Abstract
Chronic lymphocytic leukemia (CLL) is the most common form of adult leukemia in the western world. High levels of Bcl-2 family anti-apoptotic proteins are responsible for apoptotic-resistance. Besides anti-apoptotic proteins, microenvironment provides substantial surviving signals to CLL leukemic cells. However, the in-depth-knowledge on the role of individual Bcl-2 family members in the context of microenvironment is still limited. We performed a comprehensive analysis of transcripts and proteins of 18 Bcl-2 family members using “apoptosis array micro fluidic card” in primary cells before and after stromal co-cultures. Our data showed that, 5 of 6 anti-apoptotic members (excluding Bcl-b), 2 of 3 pro-apoptotic members (excluding Bok) and 6 of 9 BH3-only members were present at detectable mRNA levels in CLL cells. Importantly, stromal mediated extended survival of CLL cells was in strong association with elevated global transcription. Upon co-culturing with stromal cells, there was early response of increase in anti- (2/5) and pro-apoptotic protein (3/8) transcripts on day 1, while increase in anti-apoptotic proteins were observed on day 3, with no significant change in pro-apoptotic proteins. Our study revealed a differential pattern of expression of both transcripts and proteins following stromal co-cultures, proposing significance of Bcl-2 family members in stromal microenvironment.
Keywords: apoptosis, Bcl-2 family members, chronic lymphocytic leukemia, microenvironment, mRNA, microarray
Introduction
Chronic lymphocytic leukemia is the most common form of adult leukemia in the western world. It is characterized by the uncontrolled accumulation of abnormal lymphocytes that fail to undergo apoptosis [1]. The defective apoptosis in CLL is either associated with genetic defects due to repression of miR-15a and miR-16 or molecular defects that are associated with high expression of anti-apoptotic proteins of Bcl-2 family proteins [2,3]. Increased levels of Mcl-1, Bcl-2 and Bcl-xL are in particular responsible for sustained survival of CLL cells by promoting resistance to spontaneous apoptosis or drug-induced apoptosis [4–6].
A growing body of evidence suggests that the survival of CLL cells is partly mediated by the interactions between leukemia cells and adjacent stromal cells residing in the lymphatic tissue or bone marrow microenvironment. CLL B-cells are in constant interaction with mesenchymal stromal cells or nurse like cells. Furthermore, the surface-receptors on B-cell such as BCR (B-cell receptor), CXCR4 or others are constantly activated by their respective ligands (anti IgM or CXCL12) expressed on the stromal cells or nurse like cells that keep them in attraction to each other in the homeostasis [7,8]. This eventually leads to the activation of BCR signaling pathway, where a number of downstream kinases are activated that are essential for survival, homing and retention of CLL cells [9]. Consistent to this notion, in vitro studies using representative bone marrow stromal cells demonstrated that CLL cells co-cultured on stromal cells exhibited induction of Mcl-1, an important pro-survival factor [10]. Similarly, incubation of CLL primary cells with representative lymph node microenvironment (nurse like cells) demonstrated increase in Bcl-xL and Bfl-1, the other anti-apoptotic members of the Bcl-2 family proteins [11,12][13].
Collectively, this context-dependent elevation of anti-apoptotic proteins suggests that the Bcl-2 family proteins per se play a major role in the stromal cell mediated survival advantage.
Bcl-2 family members of 26 proteins (14 were of human origin) were categorized into three subfamilies, on the basis of their function and the composition of their Bcl-2 homology (BH) domains [14]. Anti-apoptotic members that antagonize the apoptosis process include Bcl-2, Bcl-xL, Bcl-b, Bcl-w, Bfl-1, and Mcl-1. Pro-apoptotic Bcl-2 family members that facilitate the apoptotic cascade include Bak, Bax, Bok. The third group of the proteins called BH3 only pro-apoptotic proteins include activator BH3 only proteins (Bim, Bid) or sensitizers of apoptosis that consists of Bad, Bik, Bmf, Hrk, Noxa, Bcl-rambo, and Puma [15].
Despite the notion that Bcl-2 family proteins are the central regulators of apoptosis in general and stromal-mediated survival in particular, the depth-in-knowledge of understanding the role of individual Bcl-2 family members in context of microenvironment and associated sustained survival is still limited. To address this question, using a real-time PCR mRNA array analysis, we investigated the impact of bone marrow stromal microenvironment on the gene transcripts of Bcl-2 family pro- and anti-apoptotic proteins that are involved in apoptotic pathway in CLL cells. Using CLL primary cells and the mRNA array analysis, we screened the transcripts and protein levels of 18 Bcl-2 family proteins before and after co-culturing on stromal cells. Our data show that CLL cells were significantly rescued from spontaneous apoptosis and showed a concomitant significant increase in the global RNA synthesis in the continuous support of stroma. Importantly, the percent survival and rate of RNA synthesis for the same patient samples demonstrated a linear correlation. Investigations on individual transcripts and proteins of Bcl-2 family members showed a differential pattern of expressions after incubation with stromal cells. Collectively, our data provide a comprehensive profiling of Bcl-2 family members at transcript and protein levels and the impact of bone-marrow stromal microenvironment on their expressions in CLL.
Patients and methods
Patient Samples
The present study was carried out using leukemic lymphocytes isolated from peripheral blood samples obtained from patients with CLL. All patients signed written informed consent forms in accordance with the Declaration of Helsinki, and the laboratory protocol was approved by the institutional review board at the University of Texas MD Anderson Cancer Center.
Isolation of lymphocytes
Whole blood was collected in heparinized tubes, diluted 1:3 with cold PBS (0.135 M NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8 mM Na2HPO4 [pH 7.4]), and layered onto Ficoll-Hypaque (specific gravity, 1.086; Life Technologies, Grand Island, NY). The blood was then centrifuged at 433g for 20 min, and mononuclear cells were removed from the interphase. Cells were washed twice with cold PBS and resuspended in 10 mL RPMI 1640, supplemented with 10% Fetal Bovine Serum (FBS) and fresh cells were used for all experiments. A Coulter channelyzer (Coulter Electronics, Hialeah, FL) was used to determine cell numberand the mean cell volume.
