Abstract
Polyaromatic hydrocarbons, heterocyclic aromatic amines and dioxin-like compounds are environmental carcinogens shown to initiate cancer in a number of tissue types including prostate and breast. These environmental carcinogens elicit their effects through interacting with the aryl hydrocarbon receptor (AhR), a ligand activated transcription factor. Naturally occurring compounds found in fruits and vegetables shown to have anti-carcinogenic effects also interact with the AhR. This review explores dietary and environmental exposure to chemical carcinogens and beneficial natural compounds whose effects are elicited by the AhR.
Keywords: Aryl hydrocarbon receptor, Polyaromatic hydrocarbon, Dioxin-like compound, Heterocyclic aromatic amine, Flavonoid, Stilbene, Curcumin
Introduction
Environmental and dietary exposures are considered risk factors for prostate and breast cancer. Excluding basal cell and squamous cell skin cancers; prostate and breast cancers are the 2 most commonly diagnosed cancer types and account for 28% of all new cancer cases [1]. Compared to cancer incidence in 2003, there is a slight increase in the number of new prostate and breast cancer cases without any significant change in death rates [2]. The increase of new cancer cases may be attributed to exposure to chemical carcinogens.
The relationship between diet and cancer incidence has been a major topic of cancer prevention. Studies showing an association between meat intake and prostate cancers have been inconclusive. Some studies reveal red meat is positively associated with increased prostate cancer risk with an association with more aggressive disease states [3-5]. Despite some studies showing a 43% elevation in prostate cancer risk with high consumption of red meat, others show no association with prostate cancer risk [6-8]. There are also conflicting reports concerning the association of red meat and breast cancer. Both case control and cohort studies have revealed an increased risk of breast cancer associated with meat consumption [9,10]. Conversely, others show no association [11]. Although the role of red meat in prostate and breast cancer remain inconclusive, one explanation for the possible associations reported is the accumulation of carcinogens during the cooking process.
Joshi et al [12] showed that high fish intake was associated with an increased risk of advanced prostate cancer only when cooked at high temperatures. There was no increased risk for men who consumed fish cooked at low temperatures. Similar associations were found with red meat and poultry cooked at high temperatures. While poultry cooked at low temperatures showed an association with decreased prostate cancer risk [13]. Furthermore, data obtained via food frequency questionnaires revealed that consumption of French fries, fried chicken and fried fish at least once a week was associated with an increased risk of prostate cancer [14]. Consumption of well-done red meat was also associated with a significantly elevated risk of breast cancer within a population-based case control study [15,16]. Although, some epidemiologic reports indicate no association with cancer risk and foods cooked at high temperatures, a vast majority have shown that high intake of well-done meat prepared at high temperatures may increase the risk of human cancers [17,18]. Despite inconsistent results of epidemiological studies addressing a link between diet and cancer incidence, the production of carcinogens in certain food types is well established. Additionally, the ability of these food carcinogens to induce prostate and breast cancer has been widely studied.
It has long been thought that diet has an influence on cancer development, and part of the risk may be associated with the consumption of mutagenic substances along with the foods. Several compounds, either present as dietary components or formed during food processing, can play a role in cancer risk [19]. After ingestion most food mutagens go through metabolic activation or detoxification by different endogenous enzymes. Most mutagens begin their adverse effects at the DNA level by forming DNA adducts with carcinogenic metabolites.
Polyaromatic Hydrocarbons (PAHs), heterocyclic aromatic amines and dioxin-like compounds are human carcinogens produced in the meat cooking process. Cooking experiments have shown that certain dioxin-like compounds are produced during cooking at high temperatures [20]. Biological monitoring revealed consumption of grilled, roasted or boiled meat significantly elevated levels of PAHs [21]. Continuous and high temperature grilling was shown to directly contribute to both increased PAHs and heterocyclic aromatic amines accumulation in both fish and beef [22]. Inhalation in certain occupational settings is also a source of human exposure to environmental carcinogens. PAHs are environmental toxicants that are derived from incomplete combustion of organic material such as coal, wood, gasoline and tobacco. They are released into the environment during industrial processes such as paper manufacturing and waste incineration. Dioxin-like compounds are produced as byproducts of incomplete combustion of organic substances in contact with chlorine. They are produced during waste incineration, pulp manufacturing and other industrial processes [23]. Aromatic amines are formed from amino acids, creati (ni) ne and sugar during cooking of fish and meat at high temperatures. The amount of aromatic amines formed varies depending on the cooking time, meat type and cooking method as well as temperature. Certain aromatic amines are also formed during combustion of organic material and are present in diesel exhaust particles and tobacco smoke [24,25]. Dietary consumption is the major sources of exposure in the general population to these environmental carcinogens [26]. PAHs and aromatic amines undergo activation by phase I and phase II drug metabolizing enzymes to be carcinogenic [27]. Compounds within these chemical classes interact with the aryl hydrocarbon receptor (AhR).
AhR is historically known for its role in mediating the toxic and carcinogenic effects of a wide range of environmental contaminants [28,29]. It is a ligand-activated transcription factor that belongs to the basic helix-loop-helix (bHLH), Per-ARNT-Sim (PAS) superfamily of transcription factors [30]. The AhR protein is predominantly cytoplasmic in the majority of normal tissues and binding to exogenous ligands such as PAHs, aromatic amines and dioxin-like compounds, leads to conformational changes that result in nuclear translocation of AhR and dimerization with the AhR nuclear translocator protein (ARNT) [31,32]. The heterodimer binds to a consensus DNA sequence xenobiotic responsive element (XRE) on the enhancer regions of target genes and increases their transcription. These target genes include the cytochrome P450-1 (CYP1) family of genes, which encode enzymes responsible for activation of chemical carcinogens [33,34]. Activation of AhR leads to induction of CYP1A1, CYP1A2 and CYP1B1 genes, which encode for enzymes that metabolize PAHs to mutagenic intermediates resulting in cancer initiation [34-36]. Ligand-dependent activation of AhR not only plays a role in tumor initiation but also in tumor progression [37-39]. Following transcriptional activation, the AhR is exported back to the cytoplasm where it is degraded by calpains and proteasomes [40,41]. Substantial evidence has shown that PAH-dependent activation of AhR plays a role in a variety of cancers including those in breast, liver and lung [35,42].
There is evidence from several labs suggesting that AhR may function as a tumor suppressor gene that becomes silenced during the process of tumor formation under certain conditions [43]. AhR was significantly repressed in tumors from both mice with a liver-specific retinoblastoma protein ablation and their wild-type littermates, supporting the concept that AhR silencing may be associated with cancer progression [44]. Activation of AhR by ligands can inhibit multiple aspects of the metastatic process in a panel of breast cancer cell lines. Dioxin induced protection against breast cancer may occur via down regulation of CXCR4 and CXCL12, thereby inhibiting progression of the disease, regardless of estrogen receptor, progesterone receptor, or human epidermal growth factor receptor 2 status [45]. AhR and its agonists may confer protective effects in multiple breast cancer subtypes by inhibiting invasive and metastatic features and inducing differentiation. These results support previous epidemiological and rodent studies showing a decrease in breast cancer incidence after exposure to AhR agonists. Another study reported that N-nitrosobutyl (4-hydroxybutyl) amine (BBN) can induce bladder cancer via suppression of AhR signaling pathway [46]. Fritz et al. [47,48] found that AhR-null (AhR−/−) (60%), (AhR+/−) (43%) transgenic adenocarcinoma of the mouse prostate (TRAMP) mice developed prostate tumors with greater frequency than AhR wild-type (AhR+/+) (16%) TRAMP mice. This suggests that AhR inhibits prostate carcinogenesis. Hirabayashi and Inoue [49] also found that wild- type (AhR+/+) mice showed a significant extension of lifespan in a gene-dosage-dependent manner with a delayed onset of leukemogenicity than AHR-deficiencies (AhR+/−, AhR−/−) mice. Those data implied that AhR acts as a tumor suppressor gene under some conditions, but the underlying molecular mechanism is still unknown. Future studies are necessary to investigate the potential cell/molecular mechanisms through which the AhR regulates carcinogenesis, and how the AhR contribute to progression or prevention different kinds of tumors. Given the current challenges in treating aggressive metastatic breast cancer, the clinical development of selective AhR modulators may provide an effective, broad-based alternative to current adjuvant therapies [50].
