Abstract
Introduction
Several supplementation studies with long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) describe an increase of EPA-derived hydroxy, epoxy and dihydroxy fatty acids in blood, while changes in levels of other LC n-3 and n-6 PUFA-derived oxylipins were minor. In order to investigate the kinetics of changes in oxylipin levels in response to LC n-3 PUFA ingestion, we conducted a single dose treatment study with healthy subjects.
Subjects and methods
In the present kinetic study, we compared patterns of hydroxy, epoxy and dihydroxy fatty acids in plasma of 6 healthy men before and after 6, 8, 24, and 48 h of fish oil (1008 mg EPA and 672 mg DHA) ingestion. Levels of EPA- as well as other LC PUFA-derived hydroxy, epoxy and dihydroxy fatty acids were analyzed in plasma by LC–MS. Additionally, levels of these oxylipins were compared with their parent PUFA levels in plasma phospholipids.
Results
All EPA-derived oxylipin levels were significantly increased 6 h after LC n-3 PUFA ingestion and gradually drop thereafter reaching the baseline levels about 48 h after treatment. The relative increase in EPA plasma phospholipid levels highly correlated with the increase of plasma EPA-derived oxylipin levels at different time points. In contrast, plasma levels of arachidonic acid- and DHA-derived oxylipins as well as parent PUFA levels in plasma phospholipids were hardly changed.
Discussion and conclusions
Our findings demonstrate that a single dose of LC n-3 PUFAs can rapidly induce a shift in the EPA oxylipin profile of healthy subjects within a few hours. Taking the high biological activity of the EPA-derived epoxy fatty acids into account, even short-term treatment with LC n-3 PUFAs may cause systemic effects, which warrant further investigation.
Keywords: Oxylipins, Eicosanoids, Arachidonic acid, Eicosapentaenoic acid
1. Introduction
Long-chain omega-3 and omega-6 polyunsaturated fatty acids (LC n-6 and n-3 PUFA) are precursors for oxygenated metabolites. These oxylipins function as bioactive lipid mediators regulating inflammation, pain, cell proliferation, apoptosis, tissue repair, blood coagulation, blood vessel permeability and other biological functions [1,2]. It is believed that many actions of the LC n-3 PUFA eicosapentaenoic acid (EPA C20:5 n-3) and docosahexaenoic acid (DHA C22:6 n-3) are mediated by their corresponding oxidation products [3–5]. Oxidized PUFA metabolites originate from enzymatically catalyzed reactions involving cyclooxygenases [6] e.g. the large class of prostaglandines, peroxidation by lipoxygenases (5-LOX, 12-LOX and 15-LOX) [7,8], as well as hydroxylation and epoxidation by cytochrome p450 enzymes (CYPs, e.g. CYP2J2) [9,10]. Moreover, several of these lipid mediators are formed in multistep reactions, such as dihydroxy FA by CYP mediated formation of epoxides and subsequent hydrolysis by soluble epoxide hydrolase (sEH) [11] or generation of different prostaglandins by COX and several other enzymes i.e. PGE2-synthases [2]. In addition, autoxidation can also form a multitude of LC n-3 and n-6 PUFA metabolites [12–14].
While the formation and biology of arachidonic acid (AA C20:4n-6) derived oxylipins, especially potent prostanoids such as PGE2 [2], has been intensively studied during the past 40 years, fewer information is available about the formation, biological role and potency of hydroxy, epoxy and dihydroxy FA from EPA and DHA. Recent studies have shown that particularly epoxy FA from LC n-3 PUFA act as lipid mediators which could be responsible for some of the cardio-protective effects attributed to LC n-3 PUFA [15–17]. For example, the epoxides 17(18)-EpETE and 19(20)-EpDPE possess potent anti-arrhythmic [15], vasodilatory [16] and anti-thrombotic effects [17]. Moreover, it was recently shown, that DHA derived epoxides inhibit angiogenesis, tumor growth, and metastasis [18].
To better understand the biological role of hydroxy, epoxy and dihydroxy FA in health and disease, it is important to study the biosynthetic pathways of LC PUFA and these oxylipins in response to an increased intake of LC n-3 PUFA via the diet. A few human pilot studies showed that supplementation with LC n-3 PUFA over a few weeks results in increased total (sum of free and esterified) plasma levels of EPA and DHA oxylipins as well as free EPA and DHA oxylipin levels in serum [19–22]. The shift in circulating EPA and DHA oxylipins appears to be dependent on the initial LC n-3 PUFA status particularly for EPA [20]. Furthermore, the levels of AA-derived hydroxy, epoxy and dihydroxy FA were also modulated in response to LC n-3 PUFA treatment [19,20]. Many questions on the formation of oxylipins in response to LC n-3 PUFA treatment remain to be answered. For example, duration of treatment and required dose to affect hydroxy, epoxy and dihydroxy FA patterns is unknown. There are also no data on the kinetics of hydroxy, epoxy and dihydroxy FA following LC n-3 PUFA ingestion.