To determine purity of the B-cell population, we measured % lymphocytes in all 12 samples obtained for mRNA array analysis. The % lymphocytes in these 12 samples ranged between 85–96% (median 92%). Additionally, lymphocytes were stained with CD19 antibody (a B cell marker) to determine % B-lymphocytes, in each sample by FACScalibur. On-an-average, % B-lymphocytes was high (median 89%; range, 82–94%) in these samples.
Preparation of CLL-BMSC co-cultures
Isolated lymphocytes were either cultured in suspension (1 × 107 cells/mL) or co-cultured on confluent layers of human BMSC (NKTert; RIKEN cell bank, Tsukuba, Japan) at a ratio of 100 CLL cells to 1 BMSC. NKTert cell line was authenticated by STR DNA fingerprinting using the AmpFlSTR Identifiler kit (Applied Biosystems). To perform co-culture experiments, we maintained continuous cultures of stromal cells and plated them at a concentration of 5 × 104 cells/well 24 hr prior to adding CLL cells at a concentration of 1 × 107 per well. At the end of incubation, CLL cells from suspension culture or the free-floating CLL cells from co-cultures were carefully removed, leaving the adherent stromal layer undisturbed, and used to assess different molecular end points.
Cell viability
Determination of CLL cell viability was carried out by two standard methods. Annexin V binding, was carried out with a Detection Kit I (PharMingen, San Diego, CA) according to the manufacturer’s instructions. Briefly, cells were washed with PBS and resuspended in 200 μL of 1X annexin binding buffer obtained fromBD Biosciences, at a concentration of 1 × 106 cells/mL. Annexin V–FITC (5 μL) was added and the cells were incubated in the dark for 15 min at room temperature. To the labeled cells was then added 10 μL propidium iodide (50 μg/mL);they were analyzed immediately with a FACScaliburcytometer (Becton-Dickinson). Data from at least 10,000 events per sample were recorded and processed using Cell Quest software (Becton-Dickinson). An alternative assay was based on the analysis of mitochondrial transmembrane potential by 3,3-dihexyloxocarbocyanine iodine (DiOC6) and cell membrane permeability to propidium iodide [10]. Briefly, a 200-μL cell suspension was collected at the indicated time points and transferred to FACS tubes containing 200 μL of 60 nM DiOC6 (Molecular Probes, Eugene, OR) and 10 μg/mL propidium iodide (Molecular Probes) in RPMI with 0.5% BSA. Cells were then incubated at 37°C for 15 min and analyzed within 30 min using a FACScalibur (Becton Dickinson). Fluorescence was recorded at 525 nm (FL-1) for DiOC6 and at 600 nm (FL-3) for PI.
Global RNA synthesis
Primary CLL cells in suspension or BMSC co-culture were used to measure global RNA synthesis by [3H]uridine incorporation assay. Prior to removal of the aliquot, 10 μCi/mL [3H]uridine was added to these cultures and the incubation was continued for an additional 30 min. The labeled cells were then extracted using perchloric acid and the pellets were incubated overnight with KOH at 37°C to dissolve RNA or protein. The radioactivity was measured by scintillation counting. [3H]uridine (specific activity, 41.2 Ci/mmol) were purchased from Moravek Biochemicals (Brea, CA).
Microarray analysis
In order to evaluate changes in individual transcripts of Bcl-2 family proteins in presence of bone marrow stromal microenvironment, we used ‘human apoptotic array micro fluidic card- Applied Biosystems’ (4378701) for Real-time PCR. Briefly, CLL lymphocytes co-cultured with or without stromal cells were collected and processed for RNA extraction (Qiagen RNeasy Mini Kit, 74106). 2 μg of total RNA was converted to cDNA using High Capacity RNA-to-cDNA kit (Applied Biosystems, 4387406). 100 ng of cDNA was introduced each lane micro fluidic array card along with PCR master mixture (Applied Biosystems, 4304437). The array card was sealed and PCR reactions were completed using 7900HT sequence detection system (Applied Biosystems). The data was analyzed using software RQ Manager and Data Assist. All mRNA expressions were normalized to three control genes β-actin, GAPDH and 18S. This apoptosis array micro fluidic card contains 93 mRNA targets including 18 mRNA targets of Bcl-2 family. However, the data obtained for transcripts of Bcl-2 family members are presented in this manuscript (Table 1 lists name of these genes and respective proteins).
Table 1.
Bcl-2 family genes and proteins on human apoptotic array microfluidic card and their detection in CLL lymphocytes.