AhR has been shown to influence a number of cellular processes including differentiation, proliferation and cell cycle progression [51-56]. Activation of the receptor by chemical carcinogens has been reported to antagonize androgen receptor signaling. For example AhR ligand, 2,3,7,8-tetrachlorodibenzo-para-dioxin (TCDD) inhibited testosterone-dependent transcriptional activity and testosterone-regulated prostate specific antigen (PSA) expression in a dose dependent manner [57]. TCDD was also shown to block androgen dependent proliferation of prostate cancer cells [58]. Morrow et al. [59] demonstrated that the simultaneous activation of AhR and androgen receptor with TCDD and an androgen derivative respectively, decreased androgen receptor protein levels. This observation has been contributed to the ability of AhR to promote the proteolysis of androgen receptor through assembly of an ubiquitin ligase complex in which AhR acts as a substrate-recognition subunit to recruit the androgen receptor. This action may also explain the antiandrogenic actions of a number of PAHs.
Studies concerned with intrinsic functions of AhR have found that the receptor may promote carcinogenesis. AhR protein and mRNA expression is associated with phases of rapid proliferation and differentiation in certain tissues. AhR-defective cell lines demonstrate a reduced proliferation rate [60]. Ectopic over expression of AhR in immortalized normal mammary epithelial cells induced a malignant phenotype with increased growth and acquired invasive capabilities [61]. A separate study using a constitutively active AhR construct lacking a ligand binding domain revealed that AhR acts as a transcriptional co-regulator for the unliganded estrogen receptor. These studies showed that the endogenous estrogen receptor along with the constitutively active AhR was recruited to estrogen- responsive elements to initiate signaling in an androgen depleted environment [62].
Several independent studies have confirmed elevated levels of AhR expression in malignant mammary tissue [63,64]. Elevated levels of AhR proteins were found in highly malignant breast cell lines compared to the lower basal expression found in immortalized and primary human mammary epithelial cells and breast cancer cell lines derived from early stages [64]. A separate study also demonstrated increased AhR protein expression in an advanced prostate cancer cell line compared to a less aggressive isogenic pair [65]. Together, evidence suggest that in advanced prostate and breast cancer AhR may function in cancer progression by mechanisms other than mediating the carcinogenic effects of a number of environmental toxins including PAHs, aromatic amines and dioxin-like compounds. Apart from these environmental contaminants, the AhR can bind with a variety of structurally diverse chemicals found in plants. Several investigators have observed that different dietary components, e.g., flavonoids, resveratrol, curcumin etc., bind to the AhR and exert antagonistic activity [66,67] (Figure 1).
Figure 1.
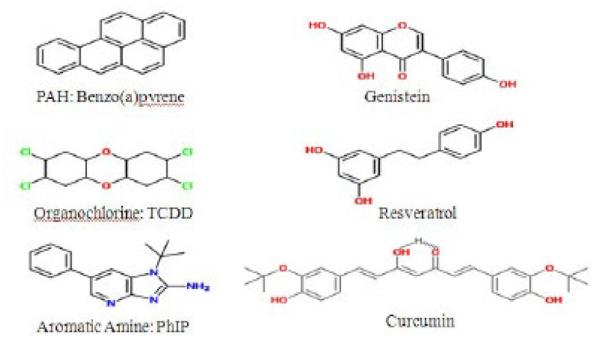
Molecular structures of AhR ligands (A-C) B(a)P, TCDD and PhIP. Molecular structures of natural compounds (D-F) genistein, resveratrol and curcumin.
Polyaromatic Hydrocarbons
PAHs are major class of environmental carcinogens and one of major health concern [68]. PAHs refer to a ubiquitous group of several hundred chemically related, environmentally persistent organic compounds of various structures and variable toxicity. PAHs are a class of lipophilic chemicals that consist of fused aromatic rings. They are solely composed of carbon and hydrogen atoms, containing two or more single or fused aromatic rings with a pair of carbon atoms shared between rings [69]. Characterized by their high hydrophobicity, PAHs are also highly resistant to natural degradation. Sources of PAHs can be both natural and anthropogenic and are largely produced as a result of incomplete combustion of hydrocarbon-containing fuels. The main anthropogenic sources of PAHs include open fires, engine exhaust emissions, manufactured gas plants by-products and domestic heating systems, and other organic substances such as tobacco and different food items [70,71]. Human exposure to these compounds can occur by the ingestion of foods, that have been contaminated by water, air, soil, industrial processing or cooked by different methods (frying, smoking, curing). The long-term intake of PAHs represents a health hazard, since they are considered potentially genotoxic and procarcinogenic [71]. PAH exposure has been linked to numerous cancer types including prostate, breast, skin, bladder and lung cancer [72,73]. The Internal Agency for Research on Cancer has classified PAH mixtures as carcinogens to humans [74,75].
The predominant source of PAHs exposure for the non-smoking general population in developed countries is the daily consumption of PAH-contaminated food. The different PAHs levels in food originate from various food processing technologies and home cooking procedures such as barbecuing meat and fish [76,77]. Grilling or broiling of meat, fish or other foods over a direct flame leads to fat dripping on hot fire and yielding gleams containing PAHs that deposit on the surface of the food materials [19,70].
The existence of PAHs has been confirmed in a wide variety of plants and aquatic organisms. However, leafy vegetables are a trivial source of PAHs in the human diet; the level of contamination is overseen by where the vegetables are grown, those situated close to roads or factories are likely to be contaminated with PAHs [76-79]. Plants may absorb PAHs from the air and this could be a more significant factor than PAHs accumulation through root absorption from soil. Also, wastewater treatment plants play an important role in reducing PAHs concentration in wastewater [80]. Incomplete combustion of hydrocarbon-containing fuels produces PAHs which can contaminate the air and water whereby plants can absorb it. In general, humans readily absorb PAHs into the body through the lung, gastrointestinal tract, and skin [81].
Several studies have confirmed the role of AhR in PAH induced toxicities and carcinogenesis. PAH exposed dams revealed extensive branching and enlargement of vessels accompanied by increased expression of antiapoptotic proteins and decreased expression of proapoptotic proteins. AhR-null fetuses did not exhibit these PAH-induced growth alterations [82]. Furthermore, PAH suppression of testicular function, especially spermatogenesis and sperm motility were absent in AhR deficient mice [83]. Curran et al [84] confirmed the presences of increased levels of PAHs in AhR-null pups demonstrating the importance of AhR-mediated expression of cytochrome P450s in detoxification. Shimizu et al [85] determined that AhR is required for PAH tumor induction. The prototypic PAH, Benzo [a]pyrene (BaP), induced expression of CYP1A1 in the skin and liver of AhR positive mice and did not induce CYP1A1 expression in AhR-null mice. All AhR positive mice exposed to BaP developed subcutaneous tumors at the site of injection. However, there were no noticeable tumors in the AhR-null mice. These experiments confirmed the carcinogenic action of BaP is mediated by the AhR. BaP is the best-characterized PAHs compound found in diet [19,86]. BaP is rated as carcinogenic to humans by the International Agency for Research on Cancer [87]. This five-ring PAH is present in virtually all PAH mixtures, and is one of the most carcinogenic of those commonly detected. BaP is a mutagenic environmental pollutant, which is suspected to contribute to several types of human cancers [88]. Its carcinogenicity is attributed primarily to its genotoxicity [89]. Although AhR-null mice are protected from BaP induced carcinogenesis, higher levels of BaP-DNA adducts are formed within them than in wild- type mice. AhR positive mice have an effective clearance of BaP metabolites which results in reduced levels of DNA adducts. The lack of a functional AhR in null mice, results in slower clearance of BaP and higher levels of DNA adducts [90]. BaP exposure can impair mismatch repair, which is important in carcinogenesis. High exposure to BaP inhibits mismatch repair activity by reducing expression of mismatch repair protein mutS homolog 6 (MSH6) [91]. It has been conclusively demonstrated in laboratory animal studies that BaP is a powerful carcinogen, which readily induces tumors in various tissues such as lung and skin at relatively low doses [87,92-95]. BaP requires metabolic activation to elicit its detrimental effects. The major end product of its diol epoxide metabolic activation pathway is r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a] pyrene (trans, anti-BaPT). Individual differences in exposure to, and metabolic activation of, carcinogenic PAHs may influence cancer risk. Measurement of PAHs metabolites in human urine could provide a direct way to assess individual differences in susceptibility to PAH-related cancer; For example, smokers have significantly higher levels of trans, anti-BaPT in their urine than do non-smokers. It may be useful as a direct phenotyping approach to assess individual differences in uptake and metabolic activation of carcinogenic PAHs [81].