In the present study we examined the effect of a single LC n-3 PUFA dose on hydroxy, epoxy, and dihydroxy FA patterns in plasma (sum of free and esterified oxylipins) of six healthy subjects at different time points. Moreover, we compared changes in hydroxy, epoxy, and dihydroxy FA levels with changes in parent PUFA levels in plasma phospholipids, a marker for the individual PUFA status.
2. Materials and methods
This investigator initiated study was designed and conducted according to the principles of the Good Clinical Practice Guidelines laid down in the Declaration of Helsinki and was approved by an ethics committee Freiburg Ethics Commission International.
2.1. Subjects
Healthy young men were recruited from the general population by an advertisement. The inclusion criteria for participating in the study were male gender, an age between 20 and 50 years and a body mass index (BMI) between 20 and 28 kg/m2. Diagnosis or suspicion of gastrointestinal disorders, high intake of oily fish (>2 times per week), intake of dietary supplements (e.g. n-3 PUFA, phytosterols, polyglucosamin) and intake of lipid-lowering drugs two month before and during intervention were defined as exclusion criteria. Inclusion and exclusion criteria were assessed via structured questionnaires. Subjects were instructed to avoid foods rich in n-3 PUFA 4 weeks before the intervention day to minimize variability in LC-PUFA status and blood levels. Restricted foods included fish, seafood, and alpha-linolenic acid (ALA, 18:3n-3)-rich vegetable oils such as linseed oil. Six healthy male volunteers were included in the study. The study collective (mean age 33 ± 5 years) was slightly overweight (25 ± 2.7 kg/m2) and showed a normal serum lipid profile (Table 1). All included subjects gave their written informed consent to take part in the study.
Table 1.
Subjects characteristics and fasting serum lipid levels at baseline.
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Age [years] | 33 ± 4.7 | 26 | 36 |
| Weight [kg] | 81.1 ± 12.1 | 67.7 | 99.4 |
| BMI [kg/m2] | 25 ± 2.7 | 22 | 29 |
| TC [mmol/l] | 5.1 ± 0.9 | 3.9 | 6.4 |
| TAG [mmol/l] | 1.5 ± 0.6 | 1.0 | 3.0 |
| LDL [mmol/l] | 2.9 ± 0.8 | 2.0 | 3.9 |
| HDL [mmol/l] | 1.5 ± 0.2 | 1.1 | 1.8 |
| LDL/HDL quotient | 1.9 ± 0.4 | 1.5 | 2.5 |
TC, total cholesterol; TAG, triacylglycerides.
2.2. Study design
During the intervention day, subjects ingested fish oil capsules at 7:00 a.m. after an overnight fast. Each capsule contained 252 mg EPA and 168 mg DHA as re-esterified triacylglycerides obtained from Dr. Loges + Co. GmbH (Winsen, Germany), a pharmaceutical company. Subjects were instructed to ingest four capsules resulting in a total dose of 1008 mg EPA and 672 mg DHA. Capsules were given together with a standardized breakfast that contained 30.1 g of fat, 29.6 g of protein, 67.9 g of carbohydrate, and 2.7 MJ of energy. The total fat intake (breakfast + capsules) was 33.5 g. During the intervention day, subjects consumed standardized meals over 24 h, which were personally adjusted according to weight. Meals were consumed 3, 5, 7, 10, 12, and 24 h after capsule ingestion. Blood samples were taken initially (predose at baseline) and 6, 8, 24, and 48 h after capsule intake. The subjects were allowed to drink water during the intervention day, no further food or other drinks were consumed.
2.3. Blood sample collection
Plasma samples were obtained by venipuncture of an arm vein into EDTA-monovettes (Sarstedt, Germany). For plasma preparation, blood samples were directly centrifuged (2000 g, 10 min, 10 °C), and plasma taken off. Serum samples were obtained by venipuncture of an arm vein into 10 ml BD Vacutainer Blood Collection Tubes (Becton Dickinson, Heidelberg, Germany). After 30 min incubation at room temperature, tubes were centrifuged for 10 min at 2000 × g and serum was transferred into 15 mL falcon tubes (Becton Dickinson). Prepared samples were stored at −80 °C upon analysis or shipment to external laboratory, where samples were shipped to on ice. Serum lipid levels were determined by the LADR laboratory, Hannover, Germany.
2.4. Fatty acid and oxylipin analyses in plasma
Plasma phospholipid FA composition was analyzed in an external laboratory (Omegametrix GmbH, München, Germany) as described previously [23] with minor modifications. Lipids were extracted using the method of Bligh and Dyer (1959) [24], and phospholipids were purified by use of a Sep-Pak C-18 mini cartridge (Waters, Eschborn, Germany). FA methyl esters were formed by acid hydrolysis, and were analyzed by capillary gas–liquid chromatography with flame ionization detection (FID) on a GC2010 Gas Chromatograph (Shimadzu, Duisburg, Germany) equipped with a SP2560, 100-m column, (Supelco, Bellefonte, PA) using hydrogen as carrier gas. FA were identified by comparison with a FA standard mixture. Results are expressed as % of total FA in plasma PL.