| Gene name | Protein name | Function | Detected in array* | |
|---|---|---|---|---|
| 1 | BCL2 | Bcl-2 | Anti-apoptotic | Y |
| 2 | BCL2A1 | Bfl-1 | Anti-apoptotic | Y |
| 3 | BCL2L1 | Bcl-xL | Anti-apoptotic | Y |
| 4 | MCL1 | Mcl-1 | Anti-apoptotic | Y |
| 5 | BCL2L10 | Bcl-b | Anti-apoptotic | N |
| 6 | BCL2L2 | Bcl-w | Anti-apoptotic | Y |
| 7 | BAK1 | Bak | Pro-apoptotic Multi-Domain | Y |
| 8 | BAX | Bax | Pro-apoptotic Multi-Domain | Y |
| 9 | BOK | Bok | Pro-apoptotic Multi-Domain | N |
| 10 | BCL2L11 | Bim | Pro-apoptotic BH3-only | Y |
| 11 | BCL2L13 | Bcl-rambo | Pro-apoptotic BH3-only | N |
| 12 | BCL2L14 | Bcl-g | Pro-apoptotic BH3-only | N |
| 13 | BAD | Bad | Pro-apoptotic BH3-only | Y |
| 14 | BBC3 | Puma | Pro-apoptotic BH3-only | Y |
| 15 | HRK | Hrk | Pro-apoptotic BH3-only | N |
| 16 | PMAIP1 | Noxa | Pro-apoptotic BH3-only | Y |
| 17 | BID | Bid | Pro-apoptotic BH3-only | Y |
| 18 | BIK | Bik | Pro-apoptotic BH3-only | Y |
Bcl-2 family members analyzed through Real-time PCR using microarray fluidic card (Applied Biosystems). (* Y = Detected in all the samples analyzed (n=12), *N= Not detected in one or more samples analyzed)
Immunoblot analysis
CLL cell pellets were washed with PBS and lysed on ice for 20 min in lysis buffer containing 25 mM HEPES (pH 7.5), 300 mM NaCl, 1.5 mM MgCl2, 0.5% sodium deoxycholate, 20 mM glycerophosphate, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.2 mM EDTA (pH 8), 0.5 mM dithiothreitol, 1 mM sodium orthovanadate (pH 10), one mini complete protease inhibitor (Roche) tablet per 10 mL of buffer. Cells were centrifuged at 14,000g for 10 min at 4°C, and the supernatant was removed and the protein content determined using a DC protein assay kit (Bio-Rad Laboratories). Aliquots (35–45 μg) of total cell protein were boiled with Laemmli sample buffer, loaded onto 4% to 12% SDS-PAGE gels, and transferred to nitrocellulose membranes (GE Osmonics Labstore). Membranes were blocked for 1 hr in PBS–Tween-20 containing 5% nonfat dried milk and then incubated with primary antibodies for 2 hr at room temperature for rabbit polyclonal antibody to Bcl2-A1 (Epitomics, CA, 1639-1), Bcl-xL (Santa Cruz, SC634), Bad (Cell Signaling, MA, 9292), Puma (Cell Signaling, MA, 4976), Noxa (Santa Cruz, CA, SC30209), Bim (Santa Cruz, CA, SC11425), Mcl-1 (Santa Cruz, CA, SC819) and mouse monoclonal antibody to Bcl-w (Millipore, MA, AB1723), Bax (Santa Cruz, CA, SC20067), Bik (Santa Cruz, CA, SC365625), Bcl-2 (Santa Cruz, CA), and Gapdh (Sigma, St. Louis, MO). After washing with PBS–Tween-20, membranes were incubated with infrared-labeled secondary antibodies (LI-COR Inc) for 1 hr, scanned, and visualized using LI-COR Odyssey Infrared Imager.
Statistical analysis
Linear correlations were derived using the GraphPad Prism5 software (GraphPad Software, Inc. San Diego, CA). r One sample t test and paired student t-tests (two tailed) were performed for statistical analyses and were mentioned at respective figure legends.
Results
CLL cells co-cultured on stromal cells demonstrate time-dependent sustainability
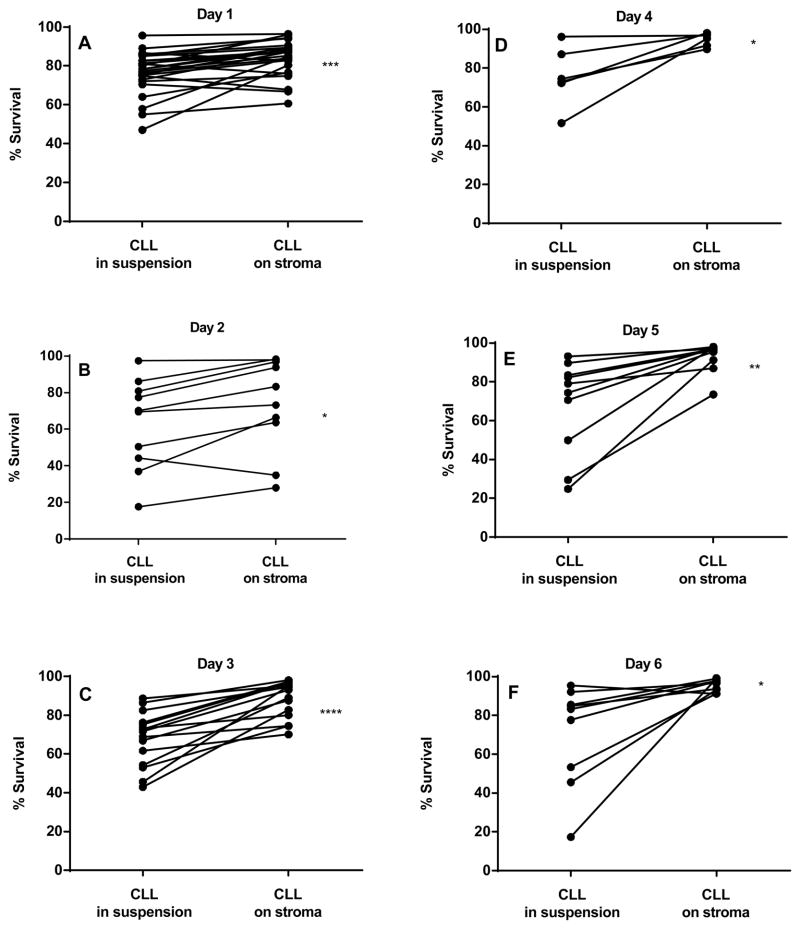
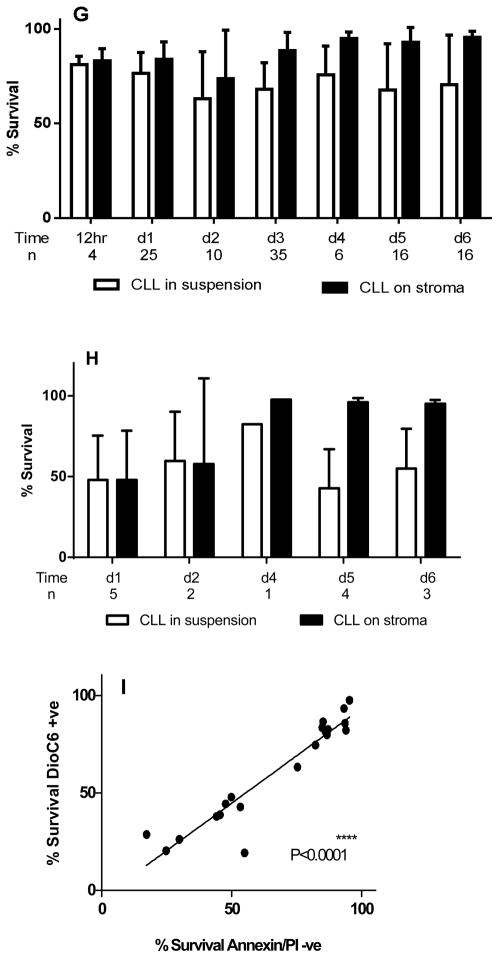