Aromatic Amines
Another class of environmental carcinogens for human is aromatic amines which can be classified into monocyclic aromatic amines, polycyclic aromatic amines and heterocyclic aromatic amines [2- amino-3,8-dimethylimidazo[4,5-f] quinoxaline (MeIQx); 2-amino-2,4-dimethylimidazo[4,5- f]quinoline (MeIQ); 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP)]. Most heterocyclic aromatic amines (HAAs), many polycyclic aromatic amines, and some monocyclic aromatic amines are mutagenic [96].
The production of dyes and other complex chemicals can produce carcinogenic aromatic amines and exposure to them happened during and by their use as antioxidants in rubber-manufacturing processes [87,97]. Some aromatic amines produce throughout the tobacco burning [98,99] and arise in the cooking oils releases [100]. Some other HAAs are also produced when tobacco is burning in high- temperature [101,102]; but, consumption of well-done cooked meats is the main source of exposure to many HAAs [103,104]. We can find HAAs also in pan-fried residues used for gravies [105,106], and arise in vapors of cooking oils [107] as well in the air during the frying or grilling of meats [108]. By using experimental laboratory animals and exposing them long-term by carcinogen bioassays, scientists found chemicals from both classes of compounds can initiate tumors at multiple sites. Certain aromatic amines are classified as human carcinogens (Group 1), and some HAAs have been recorded as probable or possible human carcinogens (Group 2A and 2B) [98,109].
HAAs for producing arylnitrenium ion (major metabolite implicated in toxicity and DNA damage) should undergo metabolic activation by N-hydroxylation of the exocyclic amine group [110,111]. During common household cooking, more than 25 HAAs have been shown to form in meats, fish, and poultry [104,112]. The concentrations of HAAs which can be created have a range from around 1 ppb (parts per billion) to more than 500 ppb [103,104,113-115]. The type of meat and the method of cooking also temperature and the duration of cooking are important factors which have effect on amount of HAAs formation during cooking [114,116]. Two major classes of HAAs which produce during heat processing of muscle foods are “pyrolytic HAAs” and “aminoimidazoarenes (AIAs)”. Pyrolytic may arise during the high-temperature pyrolysis (>250°C) of some individual amino acids, including glutamic acid and tryptophan, or during the pyrolysis of proteins [101,103,117], similarly at the low ppb concentrations, pyrolytic HAAs can form, in some cooked meats [118]. On the other hand, AIAs are formed in meats that are cooked at lower temperatures (150 - 250°C) more usually used in household kitchens. One of the most important way for formation many AIAs is the Maillard reaction (form of nonenzymatic browning resulting from a chemical reaction between an amino acid and a reducing sugar, usually requiring heat) [104,119,120]. During Maillard reaction N-methyl-imidazole-2-ylamine (portion of the molecule) can produce from creatine, and the other portions of the AIAs are supposed to arise from pyridines or pyrazines degradation [119,121]. An aldol condensation is thought to link the two molecules, to form 2-amino-3-methylimidazo [4,5-f] quinoline (IQ) and 2-amino-3- methylimidazo [4,5-f]quinoxaline (IQx)-ring-structured HAAs [122]. It should be noted that the presence of carcinogens in human’s food generation during frying and grilling, has been showed in the number of epidemiological studies. Exposure to meat carcinogens like HAAs or PAHs may increase the risk of a number of common cancers such as breast, prostate and colorectal cancer [4,18,123]. 2-amino-1- methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) is one of the most abundant HAAs detected in cooked meat.
PhIP can form in a model system containing phenylalanine, creatinine, and glucose [124]. However, PhIP can also form in the absence of sugar [104,122]. PhIP is formed in well-done cooked meats and poultry, where the concentration can reach up to 500 ppb [104,114-116,118,120,125].
The most significant gene expression changes in response to PhIP and MeIQx concern members of the AhR gene battery, including CYP1A1 and CYP1A2, which encode two enzymes closely involved in HAAs bio activation [126]. In addition, a number of genes with lower fold changes, including cancer- related genes, whose expression was differentially targeted by PhIP and MeIQx, were observed. HAAs may act in concert with other AhR-activating chemicals found in significant amounts in food and the environment, including PAHs [103,126].
Meat consumption, particularly red and processed meat consumption, has been linked to the increased risk of colorectal cancer in many epidemiological studies [127]. Mutagens such as HAAs and PAHs are formed during high-temperature cooking of meats [128]. These compounds are mutagenic in Ames/Salmonella assays and cause colon tumors in laboratory animals [129]. To exert their mutagenic action, HAAs require enzyme-catalyzed activation consisting of N-oxidation by hepatic CYP1A2 and other extrahepatic P450 isozymes, followed by O-acetylation by N-acetyltransferase 1 (NAT1) and 2(NAT2) [130]. The AhR is an important mediator for xenobiotic signaling to enhance the expression of phase I and II enzymes which affects HAAs metabolism [131].
In order to better understand the molecular basis of HAAs toxicity, Dumont et al have analyzed gene expression profiles in the metabolically competent human Hepa RG cells using pangenomic oligonucleotide microarrays, after either a single (24 hr) or a repeated (28-day) exposure to 10 μM PhIP or MeIQx. The most responsive genes to both HAAs were downstream targets of the AhR: CYP1A1 and CYP1A2 after both time points and CYP1B1 and ALDH3A1 after 28 days. Accordingly, CYP1A1/1A2 induction in HAAs-treated Hepa RG cells was prevented by chemical inhibition or small interference RNA-mediated down-regulation of the AhR. Consistently, HAAs induced activity of the CYP1A1 promoter, which contains a consensus AhR-related xenobiotic-responsive element (XRE). In addition, several other genes exhibited both time-dependent and compound-specific expression changes. These changes concerned genes mainly related to cell growth and proliferation, apoptosis, and cancer. These results identify the AhR gene battery as the preferential target of PhIP and MeIQx in Hepa RG cells and further support the hypothesis that intake of HAAs in diet might increase human cancer risk [103,126]. CYP1A2 is one of the major enzymes that bioactivate a number of procarcinogens including heterocyclic aromatic amines/amides and some natural compounds such as aristolochic acids present in several Chinese herbal medicines. Similar to CYP1A1 and 1B1, CYP1A2 is primarily regulated by the AhR [132].
Derivatives of PhIP were shown to be potential carcinogens for the prostate [133]. PhIP induced prostate carcinogenesis in mice carrying humanized CYP1A2, which activates PhIP to a carcinogen. Low grade prostatic intraepithelial neoplasia (PIN) was seen 20 weeks following administration of PhIP and high-grade PIN 30 to 50 weeks after initial dosage. The lesions induced by PhIP administration were androgen receptor positive and featured the loss of expression of the basal cell marker p63 and the tumor suppressor PTEN [134]. These studies show direct induction of prostate carcinogenesis by PhIP.
Dioxin-Like Compounds
Dioxin-like compounds are a diverse group of synthetic chemicals such as dichlorodiphenyltrichloroethane (DDT), dieldrin, hexachlorocyclohexane isomers (HCH), toxaphene, polychlorinated biphenyls (PCBs) and dioxin. Dioxins are a class of polyhalogenated compounds and belong to a group of halogenated aromatic hydrocarbons (HAHs), which have a similar chemical structure and biological effects. They include polychlorinated dibenzodioxins (PCDD), polychlorinated dibenzfurans (PCDF) and polychlorinated biphenyls (PCB). TCDD is identified as the most potent dioxin and is classified as a class I human carcinogen that has been implicated in a number of cancers [135,136]. TCDD exposure was shown to produce quinonoid metabolites of estrogen and the subsequent formation of oxidative DNA lesions through alteration of CYP1A1 and CYP1B1 expression in human breast cancer cells [137]. Kang et al [138] demonstrated that TCDD significantly increased BaP- DNA adduct formation in the absence of BRCA1. These results imply that oxidative stress is correlated with increased DNA damage in BRCA1 defective cells. Evidence suggests that TCDD might increase the risk of tumorigenesis in BRCA1 defective breast epithelial cells.