In order to determine all hydroxy, epoxy and dihydroxy FAs present in plasma including those esterified in triglycerides, e.g. in lipoproteins [25], bound oxylipins were liberated by saponification as described [26]. For saponification, 250 μl plasma were mixed with an equal volume of MeOH. After addition of 300 μL aqueous sodium hydroxide (10 M) the samples were incubated at 60 °C for 1 h. The samples were placed on ice and neutralized by addition of 275 μL acetic acid (50%). The pH of each solution was checked and adjusted to 6–7 and the samples were centrifuged (20,000 × g 10 min 4 °C) and extracted by solid phase extraction and quantified by liquid chromatography–electrospray ionization tandem mass spectrometry (LC–ESI-MS) as described [27].
2.5. Data analysis and statistics
Results for anthropometrical measures and serum lipid levels are presented as mean ± SD (Table 1), while phospholipid FA levels and oxylipin levels in plasma are presented as mean ± SE. Changes in the variables (v) were calculated for each time point (x) as Δ%, calculated by: Δ% = 100 * (vtx − vt0)/vt0. All variables of the sample sets were analyzed for normal distribution by the Kolmogorov–Smirnov test. Differences between baseline (t0) levels and different time points after capsule ingestion (t6, t8, t24, t48) were analyzed by ANOVA for repeated measurements with acceptance of statistical significance at p < 0.05. To determine statistical significance between baseline levels and each time point, t-tests for paired samples after Bonferroni correction were carried out. All statistical analyses were carried out with SPSS software (Version 21, SPSS Inc., Chicago, IL, USA).
3. Results