Primary B leukemic lymphocytes obtained from patients with CLL were co-cultured on NKtert stromal cell line (a representative human bone marrow stromal line) for different time periods and the viability of CLL lymphocytes were measured by Annexin V/PI staining (Fig. 1A–I). At the end of 12 hr incubation, there was no significant protection observed with respect to time matched controls (n=4; p=0.222; data not shown). After 24 hr, CLL cells co-cultured on stromal cells demonstrated significant viability compared to time matched controls (Fig. 1A; n=25; p=0.0004). Extended incubations on stroma further increased the viability of CLL cells significantly that include day 2 (Fig. 1B; n=10; p=0.0107), day 3 (Fig. 1C; n=15; p<0.0001), day 4 (Fig. 1D; n=6; p=0.0246), day 5 (Fig. 1E; n=10; p=0.0038), and day 6 (Fig. 1F; n=9; p=0.0232). Taken together, though there was heterogeneity among patient samples regarding percent surviving cells and the cyto-protection rendered by stroma, overall, there was significant protection of apoptosis in presence of stromal cells (Fig. 1G). To further confirm these results, DiOC6 staining method was used to measure the viable cells. DiOC6 staining data also showed similar trend of protection of CLL lymphocytes when co-cultured on stroma cells (Figure 1H). Plotting values obtained from both methods for same patients’ samples provided a significant linear correlation (P<0.0001; Pearson r = 0.94; Figure 1I).
Figure 1. CLL cells co-cultured on stromal cells demonstrate time-dependent sustainability (A-I).
CLL lymphocytes were incubated with NKTert stromal cell line at different time periods (1–6 days) and the viability of cells was measured by Annexin V/PI staining (A–F). Mean % survival of CLL lymphocytes was compared between suspension cultures and stroma co-cultures using Annexin V staining method (G) and DiOC6 staining method (H). The ‘d’ denotes ‘day’. Values obtained from two standard methods for same patient samples were correlated (Annexin V and DiOC6) to obtain a linear correlation (I). Paired student t-tests (two tailed) statistical analysis was performed (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
CLL cells co-cultured on stromal cells demonstrate time-dependent increase in rate of global RNA synthesis
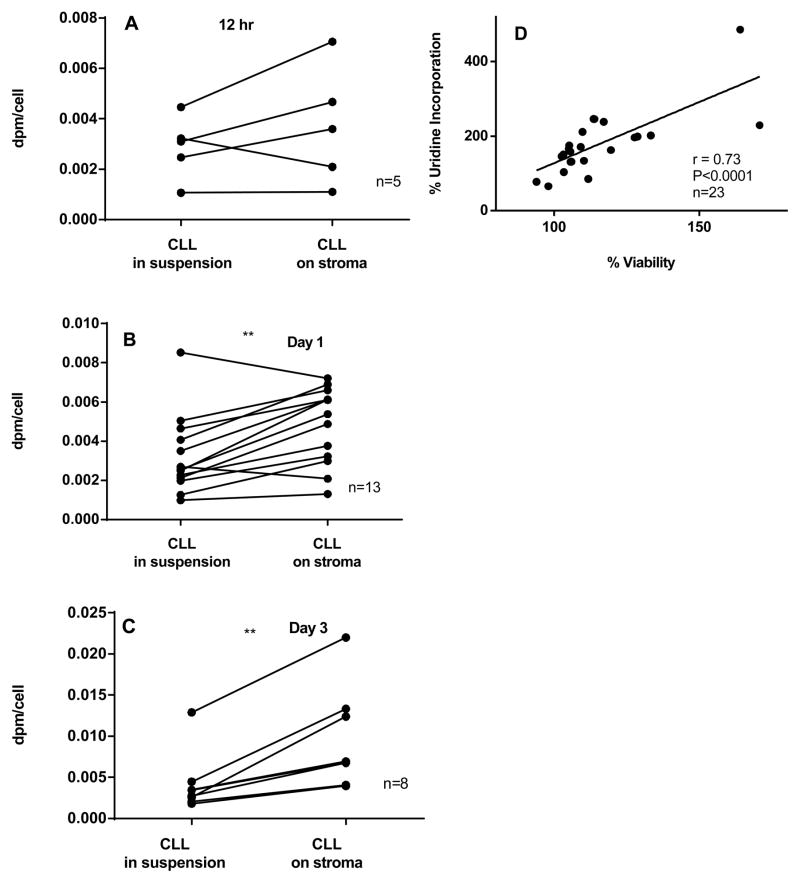
Given that CLL cells are replicationally quiescent but active in transcription, we next investigated the effect of NKtert cells on global RNA synthesis (includes mRNA, tRNA, and rRNA synthesis) in CLL lymphocytes at increasing time periods using uridine incorporation assay (12 hr, day 1 and day 3; Figure 2A–C). Consistent with viability data, our data showed no significant increase in global RNA synthesis at 12 hr (Figure 2A). However, there was a significant increase observed after 24 hr (1.7±0.5 fold; n=13, p=0.0019; Figure 2B), which was persistent until day 3 (2.5± 1.0 fold; n=8, p=0.029; Figure 2C). The mean of uridine incorporation (dpm/cell) at day 1 and day 3 were consistently high when cells were co-cultured on NKtert cells (Figure 2D). Furthermore, uridine incorporation, a measurement of total RNA synthesis, was strongly (P<0.0001) and linearly associated (r=0.73) with viability of CLL cells measured in 23 samples (Figure 2E).