Dioxins are derived from the combustion process (e.g., incineration and burning of fuels), during production and utilization of chlorinated compounds (e.g., PCBs) and bleaching of paper-pulp. PCBs enter the air, water and soil during manufacturing, use and disposal. The common features of all these above-mentioned compounds are their persistence in the environment, their bioaccumulation in adipose tissue and in food chains due to their lipophilic character, and their resistance to metabolism. Humans are exposed to dioxins mainly through the consumption of contaminated foods [139]. Among these compounds, TCDD is the most persistent lipophilic environmental contaminant (half-life ~7years) and considered as the prototype chemical [19].
TCDD has the ability to bind to and activate the ligand-activated transcription factor, AhR. Structurally related compounds that bind to the AhR and exhibit biological actions similar to TCDD are commonly referred to as dioxin-like compounds. Ambient human exposure to dioxins occurs through the ingestion of foods containing residues that bioconcentrate through the food chain. The main sources of TCDD released into the environment are from metal smelting, refining, and processing; combustion and incineration sources; chemical manufacturing and processing; biological and photochemical processes; and existing reservoir sources that reflect past releases [140]. Toxic chemicals like TCDD, BaP and PCBs can activate AhR, which subsequently induce CYP1A1 and CYP1B1 expression. Interestingly, estradiol is metabolized by CYP1A1 and CYP1B1, which also activate BaP to reactive DNA-binding intermediates [141-143].
Dietary Antagonists of AhR
Several classes of beneficial dietary compounds have been described as health-promoting or disease-preventing. Interestingly, many dietary compounds that have chemopreventive properties have been found to also act as antagonist of the AhR signaling pathway. The chemopreventive effect may be due to inhibiting the effects of environmental and food carcinogens. Therefore, dietary ligands could be effective tools in reducing cancer incidence.
Flavonoids are naturally occurring polyphenols present in many fruits and vegetables [144]. These polyphenolic compounds have attracted renewed attention as potential anticarcinogens, and the molecular mechanisms of their anticarcinogenic effects and their bioavailability have been extensively explored. The major dietary flavonoids are flavones, flavonols, and flavan-3-ols (catechins), and they play important roles in cancer prevention. After absorption with or without metabolic conjugation, flavonoids are transported to target organs where they exert their anticarcinogenic activity. The molecular mechanisms of the anticarcinogenic effects of flavonoids include their antagonistic effect on the AhR, and regulation of phase I and II drug metabolizing enzymes and phase III transporters.
Experimental evidence suggests that flavonoids modulate signal transduction pathways at each stage of carcinogenesis [145]. Dietary flavonoid 5,7, dimethoxyflavone significantly inhibited BaP-induced adduct formation and CYP1B1 expression [146]. Heiden et al [147] investigated the genetic-, time-, dose-, species- and tissue-dependent AhR-mediated agonistic/antagonistic activities of three food flavonoids: quercetin, chrysin and genistein. Human hepatoma (HepG2-Luc) and human breast tumour (T-47D- Luc) cells were compared for tissue-dependent effects. Rat hepatoma (H4IIE-ULg) and human hepatoma (HepG2-Luc) cells were compared for species-dependent activities. Evidence showed that quercetin, chrysin and genistein act in a time-, dose species- and tissue-specific way. For example, genistein displayed agonistic activities when exposed to rat hepatoma cells during 6h but not after 24 h.
Flavonoids displayed agonistic/antagonistic activities in human breast tumour cells, depending on the exposure time, while in human hepatoma cells, only antagonistic activities of flavonoids were measured. Induction of CYP1A1 by flavonoids proceeds by various mechanisms, including the direct stimulation of gene or mRNA stabilization. Some flavonoids induce CYPs through binding to AhR. Generally, substrates for AhR are planar aromatic compounds with few bulky substituent groups. That might partly explain the activity of flavonoids, which have similar planar structures as AhR ligands.
Other flavonoids have been shown to directly inhibit CYP1A1 activity, commonly demonstrated to be a competitive-type of inhibition, and to affect CYP1A1 transcription. The most abundant flavonoids, flavonols quercetin and kaempferol, are both dietary ligands of the AhR, but they exert different effects on CYP1A1 expression. Treatment of MCF-7 cells with quercetin resulted in a concentration and time dependent increase in the amount of CYP1A1 mRNA. Kaempferol, by itself, does not affect CYP1A1 expression, but it can interact with the AhR, and act as an antagonist of TCDD induced CYP1A1 transcription. Despite the structural similarity between quercetin and kaempferol, their differential effects might be due to the absence of an additional hydroxyl group on kaempferol, preventing it from achieving an optimal fit into the binding site on AhR to produce transcriptional activation. The binding of kaempferol may block the binding of AhR ligands, and thus inhibit the activity of other ligands such as TCDD [148,149].
Prenylflavone, icaritin, suppressed estrogen stimulated cell proliferation and gene expression in breast cancer cells. Icaritin exposure destabilizes estrogen receptor protein and restricts estrogen receptor-positive cell growth through direct interaction with AhR [150]. 4′,5,7-trihydroxyisoflavone, also known as genistein, is considered a major soy isoflavone. Genistein directly interacts with AhR to inhibit cytochrome P450 enzyme expression [150]. Via this direct interaction, genistein decreased viability of prostate cancer cells [151]. 3,4,5-trihydroxystilbene (resveratrol) a natural component of grape skin and wine, is also a dietary ligand for AhR [152]. Activation of AhR in breast cancer cells inhibits estrogen dependent transcription of tumor suppressor, BRCA1. The addition of resveratrol prevents AhR-mediated epigenetic silencing of BRCA-1 by promoter hyperphosphorylation [153]. Resveratrol regulation of AhR signaling is estrogen receptor independent. Resveratrol inhibited dioxin induced CYP1B1 expression in estrogen receptor-positive and estrogen receptor-negative breast cancer cells [154].
Diferuloylmethane, also known as curcumin is a dietary yellow pigment of Curcuma longa. The anti-carcinogenic properties of curcumin have been established but the mechanisms of action are not fully understood [155-157]. Evidence suggests that curcumin’s beneficial effects may be mediated through the AhR. Pretreatment of mice with curcumin inhibited BaP induced CYP1A1 expression. Decreased CYP1A1 transcription was attributed to decrease nuclear translocation and DNA binding of AhR [158]. Curcumin was also shown to induce significant inhibition of androgen receptor expression in a hormone sensitive prostate cancer cell line. Curcumin attenuated phosphorylation of Akt but increased phosphorylation of beta-catenin. Also beta-catenin target genes, cyclin D1 and c-myc, were also decreased in these studies, suggesting that curcumin’s inhibitory effect is through modulation of the Wnt/beta-catenin signaling pathway, possibly following direct interaction with AhR [159].
Ligands for the AhR have been shown to influence cell proliferation, differentiation and apoptosis. Typically, environmental toxins increase proliferation and differentiation while inhibiting apoptosis. Conversely, dietary antagonists for AhR inhibit proliferation and differentiation and induce apoptosis (Figure 2). However, the mechanism used by AhR to exert these effects is not known and cannot be attributed to its ability to induce drug metabolizing enzymes. The consumption of diet containing carcinogens, including PAHs, dioxin-like compounds and heterocyclic aromatic amines is associated with increased cancer risk. Increasing evidence suggests the consumption of dietary compounds found in fruits and vegetables can decrease cancer incidence. The aryl hydrocarbon receptor is a highly conserved transcription factor whose activity is regulated positively by environmental toxins and negatively by dietary antagonist. However, more studies are needed to confirm the species and tissue specific role of AhR and the dietary compounds that interact with the receptor in cancer initiation and progression.
Figure 2.