The LC n-3 PUFA capsules were well tolerated. Blood samples were drawn from all subjects at all six time points. All variables were normally distributed.
3.1. Fatty acid composition of plasma phospholipids
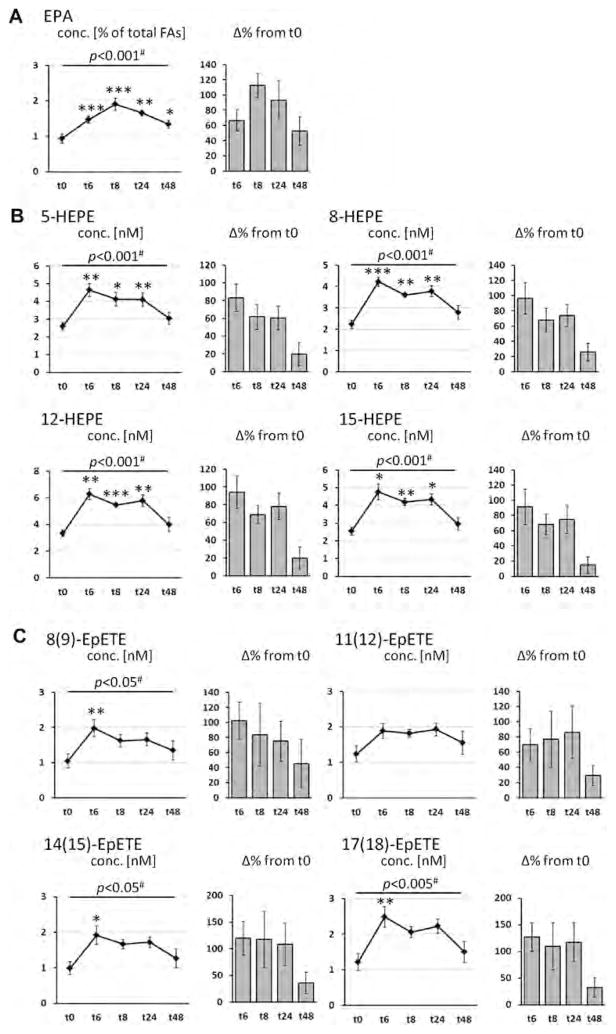
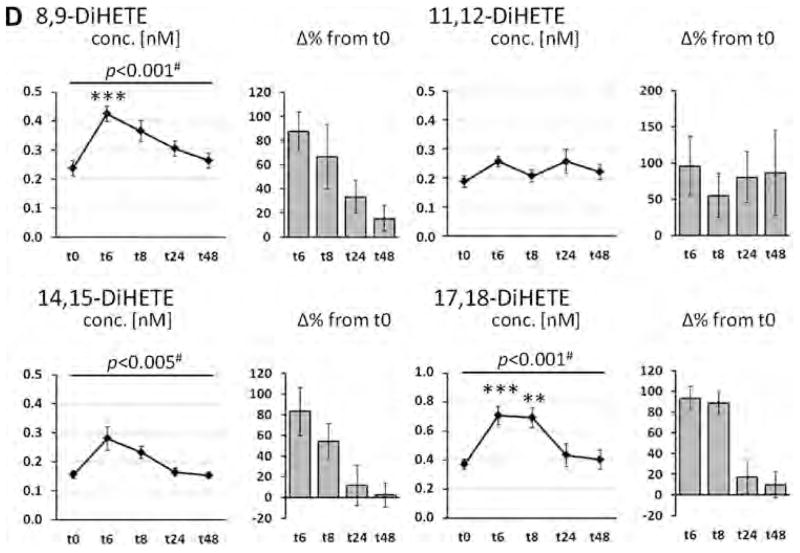
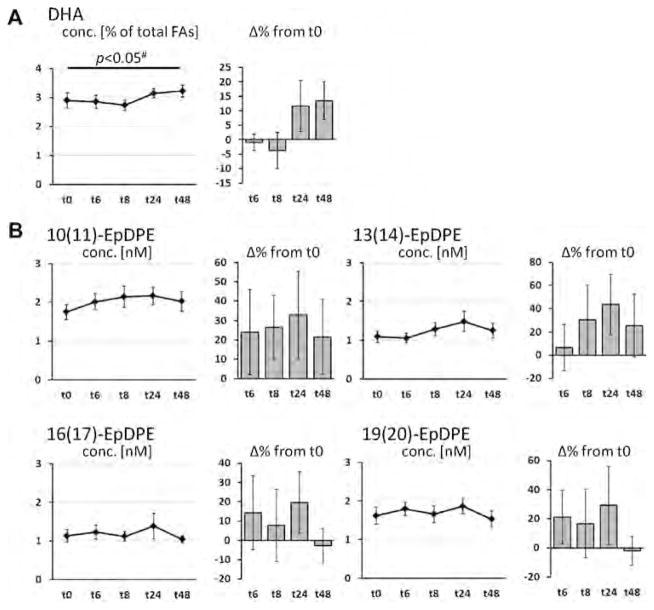
The course of LC-PUFA levels in plasma phospholipid as well as levels of corresponding hydroxy, epoxy and dihydroxy FA in plasma are shown in Fig. 1 (EPA), Fig. 2 (DHA), and Fig. 3 (AA). EPA levels were significantly increased with treatment at all time points and showed a u-shaped profile (Fig. 1A). Highest EPA levels - with an increase by 113% from baseline - were observed 8 h after LC n-3 PUFA capsule ingestion. Whereas ALA levels were significantly decreased (Table S1), DHA levels decreased initially and increased 24 and 48 h after LC n-3 PUFA treatment (Fig. 2A). Neither linoleic acid (LA, 18:2n-6) levels (Table S1) nor AA levels (Fig. 3A) were altered by LC n-3 PUFA treatment. Changes in LA, AA and DHA content in plasma lipids were not significant.
Fig. 1.
Change of the concentration of eicosapentaenoic acid (EPA, C20:5n-3) in plasma phospholipids and EPA-derived oxylipins in plasma after a single dose LC n-3 PUFA treatment. For each analyte the concentration at different time points (t0–t48) as well as the relative-change compared to baseline (t0) is presented. (A) Relative EPA content in plasma phospholipids; (B–D) concentration of oxylipins in plasma: (B) hydroxy FA, (C) epoxy FA and (D) dihydroxy FA. All results are shown as mean ± SE. #By ANOVA for repeated measurements, *p < 0.0125, **p < 0.005, ***p < 0.001 by t-tests for paired samples.
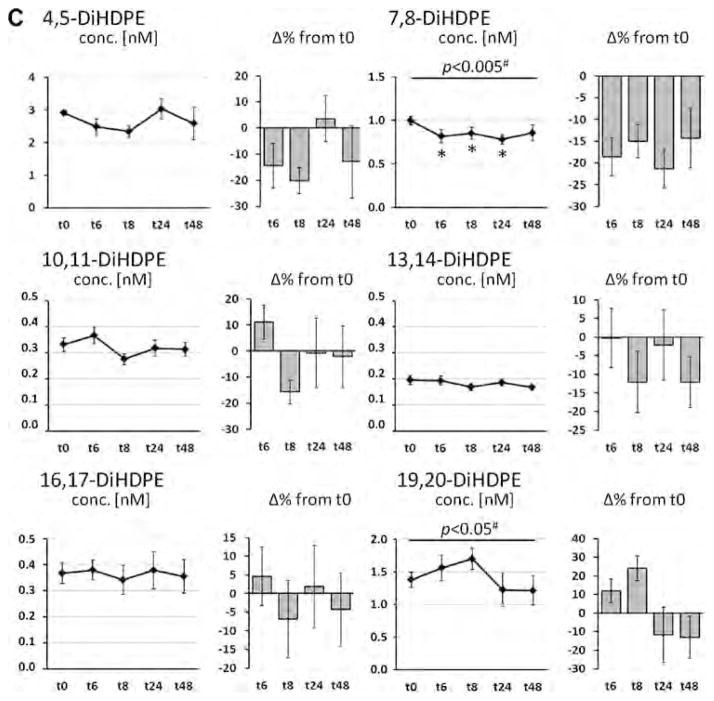
Fig. 2.