Figure 2. CLL cells co-cultured on stromal cells demonstrate time-dependent increase in rate of global RNA synthesis (A–D).
CLL lymphocytes were incubated with NKTert stromal cell line for different time periods [12 hr (A), day 1 (B) and day 3 (C)] and the global RNA synthesis was measured by uridine incorporation assay. Values obtained from viability assays and uridine incorporation assays for same patient samples was correlated to obtain a linear correlation (D; n=23; r=0.73). Paired student t-tests (two tailed) analysis was performed for statistical significance (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Effect of bone marrow stromal cells on the modulation of mRNA expression of Bcl-2 family members
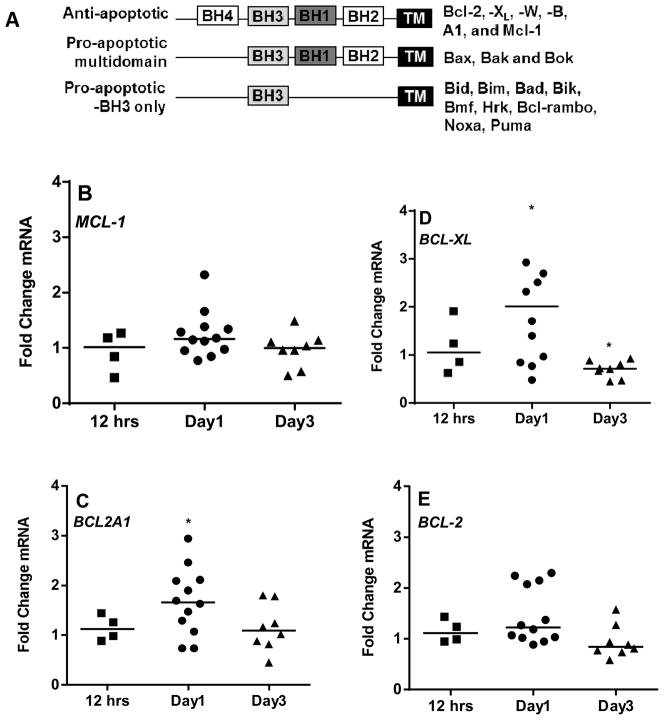
Our uridine incorporation assay showed increase in overall global RNA synthesis of CLL lymphocytes. In order to investigate which specific mRNA of Bcl-2 family members is increased in our co-culturing model system, we used Real-Time PCR apoptotic array (microfluidic card from Applied Biosystems) to determine the individual transcripts of anti- and pro-apoptotic Bcl-2 family members (given in Figure 3A) at different time periods (12 hr, day 1 and day 3; Figure 3B–F and 4A–H). We analyzed total of 18 gene transcripts of Bcl-2 family members. This array card contains six anti-apoptotic members, three pro-apoptotic multi-domain members, nine BH3- only pro-apoptotic members of the Bcl-2 family (Table 1). Of these 18 Bcl-2 family members, majority (13 of them) were expressed endogenously in CLL lymphocytes. However, transcript levels of anti-apoptotic gene - BCL2L10 (BCL-B) and four pro-apoptotic gene - BCL2L13 (BCL-RAMBO), BCL2L14, BOK, and HRK, were below the limit of detection in most of the samples analyzed (data not shown).
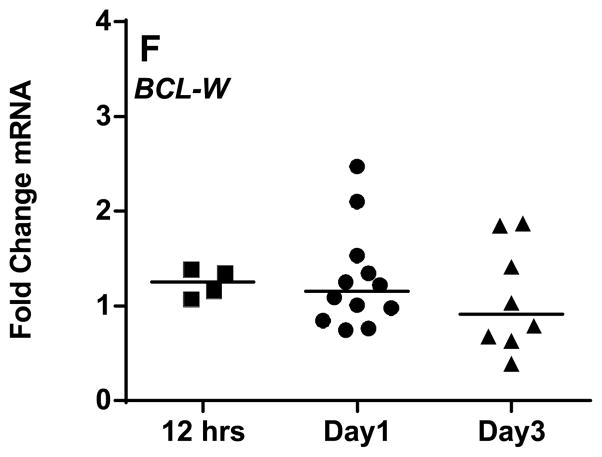
Figure 3. Comparison of mRNA expression of Bcl-2 family anti-apoptotic members in CLL cells between suspension cultures and stroma co-cultures (B–F).