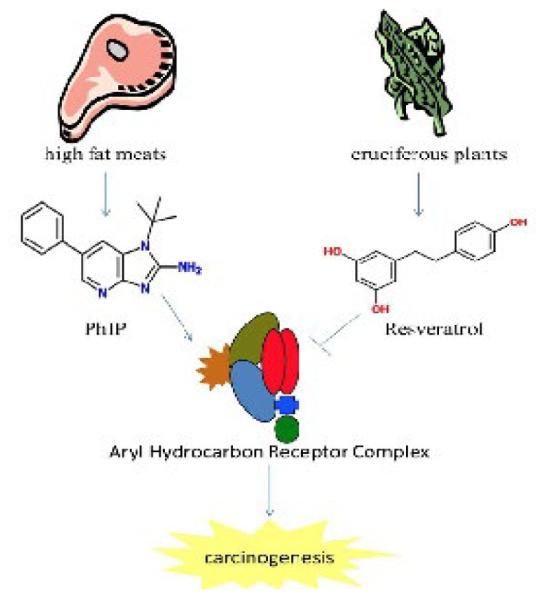
AhR signaling is activated by environmental carcinogens (e.g. PhIP) and inhibited by natural compounds (e.g. resveratrol).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167:925–934. doi: 10.1093/aje/kwm389. [DOI] [PubMed] [Google Scholar]
- 4.Sinha R, Park Y, Graubard BI, Leitzmann MF, Hollenbeck A, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol. 2009;170:1165–1177. doi: 10.1093/aje/kwp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punnen S, Hardin J, Cheng I, Klein EA, Witte JS. Impact of meat consumption, preparation, and mutagens on aggressive prostate cancer. PLoS One. 2011;6:e27711. doi: 10.1371/journal.pone.0027711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salem S, Salahi M, Mohseni M, Ahmadi H, Mehrsai A, et al. Major dietary factors and prostate cancer risk: a prospective multicenter case-control study. Nutr Cancer. 2011;63:21–27. doi: 10.1080/01635581.2010.516875. [DOI] [PubMed] [Google Scholar]
- 7.Wright JL, Neuhouser ML, Lin DW, Kwon EM, Feng Z, et al. AMACR polymorphisms, dietary intake of red meat and dairy and prostate cancer risk. Prostate. 2011;71:498–506. doi: 10.1002/pros.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander DD, Mink PJ, Cushing CA, Sceurman B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr J. 2010;9:50. doi: 10.1186/1475-2891-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor VH, Misra M, Mukherjee SD. Is red meat intake a risk factor for breast cancer among premenopausal women? Breast Cancer Res Treat. 2009;117:1–8. doi: 10.1007/s10549-009-0441-y. [DOI] [PubMed] [Google Scholar]
- 10.Bao PP, Shu XO, Zheng Y, Cai H, Ruan ZX, et al. Fruit, vegetable, and animal food intake and breast cancer risk by hormone receptor status. Nutr Cancer. 2012;64:806–819. doi: 10.1080/01635581.2012.707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabat GC, Cross AJ, Park Y, Schatzkin A, Hollenbeck AR, et al. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int J Cancer. 2009;124:2430–2435. doi: 10.1002/ijc.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi AD, John EM, Koo J, Ingles SA, Stern MC. Fish intake, cooking practices, and risk of prostate cancer: results from a multi-ethnic case-control study. Cancer Causes Control. 2012;23:405–420. doi: 10.1007/s10552-011-9889-2. [DOI] [PubMed] [Google Scholar]
- 13.Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, et al. Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer: results from a multiethnic case-control study. Carcinogenesis. 2012;33:2108–2118. doi: 10.1093/carcin/bgs242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stott-Miller M, Neuhouser ML, Stanford JL. Consumption of deep-fried foods and risk of prostate cancer. Prostate. 2013;73:960–969. doi: 10.1002/pros.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Z, Deming SL, Fair AM, Shrubsole MJ, Wujcik DM, et al. Well-done meat intake and meat-derived mutagen exposures in relation to breast cancer risk: the Nashville Breast Health Study. Breast Cancer Res Treat. 2011;129:919–928. doi: 10.1007/s10549-011-1538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steck SE, Gaudet MM, Eng SM, Britton JA, Teitelbaum SL, et al. Cooked meat and risk of breast cancer--lifetime versus recent dietary intake. Epidemiology. 2007;18:373–382. doi: 10.1097/01.ede.0000259968.11151.06. [DOI] [PubMed] [Google Scholar]
- 17.Sander A, Linseisen J, Rohrmann S. Intake of heterocyclic aromatic amines and the risk of prostate cancer in the EPIC-Heidelberg cohort. Cancer Causes Control. 2011;22:109–114. doi: 10.1007/s10552-010-9680-9. [DOI] [PubMed] [Google Scholar]
- 18.Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer. 2009;61:437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S, Koner BC, Ray S, Ray A. Environmental contaminants in pathogenesis of breast cancer. Indian J Exp Biol. 2006;44:597–617. [PubMed] [Google Scholar]
- 20.Wu J, Dong S, Liu G, Zhang B, Zheng M. Cooking process: a new source of unintentionally produced dioxins? J Agric Food Chem. 2011;59:5444–5449. doi: 10.1021/jf200216r. [DOI] [PubMed] [Google Scholar]
- 21.Gunier RB, Reynolds P, Hurley SE, Yerabati S, Hertz A, et al. Estimating exposure to polycyclic aromatic hydrocarbons: a comparison of survey, biological monitoring, and geographic information system-based methods. Cancer Epidemiol Biomarkers Prev. 2006;15:1376–1381. doi: 10.1158/1055-9965.EPI-05-0799. [DOI] [PubMed] [Google Scholar]
- 22.Viegas O, Novo P, Pinto E, Pinho O, Ferreira IM. Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem Toxicol. 2012;50:2128–34. doi: 10.1016/j.fct.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 23.Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7:331–339. doi: 10.1093/humupd/7.3.331. [DOI] [PubMed] [Google Scholar]
- 24.Manabe S, Kurihara N, Wada O, Izumikawa S, Asakuno K, et al. Detection of a carcinogen, 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine, in airborne particles and diesel-exhaust particles. Environ Pollut. 1993;80:281–286. doi: 10.1016/0269-7491(93)90049-t. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita M, Wakabayashi K, Nagao M, Sato S, Yamaizumi Z, et al. Detection of 2-amino-3-methylimidazo[4,5-f]quinoline in cigarette smoke condensate. Jpn J Cancer Res. 1986;77:419–422. [PubMed] [Google Scholar]
- 26.Zhang X, Lin S, Funk WE, Hou L. Environmental and occupational exposure to chemicals and telomere length in human studies. Occup Environ Med. 2013;70:743–749. doi: 10.1136/oemed-2012-101350. [DOI] [PubMed] [Google Scholar]
- 27.Turesky RJ. The role of genetic polymorphisms in metabolism of carcinogenic heterocyclic aromatic amines. Curr Drug Metab. 2004;5:169–180. doi: 10.2174/1389200043489036. [DOI] [PubMed] [Google Scholar]
- 28.Rowlands JC1, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 29.Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 30.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen HS, Perdew GH. Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex. J Biol Chem. 1994;269:27554–27558. [PubMed] [Google Scholar]
- 32.Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact. 2002;141:3–24. doi: 10.1016/s0009-2797(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 33.Smolowitz RM, Schultz ME, Stegeman JJ. Cytochrome P4501A induction in tissues, including olfactory epithelium, of topminnows (Poeciliopsis spp.) by waterborne benzo[a]pyrene. Carcinogenesis. 1992;13:2395–2402. doi: 10.1093/carcin/13.12.2395. [DOI] [PubMed] [Google Scholar]
- 34.Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 35.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 36.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 37.Diry M, Tomkiewicz C, Koehle C, Coumoul X, Bock KW, et al. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25:5570–5574. doi: 10.1038/sj.onc.1209553. [DOI] [PubMed] [Google Scholar]
- 38.Peng TL, Chen J, Mao W, Song X, Chen MH. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009;10:27. doi: 10.1186/1471-2121-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010;31:1319–1328. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J Biol Chem. 1999;274:28708–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 41.Dale YR, Eltom SE. Calpain mediates the dioxin-induced activation and down-regulation of the aryl hydrocarbon receptor. Mol Pharmacol. 2006;70:1481–1487. doi: 10.1124/mol.106.027474. [DOI] [PubMed] [Google Scholar]
- 42.Schlezinger JJ1, Liu D, Farago M, Seldin DC, Belguise K, et al. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–1187. doi: 10.1515/BC.2006.145. [DOI] [PubMed] [Google Scholar]
- 43.Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biochim Biophys Acta. 2013;1836:197–210. doi: 10.1016/j.bbcan.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Peng L, Mayhew CN, Schnekenburger M, Knudsen ES, Puga A. Repression of Ah receptor and induction of transforming growth factor-beta genes in DEN-induced mouse liver tumors. Toxicology. 2008;246:242–247. doi: 10.1016/j.tox.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu EL, Yoon D, Choi HH, Wang F, Taylor RT, et al. A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol Sci. 2007;98:436–444. doi: 10.1093/toxsci/kfm125. [DOI] [PubMed] [Google Scholar]