Change of the concentration of docosahexaenoic acid (DHA, C22:6n-3) in plasma phospholipids and DHA-derived oxylipins in plasma after a single dose LC n-3 PUFA treatment. For each analyte the concentration at different time points (t0–t48) as well as the relative-change compared to baseline (t0) is presented. (A) Relative DHA content in plasma phospholipids; (B–D) concentration of oxylipins in plasma: (B) hydroxy FA, (C) epoxy FA and (D) dihydroxy FA. All results are shown as mean ± SE. #By ANOVA for repeated measurements, *p < 0.0125, **p < 0.005, ***p < 0.001 by t-tests for paired samples.
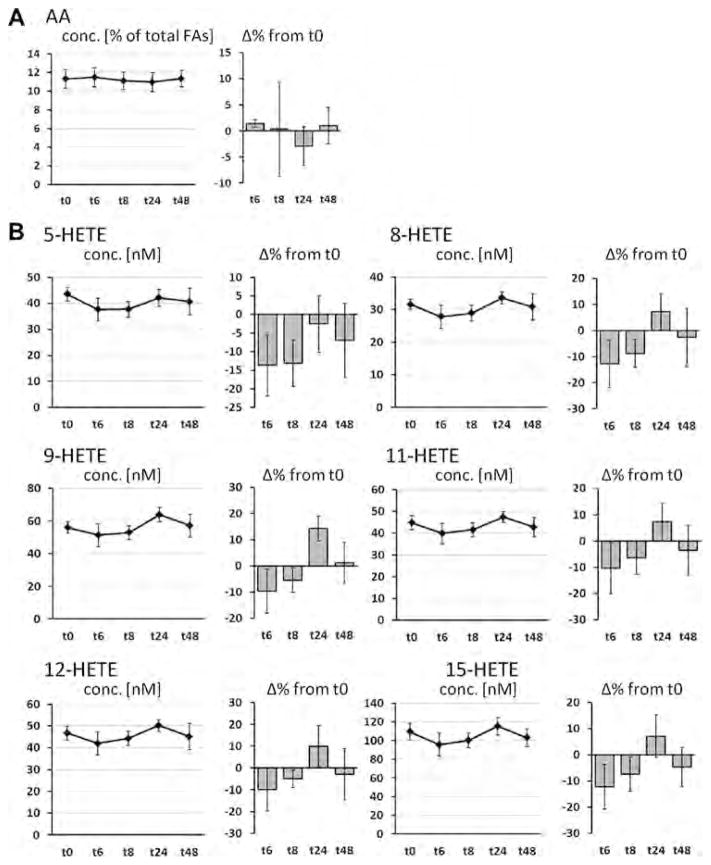
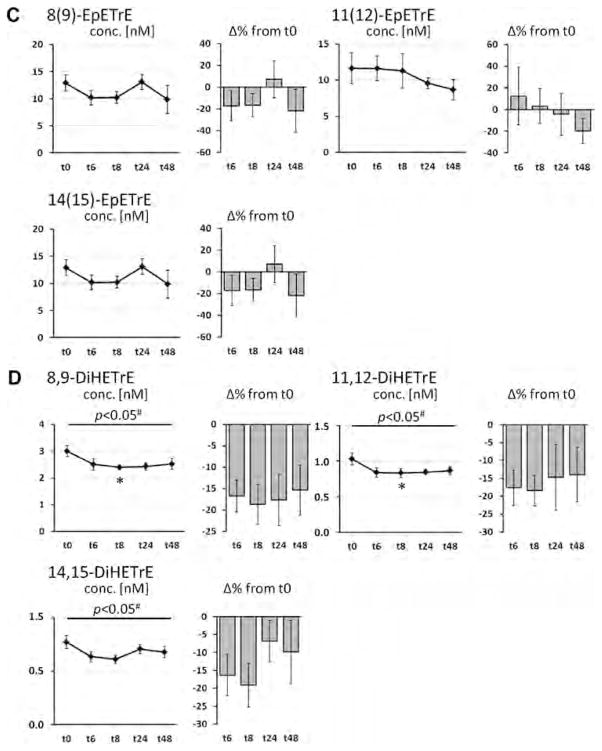
Fig. 3.
Change of the concentration of arachidonic acid (AA, C20:4n-6) in plasma phospholipids and AA-derived oxylipins in plasma after a single dose LC n-3 PUFA treatment. For each analyte the concentration at different time points (t0–t48) as well as the relative-change compared to baseline (t0) is presented. (A) Relative AA content in plasma phospholipids; (B–D) concentration of oxylipins in plasma: (B) hydroxy FA, (C) epoxy FA and (D) dihydroxy FA. All results are shown as mean ± SE. #By ANOVA for repeated measurements, *p < 0.0125, **p < 0.005, ***p < 0.001 by t-tests for paired samples.