CLL lymphocytes were incubated with NKTert stromal cell line at different time periods (12 hr, day 1, and day 3) and the individual transcript levels of Bcl-2 family proteins were determined using ‘human apoptotic array micro fluidic card’ (Applied Biosystems, 4378701) for Real-time PCR. A schematic illustration of different Bcl-2 family members is provided (A). mRNA expressions of a spectrum of anti-apoptotic Bcl-2 family proteins (B–F), were evaluated. Each symbol on the graph represents fold change in mRNA expression with respect to that after stromal co-incubations, which is normalized to respective mRNA levels of control samples. One sample t test statistical analysis was performed (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
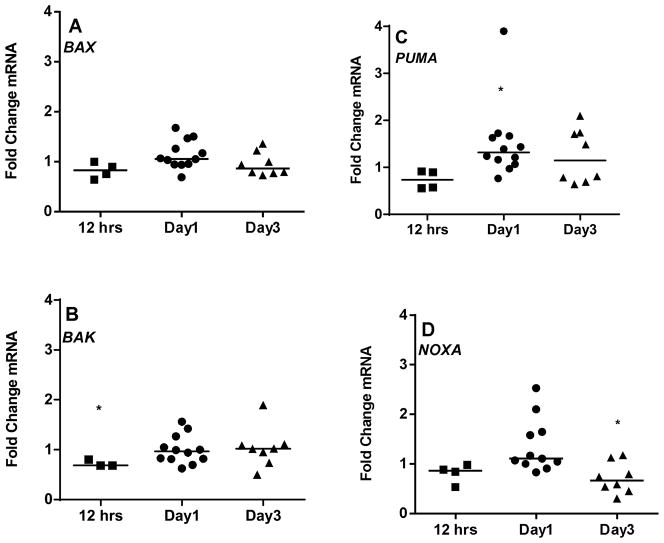
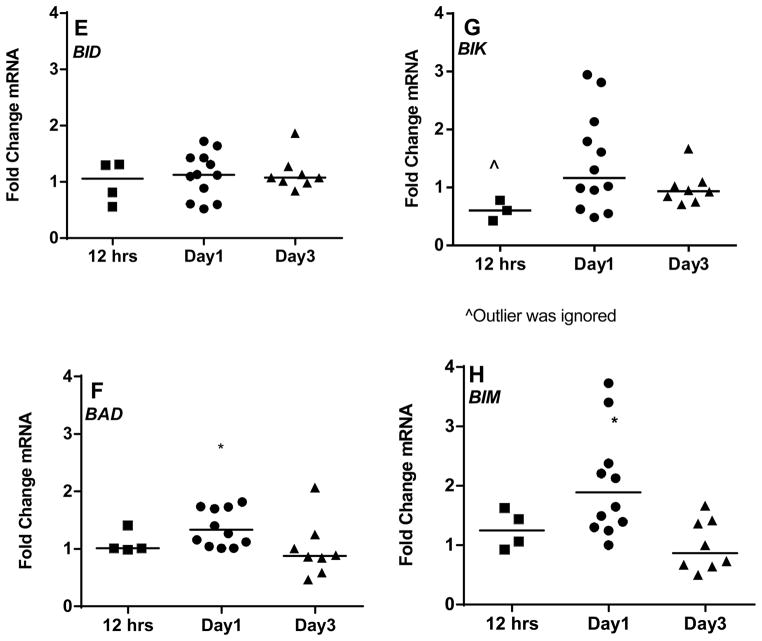
Figure 4. Comparison of mRNA expression of Bcl-2 family pro-apoptotic members in CLL between suspension cultures and stroma co-cultures (A–H).
CLL lymphocytes were incubated with NKTert stromal cell line at different time periods (12 hr, day 1, and day 3) and the individual transcript levels of pro-apoptotic Bcl-2 family proteins were determined using ‘human apoptotic array micro fluidic card’ (Applied Biosystems, 4378701) for Real-time PCR. mRNA levels of multi-domain pro-apoptotic Bcl-2 family members (A and B) and mRNA expressions of BH3-only pro-apoptotic proteins (C–H) were evaluated. Each symbol on the graph represents fold change in mRNA expression with respect to that after stromal co-incubations, which is normalized to respective mRNA levels of control samples. One sample t test statistical analysis was performed (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
In short term co-cultures on stroma (12 hr), CLL lymphocytes demonstrated modest increase in transcripts of anti-apoptotic members to a median of 1–1.25 fold (n=4) in MCL-1 (Figure 3B), BCL2A1 (Figure 3C), BCL2L1 (BCL-XL) (Figure 3D), BCL-2 (Figure 3E), and BCL2L2 (BCL-W) (Figure 3F). By day 1, this increase was significant in all the transcripts of anti-apoptotic members ranging between 1.3 to 2.1 fold (Figure 3B-F). Interestingly, the maximum increase in mRNA level was observed with BCL-XL (n=12, p=0.0179; Figure 3D) while minimum or no change was observed with BCL-W (Figure 3F). Of note, by day 3, the levels of all anti-apoptotic transcripts were returned to the basal levels (Figure 3B–F).
Bcl-2 pro-apoptotic multi domain members BAK and BAX had insignificant decrease in their transcript levels during short term co-cultures (median 0.68 and 0.82 fold respectively; 12 hr), and thereafter remained similar on day 1 and 3 (Figure 4A and B). As mentioned above, the third member of this group, BOK was below the limit of detection. Given the opposing functions of anti- and pro-apoptotic proteins, one would expect a differential pattern of expression between these two sets of proteins. To our surprise, in context to stromal microenvironment, pro-apoptotic BH3 only members indeed followed the similar pattern of mRNA increase as of anti-apoptotic proteins (Figure 4C–H), at day 1 for PUMA (n=12, p=0.0487; Figure 4C), NOXA (n=12, p=0.0505; Figure 4D), BIM (n=12, p=0.0054; Figure 4H) and BAD (n=12, p=0.0394; Figure 4F) but returned to the basal levels observed in suspension cells on day 3 (median ~1 fold). Pro-apoptotic BH3 only members - BID and BIK mRNA remained unchanged (Figure 4E and G).
Effect of bone marrow stromal cells on the modulation of protein levels of Bcl-2 family members
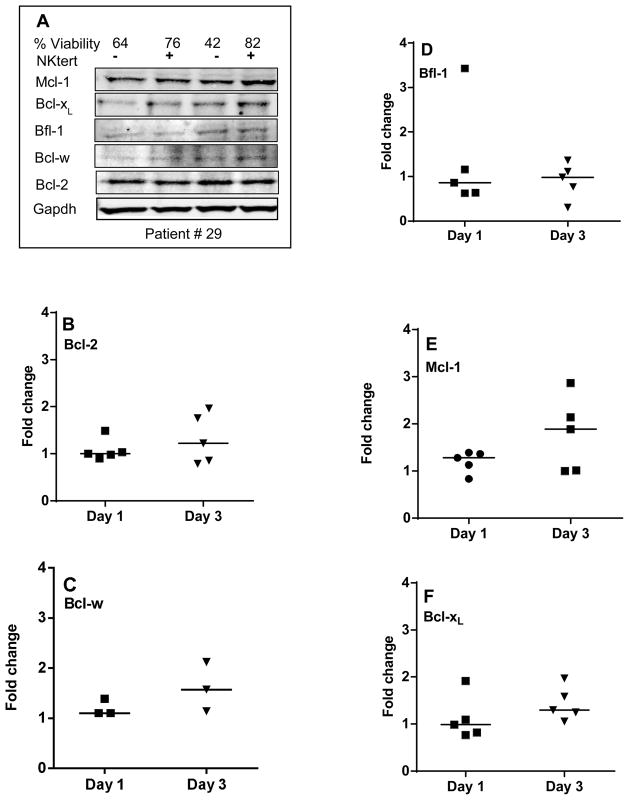
Given that Bcl-2 family transcripts exhibited variability in CLL cells co-cultured on stroma, we next investigated the protein levels of Bcl-2 family members. Figure 5A represents one representative patient data of immunoblot for anti-apoptotic proteins. Overall, anti-apoptotic proteins Bcl-2, Bfl-1, Bcl-xL, Mcl-1, Bcl-w showed either stable expression or increased expression in CLL cells when co-cultured on NKtert stromal cells on day 1 and day 3 (Figure 5B–F; n=5). Of note, CLL lymphocytes from all patient samples displayed sustainability in support of stromal cells. Bcl-2, Mcl-1, Bcl-xL, and Bcl-w protein levels increased on day 3 except Bfl-1. However, cells from patient #31 did not demonstrate increase in survival when co-cultured on NKtert cells. Corresponding to this observation, CLL lymphocytes from this patient showed decrease in almost all anti-apoptotic proteins on day 1 (data not shown).