- 46.Iida K, Mimura J, Itoh K, Ohyama C, Fujii-Kuriyama Y, et al. Suppression of AhR signaling pathway is associated with the down-regulation of UDP-glucuronosyltransferases during BBN-induced urinary bladder carcinogenesis in mice. J Biochem. 2010;147:353–360. doi: 10.1093/jb/mvp169. [DOI] [PubMed] [Google Scholar]
- 47.Fritz WA, Lin TM, Peterson RE. The aryl hydrocarbon receptor (AhR) inhibits vanadate-induced vascular endothelial growth factor (VEGF) production in TRAMP prostates. Carcinogenesis. 2008;29:1077–1082. doi: 10.1093/carcin/bgn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fritz WA, Lin TM, Cardiff RD, Peterson RE. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis. 2007;28:497–505. doi: 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- 49.Hirabayashi Y, Inoue T. Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem Pharmacol. 2009;77:521–535. doi: 10.1016/j.bcp.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 50.Hall JM, Barhoover MA, Kazmin D, McDonnell DP, Greenlee WF, et al. Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation. Mol Endocrinol. 2010;24:359–369. doi: 10.1210/me.2009-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi S, Okabe-Kado J, Honma Y, Kawajiri K. Expression of Ah receptor (TCDD receptor) during human monocytic differentiation. Carcinogenesis. 1995;16:1403–1409. doi: 10.1093/carcin/16.6.1403. [DOI] [PubMed] [Google Scholar]
- 52.Bunaciu RP, Yen A. Activation of the aryl hydrocarbon receptor AhR Promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 2011;71:2371–2380. doi: 10.1158/0008-5472.CAN-10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dever DP, Opanashuk LA. The aryl hydrocarbon receptor contributes to the proliferation of human medulloblastoma cells. Mol Pharmacol. 2012;81:669–678. doi: 10.1124/mol.111.077305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hestermann EV, Stegeman JJ, Hahn ME. Relationships among the cell cycle, cell proliferation, and aryl hydrocarbon receptor expression in PLHC-1 cells. Aquat Toxicol. 2002;58:201–213. doi: 10.1016/s0166-445x(01)00229-6. [DOI] [PubMed] [Google Scholar]
- 55.Hrubá E, Vondráček J, Líbalová H, Topinka J, Bryja V, et al. Gene expression changes in human prostate carcinoma cells exposed to genotoxic and nongenotoxic aryl hydrocarbon receptor ligands. Toxicol Lett. 2011;206:178–188. doi: 10.1016/j.toxlet.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Weiss C, Kolluri SK, Kiefer F, Göttlicher M. Complementation of Ah receptor deficiency in hepatoma cells: negative feedback regulation and cell cycle control by the Ah receptor. Exp Cell Res. 1996;226:154–163. doi: 10.1006/excr.1996.0214. [DOI] [PubMed] [Google Scholar]
- 57.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, et al. Crosstalk between 2,3,7,8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochem Biophys Res Commun. 1999;256:462–468. doi: 10.1006/bbrc.1999.0367. [DOI] [PubMed] [Google Scholar]
- 58.Barnes-Ellerbe S, Knudsen KE, Puga A. 2,3,7,8-Tetrachlorodibenzo-pdioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol Pharmacol. 2004;66:502–511. doi: 10.1124/mol.104.000356. [DOI] [PubMed] [Google Scholar]
- 59.Morrow D, Qin C, Smith R, Safe S. Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J Steroid Biochem Mol Biol. 2004;88:27–36. doi: 10.1016/j.jsbmb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Wang W, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenicity in MCF-7 cells: modulation of hormone-induced cell cycle enzymes. Arch Biochem Biophys. 1998;356:239–248. doi: 10.1006/abbi.1998.0782. [DOI] [PubMed] [Google Scholar]
- 61.Brooks J, Eltom SE. Malignant transformation of mammary epithelial cells by ectopic overexpression of the aryl hydrocarbon receptor. Curr Cancer Drug Targets. 2011;11:654–669. doi: 10.2174/156800911795655967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohtake F, Baba A, Fujii-Kuriyama Y, Kato S. Intrinsic AhR function underlies cross-talk of dioxins with sex hormone signalings. Biochem Biophys Res Commun. 2008;370:541–546. doi: 10.1016/j.bbrc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 63.Eltom SE, Gasmelseed A, Saoudi-Guentri D. The aryl hydrocarbon receptor is over- expressed and constitutively activated in advanced breast carcinoma. Proc Amer Assoc Cancer Research. 2006 [Google Scholar]
- 64.Yang X, Solomon S, Fraser LR, Trombino AF, Liu D, et al. Constitutive regulation of CYP1B1 by the aryl hydrocarbon receptor (AhR) in pre-malignant and malignant mammary tissue. J Cell Biochem. 2008;104:402–417. doi: 10.1002/jcb.21630. [DOI] [PubMed] [Google Scholar]
- 65.Tran C, Richmond O, Aaron L, Powell JB. Inhibition of constitutive aryl hydrocarbon receptor (AhR) signaling attenuates androgen independent signaling and growth in (C4-2) prostate cancer cells. Biochem Pharmacol. 2013;85:753–762. doi: 10.1016/j.bcp.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 67.Amakura Y, Tsutsumi T, Sasaki K, Yoshida T, Maitani T. Screening of the inhibitory effect of vegetable constituents on the aryl hydrocarbon receptor-mediated activity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol Pharm Bull. 2003;26:1754–1760. doi: 10.1248/bpb.26.1754. [DOI] [PubMed] [Google Scholar]
- 68.Currier N, Solomon SE, Demicco EG, Chang DL, Farago M, et al. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol Pathol. 2005;33:726–737. doi: 10.1080/01926230500352226. [DOI] [PubMed] [Google Scholar]
- 69.Riachi LG, Santos A, Moreira RF, De Maria CA. A review of ethyl carbamate and polycyclic aromatic hydrocarbon contamination risk in cachaça and other Brazilian sugarcane spirits. Food Chem. 2014;149:159–169. doi: 10.1016/j.foodchem.2013.10.088. [DOI] [PubMed] [Google Scholar]
- 70.Jägerstad M, Skog K. Genotoxicity of heat-processed foods. Mutat Res. 2005;574:156–172. doi: 10.1016/j.mrfmmm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 71.Lau EV1, Gan S, Ng HK, Poh PE. Extraction agents for the removal of polycyclic aromatic hydrocarbons (PAHs) from soil in soil washing technologies. Environ Pollut. 2014;184:640–649. doi: 10.1016/j.envpol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 72.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 73.Gammon MD, Santella RM. PAH, genetic susceptibility and breast cancer risk: an update from the Long Island Breast Cancer Study Project. Eur J Cancer. 2008;44:636–640. doi: 10.1016/j.ejca.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 74.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, et al. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol. 2005;6:931–932. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- 75.Merletti F, Heseltine E, Saracci R, Simonato L, Vainio H, et al. Target organs for carcinogenicity of chemicals and industrial exposures in humans: a review of results in the IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Cancer Res. 1984;44:2244–2250. [PubMed] [Google Scholar]
- 76.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 77.Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, et al. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- 78.Meador JP, Stein JE, Reichert WL, Varanasi U. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Rev Environ Contam Toxicol. 1995;143:79–165. doi: 10.1007/978-1-4612-2542-3_4. [DOI] [PubMed] [Google Scholar]
- 79.Guillén MD, Sopelana P, Partearroyo MA. Food as a source of polycyclic aromatic carcinogens. Rev Environ Health. 1997;12:133–146. doi: 10.1515/reveh.1997.12.3.133. [DOI] [PubMed] [Google Scholar]
