wolf MS, Curtis LM, Wilson EAH, Revelle W, Waite KR, Smith SG, Weintraub S, Borosh B, Rapp DN, Park DC, Deary IC, Baker DW. Literacy, cognitive function, and health: Results of the LitCog Study. J Gen Intern Med. 2012;27:1300–1307. doi: 10.1007/s11606-012-2079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker DW, Wolf MS, Feinglass J, Thompson JA. Health literacy, cognitive abilities, and mortality among elderly persons. J Gen Intern Med. 2008;23:723–726. doi: 10.1007/s11606-008-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudore RL, Yaffe K, Satterfield S, Harris TB, Mehta KM, Simonsick EM, Newman AB, Rosano C, Rooks R, Rubin SM, Ayonayon HN, Schillinger D. Limited literacy and mortality in the elderly: The Health, Aging, and Body Composition study. J Gen Intern Med. 2006;21:806–812. doi: 10.1111/j.1525-1497.2006.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manly JJ, Touradji P, Tang M-X, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25:680–690. doi: 10.1076/jcen.25.5.680.14579. [DOI] [PubMed] [Google Scholar]
- 45.Cairns CB, Maier RV, Adeoye O, Bapiste D, Barsan WG, Blackbourne L, Burd R, Carpenter C, Chang D, Cioffi W, Cornwell E, Dean JM, Dyer C, Jaffe D, Manley G, Meurer WJ, Neumar R, Silbergleit R, Stevens M, Wang M, Weiner D, Wright D. NIH roundtable on emergency trauma research. Ann Emerg Med. 2010;56:538–550. doi: 10.1016/j.annemergmed.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Kirk JK, Grzywacz JG, Arcury TA, Ip EH, Nguyen HT, Bell RA, Saldana S, Quandt SA. Performance of health literacy tests among older adults with diabetes. J Gen Intern Med. 2011;27:534–540. doi: 10.1007/s11606-011-1927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carpenter CR, Kaphingst KA, Goodman M, Lin MJ, Melson A, Griffey RT. Feasibility and diagnostic accuracy of brief health literacy and numeracy screening instruments in an urban emergency department. Acad Emerg Med. doi: 10.1111/acem.12315. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayeaux EJ, Jr., Davis TC, Jackson RH, Henry D, Patton P, Slay L, Sentell T. Literacy and self-reported educational levels in relation to Mini-mental State Examination scores. Fam Med. 1995;27:658–662. [PubMed] [Google Scholar]
- 49.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 50.Carpenter C, Palatnik M, Koch S, Skebba T, Twaalfhoven E, Godbout A, Morris JC. Detecting mild cognitive impairment in geriatric emergency department patients: prevalence and diagnostic accuracy of three brief screening instruments. Acad Emerg Med. 2011;18:S95. [Google Scholar]
- 51.Wong C, Holroyd-Leduc J, Simel D, Straus S. Does this patient have delirium? Value of bedside instruments. JAMA. 2010;304:779–806. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 52.Mancuso JM. Assessment and measurement of health literacy: An integrative review of the literature. Nursing and Health Sciences. 2009;11:77–89. doi: 10.1111/j.1442-2018.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 53.Baker DW. The meaning and the measure of health literacy. J Gen Intern Med. 2006;21:878–883. doi: 10.1111/j.1525-1497.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams MV, Baker DW, Parker RM, Coates W, Nurss JR. The impact of inadequate functional health literacy on patients' understanding of diagnosis, prescribed medications, and compliance. Acad Emerg Med. 1995;2:386. [Google Scholar]
- 55.Petersen R, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 56.DeWalt DA, Callahan LF, Hawk V, Broucksou KA, Hink A, Rudd RE, et al. Health literacy universal precautions toolkit. Agency for Healthcare Research and Quality; Rockville, MD: 2010. [Google Scholar]