Figure 5. Comparison of protein expression of Bcl-2 family anti-apoptotic members in CLL cells between suspension cultures and stroma co-cultures (A–F).
CLL lymphocytes were incubated with NKTert stromal cell line at different time periods (day 1 and day 3) and the individual protein levels of Bcl-2 family anti-apoptotic members were determined by immunoblotting experiments. One representative patient data for a panel of anti-apoptotic proteins (A) and quantitative analysis of all the samples evaluated for anti-apoptotic proteins is provided (B–F). Each symbol on the graph represents fold change in protein expression with respect to that after stromal co-incubations, which is normalized to respective protein levels of control samples. One sample t test statistical analysis was performed.
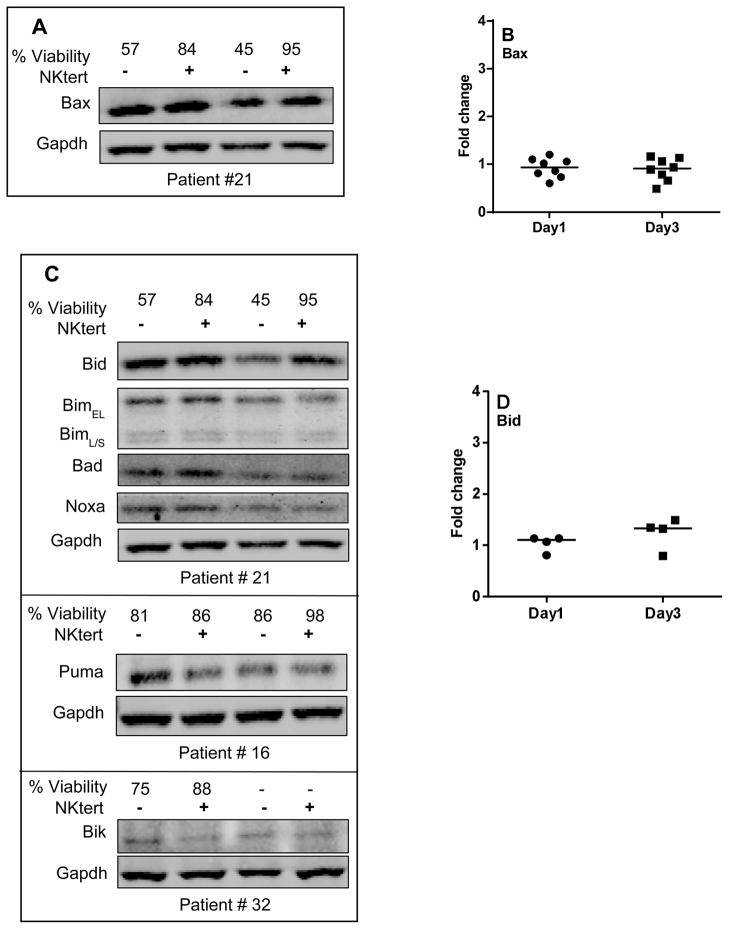
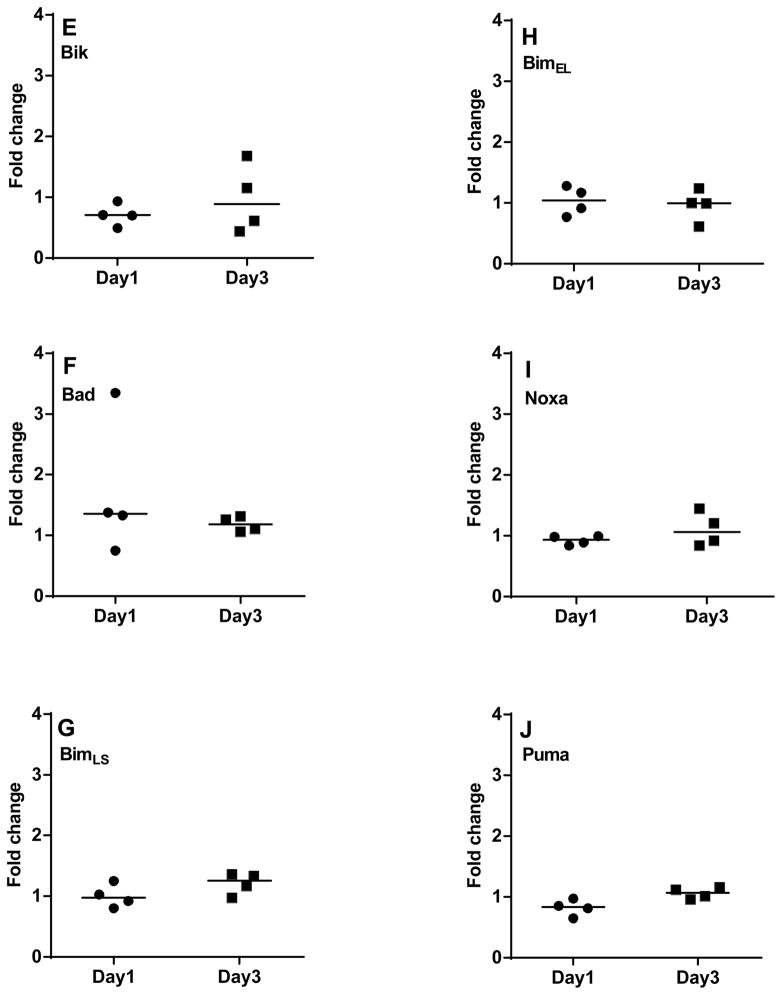
With regard to pro-apoptotic proteins, one representative patient data for pro-apoptotic multi domain protein Bax is provided in Figure 6A. Consistent with the transcript data, additional samples evaluated for Bax expression showed no change in protein levels at day 1 and day 3 (Figure 6B; n=8). Bak expression was not measured in these experiments. Figure 6C represents one representative patient data for BH3-only pro-apoptotic proteins. Additional samples evaluated for these proteins demonstrated that Bid remained unchanged (Figure 6D; n=4), while Bik decreased on day 1 (n=4, P=0.043; Figure 6E). Bad increased on day 1 (3/4 patients) but remained unchanged on day 3 (Figure 6F). Short and long isoforms of Bim, and Noxa displayed no change (Figure 6G–I) while Puma slightly decreased (n=4, p=0.072) on day 1; but the levels were regained on day 3 (Figure 6J).