3.2. Plasma oxylipin levels
Levels of total hydroxy, epoxy and dihydroxy FA after liberation of ester-bound oxylipins at baseline and the different time points are shown in Figs. 1–3(B–C) and Table S2–S6. All EPA-derived hydroxy, epoxy and dihydroxy FA levels were significantly increased (> 80% except 11(12)-EpETE) 6 h after LC n-3 PUFA ingestion and gradually declined thereafter. Compared to baseline, levels of all EPA oxylipins were still significantly increased by >50% 8 and 24 h post-treatment (except 11,12-DiHETE and 14,15-DiHETE at t8 and 8,9-DiHETE, 14,15-DiHETE and 17,18-DiHETE at t24), while concentrations approximately reach baseline again after 48 h. The relative increase in EPA plasma phospholipid levels highly correlated with the increase of plasma EPA-derived oxylipin levels at different time points (Table S7). Regarding the absolute concentration, the levels of HEPEs were increased up to 6.27 ± 0.41 nM (12-HEPE, t6), of EpETEs up to 2.48 ± 0.29 nM (17(18)-EpETE, t6) and of DiHETEs up to 0.71 ± 0.06 nM (17,18-DiHETE, t6). These levels were 5–20 fold lower than the AA derived oxylipin concentrations at baseline ranging between 30 and 100 nM for HETEs, 11–13 nM for EpETrEs and 0.8–3.0 nM for DiHETrEs (Fig. 1, Table S1).
The supplementation with LC n-3 PUFA hardly changed levels of AA- and DHA-derived oxylipins, similar to the parent FA levels in plasma PL. Except 11(12)-EpETrE all AA-derived hydroxy and epoxy FA showed a similar profile in the course of time: During time point t6 and t8, levels were decreased and slightly elevated at t24 and dropped again at t48. AA-derived dihydroxy FA were generally lowered in comparison to baseline levels. However, with a few exceptions all described changes in concentrations were not significant.
DHA-derived epoxy FA were slightly elevated (10–40%) during all four time points, however, variations between subjects were high and changes were not significant. The differences of DHA-derived dihydroxy FA levels were inconsistent (increase and decrease) between different oxylipins with inter-individual variations and no significant changes of concentrations were observed. Only 7,8-DiHDPE showed a decrease in concentrations (15–20%) at all time points, which was significant at t6, t8 and t24.
4. Discussion
Dietary long-chain (LC) n-3 PUFA have gained increasing attention as preventive and therapeutic agents in chronic inflammatory diseases such as rheumatoid arthritis [28], neuropsychiatric diseases [29,30], atherosclerosis and cardiovascular diseases [31,32]. However, the underlying molecular mechanisms by which LC n-3 PUFA exert their effects are versatile and not completely understood. It is believed that many actions of LC n-3 PUFA are mediated by their bioactive lipid metabolites [3–5]. In the mammalian body LC PUFA undergo oxygenation to form oxylipins–including the important class of eicosanoids (C20), which serve as important lipid mediators in multiple physiological and pathophysiological processes [2]. Only few studies investigated the effect of LC n-3 PUFA supplementation on patterns of hydroxy, epoxy and dihydroxy FA in human blood [19–22]. Following a long-term treatment of several weeks, in all studies a considerable increase of EPA-derived hydroxy, epoxy and dihydroxy FA levels were observed, while the increase of DHA-metabolites was minor. Moreover, the LC n-3 PUFA supplementation caused an inconsistent reduction of ALA- and AA-derived oxylipin levels [19–22]. No information about the short time effects of LC n-3 PUFA supplementation on the oxylipin pattern or the kinetics of the observed shifts are available.
The findings of the present study demonstrate that even a single dose of an LC n-3 PUFA rich fish oil can induce a shift in the hydroxy, epoxy and dihydroxy FA profile of healthy subjects within a few hours. The determined levels of all EPA-derived metabolites were significantly elevated. The shift in EPA oxylipins highly correlated with the increase of relative EPA levels in plasma phospholipids suggesting that the formation of hydroxy, epoxy and dihydroxy EPA directly depends on the substrate. The concentration of 5-HEPE, 12-HEPE and 15-HEPE, which can be formed by 5-LOX, 12-LOX and 15-LOX respectively [27], were increased by 80–100%. Interestingly, 8-HEPE, for which no enzymatically formation is described so far, was elevated to the same extend, suggesting that autoxidation plays an important role for the observed formation of the HEPEs. The epoxy-EPAs and dihydroxy-EPAs were elevated relatively to the same extend reaching a maximal concentration of 2–3 nM (Fig. 1). Here, the formation of 17(18)-EpETE and its hydrolysis product 17,18-DiHETE prevailed over the formation of the other regioisomers. This is well in line with preference of the CYP2J and CYP2 for epoxidizing the double bound at position n-3 [26], indicating that these metabolites are formed enzymatically in the CYP pathway of the AA cascade. Several CYP enzymes prefer DHA and EPA as substrate, for example the turnover rates CYP2J2 are 4-fold (DHA) to 17-fold (EPA) higher compared to AA [26]. This might also explain why the relative increase in the EPA epoxides is more pronounced than in the EPA levels (Fig. 1). However, based on this data, the route of formation of the formed hydroxy, epoxy and dihydroxy FA cannot be elucidated. Thus, all formation routes have to be considered, which are, for example, for the class of epoxides aside from CYP action, hemoglobin catalyzed formation and autoxidation [33].