- 80.Li J, Shang X, Zhao Z, Tanguay RL, Dong Q, et al. Polycyclic aromatic hydrocarbons in water, sediment, soil, and plants of the Aojiang River waterway in Wenzhou, China. J Hazard Mater. 2010;173:75–81. doi: 10.1016/j.jhazmat.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhong Y, Carmella SG, Hochalter JB, Balbo S, Hecht SS, et al. Analysis of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine: a biomarker for directly assessing carcinogenic polycyclic aromatic hydrocarbon exposure plus metabolic activation. Chem Res Toxicol. 2011;24:73–80. doi: 10.1021/tx100287n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Detmar J, Rennie MY, Whiteley KJ, Qu D, Taniuchi Y, et al. Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. Am J Physiol Endocrinol Metab. 2008;295:E519–30. doi: 10.1152/ajpendo.90436.2008. [DOI] [PubMed] [Google Scholar]
- 83.Izawa H, Kohara M, Watanabe G, Taya K, Sagai M. Diesel exhaust particle toxicity on spermatogenesis in the mouse is aryl hydrocarbon receptor dependent. J Reprod Dev. 2007;53:1069–1078. doi: 10.1262/jrd.19025. [DOI] [PubMed] [Google Scholar]
- 84.Curran CP, Nebert DW, Genter MB, Patel KV, Schaefer TL, et al. In utero and lactational exposure to PCBs in mice: adult offspring show altered learning and memory depending on Cyp1a2 and Ahr genotypes. Environ Health Perspect. 2011;119:1286–1293. doi: 10.1289/ehp.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldman R, Shields PG. Food mutagens. J Nutr. 2003;133(Suppl 3):965S–973S. doi: 10.1093/jn/133.3.965S. [DOI] [PubMed] [Google Scholar]
- 87.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 88.Catalani S. [IARC revision on dioxin and some dioxin-like compounds] G Ital Med Lav Ergon. 2010;32:79–81. [PubMed] [Google Scholar]
- 89.Machala M, Vondrácek J, Bláha L, Ciganek M, Neca JV. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat Res. 2001;497:49–62. doi: 10.1016/s1383-5718(01)00240-6. [DOI] [PubMed] [Google Scholar]
- 90.Sagredo C, Mollerup S, Cole KJ, Phillips DH, Uppstad H, et al. Biotransformation of benzo[a]pyrene in Ahr knockout mice is dependent on time and route of exposure. Chem Res Toxicol. 2009;22:584–591. doi: 10.1021/tx8003664. [DOI] [PubMed] [Google Scholar]
- 91.Chen Y, Huang C, Bai C, Gao H, Ma R, et al. Benzo[α]pyrene repressed DNA mismatch repair in human breast cancer cells. Toxicology. 2013;304:167–172. doi: 10.1016/j.tox.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 92.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 93.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 94.Polycyclic aromatic hydrocarbons, 15 listings. Rep Carcinog. 2004;11:III220–222. [PubMed] [Google Scholar]
- 95.Polynuclear aromatic compounds, Part 1, Chemical, environmental and experimental data. IARC Monogr Eval Carcinog Risk Chem Hum. 1983;32:1–453. [PubMed] [Google Scholar]
- 96.DeBruin LS, Josephy PD. Perspectives on the chemical etiology of breast cancer. Environ Health Perspect. 2002;110(Suppl 1):119–128. doi: 10.1289/ehp.02110s1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987;7:1–440. [PubMed] [Google Scholar]
- 98.Tobacco smoking. IARC Monogr Eval Carcinog Risk Chem Hum. 1986;38:35–394. [PubMed] [Google Scholar]
- 99.Patrianakos C, Hoffman D. Chemical studies on tabacco smoke-On the analysis of aromatic amines in cigarette smoke. J Assoc Off Anal Chem. 1979:150–154. [Google Scholar]
- 100.Chiang TA, Pei-Fen W, Ying LS, Wang LF, Ko YC. Mutagenicity and aromatic amine content of fumes from heated cooking oils produced in Taiwan. Food Chem Toxicol. 1999;37:125–134. doi: 10.1016/s0278-6915(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 101.Matsumoto T, Yoshida D, Tomita H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer Lett. 1981;12:105–110. doi: 10.1016/0304-3835(81)90045-8. [DOI] [PubMed] [Google Scholar]
- 102.Manabe S, Tohyama K, Wada O, Aramaki T. Detection of a carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), in cigarette smoke condensate. Carcinogenesis. 1991;12:1945–1947. doi: 10.1093/carcin/12.10.1945. [DOI] [PubMed] [Google Scholar]
- 103.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Felton JS, et al. Contents in foods, beverages and tobacco. Food Borne Carcinogens. 2000:31–71. [Google Scholar]
- 105.Laser Reutersward A, Skog K, Jagerstad M. Effects of creatine and creatinine content on the mutagenic activity of meat extracts, bouillons and gravies from different sources. Food Chem Toxicol. 1987;25:747–54. doi: 10.1016/0278-6915(87)90229-8. [DOI] [PubMed] [Google Scholar]
- 106.Gross GA, Turesky RJ, Fay LB, Stillwell WG, Skipper PL, et al. Heterocyclic aromatic amine formation in grilled bacon, beef and fish and in grill scrapings. Carcinogenesis. 1993;14:2313–2318. doi: 10.1093/carcin/14.11.2313. [DOI] [PubMed] [Google Scholar]
- 107.Yang CC, Jenq SN, Lee H. Characterization of the carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in cooking aerosols under domestic conditions. Carcinogenesis. 1998;19:359–363. doi: 10.1093/carcin/19.2.359. [DOI] [PubMed] [Google Scholar]
- 108.Thiébaud HP, Knize MG, Kuzmicky PA, Hsieh DP, Felton JS. Airborne mutagens produced by frying beef, pork and a soy-based food. Food Chem Toxicol. 1995;33:821–828. doi: 10.1016/0278-6915(95)00057-9. [DOI] [PubMed] [Google Scholar]
- 109.International Agency for Research on Cancer, Some naturally occurring subsgtances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. 1993:56. [Google Scholar]
- 110.Scribner JD, Fisk SR, Scribner NK. Mechanisms of action of carcinogenic aromatic amines: an investigation using mutagenesis in bacteria. Chem Biol Interact. 1979;26:11–25. doi: 10.1016/0009-2797(79)90090-5. [DOI] [PubMed] [Google Scholar]
- 111.Hatch FT, Knize MG, Colvin ME. Extended quantitative structure-activity relationships for 80 aromatic and heterocyclic amines: structural, electronic, and hydropathic factors affecting mutagenic potency. Environ Mol Mutagen. 2001;38:268–291. doi: 10.1002/em.10028. [DOI] [PubMed] [Google Scholar]
- 112.Alaejos Maite Sanz, Afonso Ana M. Factos that affect the content of heterocyclic aromatic amines in foods. Comprehensive Reviews in Food Science and Food Safety. 2011;10:52–108. [Google Scholar]
- 113.Knize MG, Felton JS. Formation and human risk of carcinogenic heterocyclic amines formed from natural precursors in meat. Nutr Rev. 2005;63:158–165. doi: 10.1111/j.1753-4887.2005.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 114.Sinha R, Rothman N, Brown ED, Salmon CP, Knize MG, et al. High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo- [4,5-b] pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res. 1995;55:4516–4519. [PubMed] [Google Scholar]
- 115.Ni W, McNaughton L, LeMaster DM, Sinha R, Turesky RJ. Quantitation of 13 heterocyclic aromatic amines in cooked beef, pork, and chicken by liquid chromatography-electrospray ionization/tandem mass spectrometry. J Agric Food Chem. 2008;56:68–78. doi: 10.1021/jf072461a. [DOI] [PubMed] [Google Scholar]
- 116.Knize MG, Dolbeare FA, Carroll KL, Moore DH, 2nd, Felton JS. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem Toxicol. 1994;32:595–603. doi: 10.1016/0278-6915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 117.Yoshida D, Matsumoto T, Yoshimura R, Matsuzaki T. Mutagenicity of amino-alpha-carbolines in pyrolysis products of soybean globulin. Biochem Biophys Res Commun. 1978;83:915–920. doi: 10.1016/0006-291x(78)91482-1. [DOI] [PubMed] [Google Scholar]
- 118.Skog K, Solyakov A, Arvidsson P, Jägerstad M. Analysis of nonpolar heterocyclic amines in cooked foods and meat extracts using gas chromatography-mass spectrometry. J Chromatogr A. 1998;803:227–233. doi: 10.1016/s0021-9673(97)01266-1. [DOI] [PubMed] [Google Scholar]
- 119.Jägerstad M, Skog K, Grivas S, Olsson K. Formation of heterocyclic amines using model systems. Mutat Res. 1991;259:219–233. doi: 10.1016/0165-1218(91)90119-7. [DOI] [PubMed] [Google Scholar]
- 120.Skog KI, Johansson MA, Jägerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food Chem Toxicol. 1998;36:879–896. doi: 10.1016/s0278-6915(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 121.Milic BL, Djilas SM, Candadanoic-Brunet JM. Synthesis of some heterocyclic aminoimidazoarenes. Food Chemistry. 1993;46:273–276. [Google Scholar]