Regardless the route of formation, the strong increase of these oxylipins could be of biological relevance, since n-3 epoxy FA such as 17(18)-EpETE act as potent cardio-protective and analgesic mediators. Because hydroxy, epoxy and dihydroxy FA levels in plasma were analyzed after saponification, it is also impossible to distinguish, whether the plasma concentration of the free or esterified EPA-derived hydroxy, epoxy and dihydroxy FA are elevated. From animal studies it is known that >90% of whole plasma hydroxy, epoxy and dihydroxy FA, particularly, the epoxy FA are esterified in cell lipids such as phospholipids and triglycerides [34,35]. However, comparing free serum and plasma oxylipin levels of healthy human subjects with those after conjugated cleavage, several free n-6 and n-3 oxylipins, particularly dihydroxy FA, were observed in the same range [27]. Thus, the increase of EPA-derived hydroxy, epoxy, and dihydroxy FA levels, as observed in this study, might be the result of an increase of both free and esterified oxylipins.
In contrast to EPA, changes in relative DHA and AA levels in plasma phospholipids as well as corresponding plasma oxylipin concentrations were minor and inconsistent. Although subjects ingested a dose of 672 mg DHA, the changes in relative DHA levels in plasma phospholipids were negligible and only tend to increase after 24 and 48 h (not significant). Beside a lower dose compared to EPA (1008 mg), this can be most likely explained by the high level of DHA at baseline compared to EPA. The same reason is likely to be responsible for the unchanged levels of AA in plasma phospholipids. Initial AA levels in plasma phospholipids were 12-times higher compared to EPA. In long-term studies it is a well-known that EPA + DHA supplementation results in decreased AA blood levels [36]. However, the single dose of EPA + DHA in this study seems to be insufficient to reduce AA content in plasma phospholipids. However, although AA levels in plasma phospholipids were unchanged, there seems to be a trend for decreased AA oxylipin levels 6 and 8 h after capsule ingestion (significant for dihydroxy-AAs, Fig. 3). These findings might be the result of a displacement of AA as substrate for converting enzymes by EPA.
5. Conclusion
Our results demonstrate that plasma levels of EPA-derived oxylipins are induced within a few hours in response to a single treatment with LC n-3 PUFA suggesting a fast conversion to hydroxy, epoxy and dihydroxy FA metabolites. In contrast, the effect on DHA-levels in plasma phospholipids as well as DHA oxylipin levels in plasma was moderate and absent. Taking the high biological activity of n-3 epoxy FA into account, future studies should be taken out investigating the kinetics of the shifts in comprehensive oxylipin patterns in repeated LC n-3 PUFA supplementation over several days. Particularly, modulation of endogenous patterns of oxidized LC n-3 and n-6 PUFA in different compartments such as free and esterified oxylipins in phospholipids, triglycerides of lipoproteins as well as cell membranes in blood cells and tissues has to be elucidated.
Supplementary Material
Table S1: Concentration of LC PUFA in plasma phospholipids [in % of total fatty acids] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S2: Concentration of linoleic acid (LA, 18:2n-6) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S3: Concentration of arachidonic acid (AA, 20:4n-6) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S4: Concentration of alpha-linolenic acid (ALA, 18:3n-3) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S5: Concentration of eicosapentaenoic acid (EPA, 20:5n-3) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S6: Concentration of docosahexaenoic acid (DHA, 22:2n-3) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S7: Correlation between relative change of eicosapentaenoic acid (EPA, C20:5n-3) concentrations in plasma phospholipids and relative change in plasma concentrations of A) hydroxy FA, B) epoxy FA and C) dihydroxy FA. Only those oxylipins are presented, where a significant correlation (correlation coefficient ≥ 0.5 and p<0.05) was observed.
Acknowledgments
This study was supported by a Marie Curie Career Integration Grant to NHS, a Kekulé Ph.D. fellowship of the Fonds der Chemischen Industry to IW and a grants from US National Institutes of Health (NIH), Enviromental Health (NIEHS, P42 ES004699 and R01 ES002710) and Diabetes and Digestive and Kidney Diseases (NIDDK, U24 DK097154) and the West Coast Central Comprehensive Metabolomics Resource Core (WC3MRC) to BDH. BDH is a George and Judy Marcus Senior Fellow of the American Asthma Association. The provision of the fish oil supplement by Dr. Loges + Co. GmbH (Winsen, Germany) is kindly acknowledged. The authors are solely responsible for the design and conduct of the study, collection, management, analysis, and interpretation of the data, as well as preparation of the manuscript. All authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written. We would like to thank the participants who contributed their time to this project.
Abbreviations
- AA
arachidonic acid
- ALA
alpha-linoleic acid
- COX
cyclooxy-genase
- CYP
cytochrome P450
- DHA
docosahexaenic acid
- DiHDPE
dihydroxy docosapentaenoic acid
- DiHETE
dihydroxy eicosatetraenoic acid
- EPA
eicosapen-taenoic acid
- EpDPE
epoxy docosapentaenoic acid
- EpETE
epoxy eicosatetraenoic acid
- EpETrE
epoxy eicosatrienoic acid
- FA
fatty acid
- HEPE
hydroxy eicosapen-taenoic acid
- LC
long-chain
- LOX
lipoxygenase
- n-3
omega-3
- n-6
omega-6
- PL
phospholipids
- PUFA
polyunsaturated fatty acid
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.prostaglandins.2014.03.001.
References
- 1.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62(3):536–47. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 2.Buczynski MW, Dumlao DS, Dennis EA. Thematic review series: proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50(6):1015–38. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powell WS, Gravel S, Gravelle F. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J Lipid Res. 1995;36(12):2590–8. [PubMed] [Google Scholar]
- 4.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8(2):115–21. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Morisseau C, Inceoglu B, Schmelzer K, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50(Suppl):S29–34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuelsson B, Dahlén SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237(4819):1171–6. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 8.Rådmark O, Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. J Lipid Res. 2009;50(Suppl):S40–5. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82(1):131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 10.Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 2002;68–69:325–44. doi: 10.1016/s0090-6980(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 11.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111(10):5944–72. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 13.Spickett CM, Wiswedel I, Siems W, Zarkovic K, Zarkovic N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic Res. 2010;44(10):1172–202. doi: 10.3109/10715762.2010.498476. [DOI] [PubMed] [Google Scholar]
- 14.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34(2):171–80. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- 15.Westphal C, Konkel A, Schunck W. CYP-eicosanoids—a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011;96(1–4):99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Agbor LN, Walsh MT, Boberg JR, Walker MK. Elevated blood pressure in cytochrome P4501A1 knockout mice is associated with reduced vasodilation to omega-3 polyunsaturated fatty acids. Toxicol Appl Pharmacol. 2012;264(3):351–60. doi: 10.1016/j.taap.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung F, Schulz C, Blaschke F, et al. Effect of cytochrome P450-dependent epoxye-icosanoids on Ristocetin-induced thrombocyte aggregation. Clin Hemorheol Microcirc. 2012;52(2–4):403–16. doi: 10.3233/CH-2012-1614. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Panigrahy D, Mahakian LM, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci USA. 2013;110(16):6530–5. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51(8):2074–81. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res. 2012;53(8):1662–9. doi: 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundström SL, Yang J, Brannan JD, Haeggström JZ, Hammock BD, Nair P. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol Nutr Food Res. 2013;57(8):1378–89. doi: 10.1002/mnfr.201200827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuchardt JP, Schmidt S, Kressel G, et al. Modulation of blood oxylipin levels by long-chain omega-3 fatty acid supplementation in hyper- and normolipidemic men. Prostaglandins Leukot Essent Fatty Acids. 2014;90(2–3):27–37. doi: 10.1016/j.plefa.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaminski WE, Jendraschak E, Kiefl R, von Schacky C. Dietary omega-3 fatty acids lower levels of platelet-derived growth factor mRNA in human mononuclear cells. Blood. 1993;81(7):1871–9. [PubMed] [Google Scholar]
- 24.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Newman JW, Kaysen GA, Hammock BD, Shearer GC. Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J Lipid Res. 2007;48(8):1792–800. doi: 10.1194/jlr.M700146-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010;285(43):32720–33. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuchardt JP, Schmidt S, Kressel G, Dong H, Willenberg I, Hammock BD. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot Essent Fatty Acids. 2013;89(1):19–29. doi: 10.1016/j.plefa.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107(Suppl 2):S171–84. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- 29.Samieri C, Féart C, Letenneur L, et al. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008;88(3):714–21. doi: 10.1093/ajcn/88.3.714. [DOI] [PubMed] [Google Scholar]
- 30.Lin P, Su K. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056–61. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 31.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr. 2012;107(Suppl 2):S201–13. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–67. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Zhu AG, Mamczur M, et al. Hydrolysis of cis- and trans-epoxyeicosatrienoic acids by rat red blood cells. J Pharmacol Exp Ther. 2008;326(1):330–7. doi: 10.1124/jpet.107.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids. 2008;79(6):215–22. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shearer GC, Newman JW. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr Atheroscler Rep. 2009;11(6):403–10. doi: 10.1007/s11883-009-0061-3. [DOI] [PubMed] [Google Scholar]
- 36.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52(12):2265–72. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Concentration of LC PUFA in plasma phospholipids [in % of total fatty acids] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S2: Concentration of linoleic acid (LA, 18:2n-6) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S3: Concentration of arachidonic acid (AA, 20:4n-6) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S4: Concentration of alpha-linolenic acid (ALA, 18:3n-3) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S5: Concentration of eicosapentaenoic acid (EPA, 20:5n-3) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S6: Concentration of docosahexaenoic acid (DHA, 22:2n-3) derived oxylipins in plasma [nM] at baseline (t0) and different time points (t6-t48) following a single dose of LC n-3 PUFA treatment.
Table S7: Correlation between relative change of eicosapentaenoic acid (EPA, C20:5n-3) concentrations in plasma phospholipids and relative change in plasma concentrations of A) hydroxy FA, B) epoxy FA and C) dihydroxy FA. Only those oxylipins are presented, where a significant correlation (correlation coefficient ≥ 0.5 and p<0.05) was observed.