- 122.Murkovic M. Formation of heterocyclic aromatic amines in model systems. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:3–10. doi: 10.1016/j.jchromb.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 123.Rohrmann S, Lukas Jung SU, Linseisen J, Pfau W. Dietary intake of meat and meat-derived heterocyclic aromatic amines and their correlation with DNA adducts in female breast tissue. Mutagenesis. 2009;24:127–132. doi: 10.1093/mutage/gen058. [DOI] [PubMed] [Google Scholar]
- 124.Shioya M, Wakabayashi K, Sato S, Nagao M, Sugimura T. Formation of a mutagen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine (PhIP) in cooked beef, by heating a mixture containing creatinine, phenylalanine and glucose. Mutat Res. 1987;191:133–138. doi: 10.1016/0165-7992(87)90143-6. [DOI] [PubMed] [Google Scholar]
- 125.Sinha R, Rothman N, Salmon CP, Knize MG, Brown ED, et al. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol. 1998;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 126.Dumont J, Jossé R, Lambert C, Anthérieu S, Laurent V, et al. Preferential induction of the AhR gene battery in HepaRG cells after a single or repeated exposure to heterocyclic aromatic amines. Toxicol Appl Pharmacol. 2010;249:91–100. doi: 10.1016/j.taap.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 127.Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98:241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 128.Sinha R, Cross A, Curtin J, Zimmerman T, McNutt S, et al. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res. 2005;49:648–655. doi: 10.1002/mnfr.200500018. [DOI] [PubMed] [Google Scholar]
- 129.Vineis P, McMichael A. Interplay between heterocyclic amines in cooked meat and metabolic phenotype in the etiology of colon cancer. Cancer Causes Control. 1996;7:479–486. doi: 10.1007/BF00052675. [DOI] [PubMed] [Google Scholar]
- 130.Nowell SA, Ahn J, Ambrosone CB. Gene-nutrient interactions in cancer etiology. Nutr Rev. 2004;62:427–438. doi: 10.1111/j.1753-4887.2004.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 131.Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- 132.Wang B, Zhou SF. Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16:4066–4218. doi: 10.2174/092986709789378198. [DOI] [PubMed] [Google Scholar]
- 133.Sinha R, Gustafson DR, Kulldorff M, Wen WQ, Cerhan JR, et al. 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a carcinogen in high-temperature-cooked meat, and breast cancer risk. J Natl Cancer Inst. 2000;92:1352–1354. doi: 10.1093/jnci/92.16.1352. [DOI] [PubMed] [Google Scholar]
- 134.Li G, Wang H, Liu AB, Cheung C, Reuhl KR, et al. Dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer Prev Res (Phila) 2012;5:963–972. doi: 10.1158/1940-6207.CAPR-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giri AK. Mutagenic and genotoxic effects of 2,3,7,8-tetrachlorodibenzop-dioxin, a review. Mutat Res. 1986;168:241–248. doi: 10.1016/0165-1110(86)90022-9. [DOI] [PubMed] [Google Scholar]
- 136.Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part F: chemical agents and related occupations. Lancet Oncol. 2009;10:1143–1144. doi: 10.1016/s1470-2045(09)70358-4. [DOI] [PubMed] [Google Scholar]
- 137.Chen ST, Chen DR, Fang JP, Lin PH. 2,3,7,8-Tetrachlorodibenzo-pdioxin modulates estradiol-induced aldehydic DNA lesions in human breast cancer cells through alteration of CYP1A1 and CYP1B1 expression. Breast Cancer. 2013 doi: 10.1007/s12282-013-0476-0. [DOI] [PubMed] [Google Scholar]
- 138.Kang HJ, Hong YB, Yi YW, Cho CH, Wang A, et al. Correlations between BRCA1 defect and environmental factors in the risk of breast cancer. J Toxicol Sci. 2013;38:355–361. doi: 10.2131/jts.38.355. [DOI] [PubMed] [Google Scholar]
- 139.Mitra AK, Faruque FS, Avis AL. Breast cancer and environmental risks: where is the link? J Environ Health. 2004;66:24–32. 40. [PubMed] [Google Scholar]
- 140.Toxicology and carcinogenesis studies of a mixture of TCDD, PeCDF and PCB126 in female Harlan Sprague-Dawley rats. Natl Toxicol Program Tech Rep Ser. 526:1–180. [PubMed] [Google Scholar]
- 141.Lin P, Chang JT, Ko JL, Liao SH, Lo WS, et al. Reduction of androgen receptor expression by benzo[alpha]pyrene and 7,8- dihydro-9,10-epoxy-7,8,9,10-tetrahydrobenzo[alpha]pyrene in human lung cells. Biochem Pharmacol. 2004;67:1523–1530. doi: 10.1016/j.bcp.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 142.Uno S, Dalton TP, Shertzer HG, Genter MB, Warshawsky D, et al. Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1(−/−) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem Biophys Res Commun. 2001;289:1049–1056. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- 143.Russo J, Tahin Q, Lareef MH, Hu YF, Russo IH. Neoplastic transformation of human breast epithelial cells by estrogens and chemical carcinogens. Environ Mol Mutagen. 2002;39:254–263. doi: 10.1002/em.10052. [DOI] [PubMed] [Google Scholar]
- 144.Hollman PC, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother. 1997;51:305–310. doi: 10.1016/s0753-3322(97)88045-6. [DOI] [PubMed] [Google Scholar]
- 145.Nishiumi S, Miyamoto S, Kawabata K, Ohnishi K, Mukai R, et al. Dietary flavonoids as cancer-preventive and therapeutic biofactors. Front Biosci (Schol Ed) 2011;3:1332–1362. doi: 10.2741/229. [DOI] [PubMed] [Google Scholar]
- 146.Wen X, Walle T. Cytochrome P450 1B1, a novel chemopreventive target for benzo[a]pyrene-initiated human esophageal cancer. Cancer Lett. 2007;246:109–114. doi: 10.1016/j.canlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 147.Van der Heiden E, Bechoux N, Muller M, Sergent T, Schneider YJ, et al. Food flavonoid aryl hydrocarbon receptor-mediated agonistic/antagonistic/ synergic activities in human and rat reporter gene assays. Anal Chim Acta. 2009;637:337–345. doi: 10.1016/j.aca.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 148.Wen X, Walle UK, Walle T. 5,7-Dimethoxyflavone downregulates CYP1A1 expression and benzo[a]pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis. 2005;26:803–809. doi: 10.1093/carcin/bgi015. [DOI] [PubMed] [Google Scholar]
- 149.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 150.Tiong CT, Chen C, Zhang SJ, Li J, Soshilov A, et al. A novel prenylflavone restricts breast cancer cell growth through AhR-mediated destabilization of ERα protein. Carcinogenesis. 2012;33:1089–1097. doi: 10.1093/carcin/bgs110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oh HY, Leem J, Yoon SJ, Yoon S, Hong SJ. Lipid raft cholesterol and genistein inhibit the cell viability of prostate cancer cells via the partial contribution of EGFR-Akt/p70S6k pathway and down-regulation of androgen receptor. Biochem Biophys Res Commun. 2010;393:319–324. doi: 10.1016/j.bbrc.2010.01.133. [DOI] [PubMed] [Google Scholar]
- 152.Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–143. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- 153.Papoutsis AJ, Lamore SD, Wondrak GT, Selmin OI, Romagnolo DF. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J Nutr. 2010;140:1607–1614. doi: 10.3945/jn.110.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Macpherson L, Matthews J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells. Cancer Lett. 2010;299:119–129. doi: 10.1016/j.canlet.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deshpande SS, Ingle AD, Maru GB. Chemopreventive efficacy of curcumin-free aqueous turmeric extract in 7,12-dimethylbenz[a]anthracene-induced rat mammary tumorigenesis. Cancer Lett. 1998;123:35–40. doi: 10.1016/s0304-3835(97)00400-x. [DOI] [PubMed] [Google Scholar]
- 156.Azuine MA, Kayal JJ, Bhide SV. Protective role of aqueous turmeric extract against mutagenicity of direct-acting carcinogens as well as benzo [alpha] pyrene-induced genotoxicity and carcinogenicity. J Cancer Res Clin Oncol. 1992;118:447–452. doi: 10.1007/BF01629428. [DOI] [PubMed] [Google Scholar]
- 157.Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997;116:197–203. doi: 10.1016/s0304-3835(97)00187-0. [DOI] [PubMed] [Google Scholar]
- 158.Garg R, Gupta S, Maru GB. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 159.Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010;13:343–349. doi: 10.1038/pcan.2010.26. [DOI] [PubMed] [Google Scholar]