Figure 6. Comparison of protein expression of Bcl-2 family pro-apoptotic members in CLL cells between suspension cultures and stroma (A–J).
CLL lymphocytes were incubated with NKTert stromal cell line at different time periods (day 1 and day 3) and the individual protein levels of Bcl-2 family pro-apoptotic proteins were determined by immunoblotting experiments. One representative patient data for expression of multi-domain pro-apoptotic protein Bax (A) and quantitative analysis of all the samples evaluated for Bax is provided (B). One representative patient data for a panel of BH3 only pro-apoptotic proteins (C) and quantitative analysis of all the samples evaluated for BH3 only pro-apoptotic proteins is provided (D–J). Each symbol on the graph represents fold change in protein expression with respect to that after stromal co-incubations, which is normalized to respective protein levels of control samples. One sample t test statistical analysis was performed.
Discussion
CLL is inherently characterized by the defective cellular apoptotic machinery. Bcl-2 family anti-apoptotic proteins, which are the major road blocks of apoptotic pathway, are expressed high in B-CLL malignant cells compared to normal B-mature lymphocytes [3,16,17]. Of 6 members of anti-apoptotic proteins, Bcl-2 is required for maintenance of peripheral lymphocyte populations in adulthood [18] and Bcl-xL is required for survival of immature thymocytes [19]. Anti-apoptotic Mcl-1 is needed for survival of hematopoietic stem cells, as well as for development of mature lymphocytes. [20,21]. In corollary to this transgenic mice overexpressing Mcl-1 develops B-cell lymphomas [22]. mRNA levels of anti-apoptotic proteins significantly correlated with measure of cell death as knocking down Bfl-1 with siRNA promoted apoptosis [23]. Another member, Bcl-w, is not associated with hematopoetic cells rather is present in sperm cells. [24]. Together, these reports provide individual expression of Bcl-2 family anti-apoptotic proteins and their important role in resistance to apoptosis of CLL cells [25,26]. Consistent to these reports, our comprehensive evaluation of Bcl-2 family anti-apoptotic proteins in CLL primary cells demonstrated presence of 5 of 6 anti-apoptotic members at mRNA (Table 1 and Figure 3) and protein levels (Figure 5–6). While Bfl-1 was not affected Bcl-xL, Mcl-1 and Bcl-w protein levels were significantly induced with stromal cell incubations.
As opposed to Bcl-2 family anti-apoptotic proteins, pro-apoptotic proteins Bax and Bak promote apoptosis through the release of cytochrome c from mitochondria in cancer cells [27–29]. Bax is required for the programmed cell death of mature lymphocytes [30]. Pro-apoptotic Bak helps to govern the homeostasis of B-cell populations in vivo and bak−/− platelets have extended half-life [31]. Our evaluation using apoptotic microarray demonstrated endogenous presence of Bax and Bak, (but not Bok) in CLL cells (Figure 4), and compared to control cells, mRNA and protein levels did not change when CLL cells were cocultured on stroma.
The third group of Bcl-2 family proteins is BH3 only proteins, which either activate or sensitize leukemic cells to apoptosis depending on their structural and functional homology. Bid regulates turnover of some populations of myeloid cells [32]; Bim is required for programmed cell death of memory B cells and plasma cells (or their progenitors) [33–36]; and Bad has tumor suppressor role in mature B cells [37]. In our evaluations, six of nine BH3 only proteins, were present at mRNA level in CLL cells (Table 1 and Figure 4) and six were expressed at protein level (Figure 6).
While the circulating lymphocytes are in constant uphold by intrinsic Bcl-2 family proteins, lymphocytes that are homing in bone marrow or lymph nodes are essentially retained by signals from extrinsic microenvironment that promote survival advantage to spontaneous and drug-induced apoptosis [38]. Consistent with this, our data showed an increase in BFL-1 mRNA in cells co-cultured on stroma. Reports reveal that co-culturing CLL cells on stroma decreased the apoptotic priming in a leukemic cell, evidencing the significance of Bcl-2 family anti-apoptotic proteins in context to tumor microenvironment[39]. Mcl-1 is induced in cells in contact with follicular dendritic cell line, while Bcl-xL was induced in cells co-cultured with CD-40 ligand (CD-154)-expressing fibroblast cell line [12,40]. Bone biopsy derived marrow stromal elements facilitated angiogenic switch by increasing Mcl-1 and Bcl-2 in CLL [41]. Importantly, several chemotherapeutics that induce apoptosis in CLL suspension cells, partially fail to promote apoptosis in cells co-cultured with stroma [42]. This suggests that the high levels of anti-apoptotic proteins induced in association with microenvironment could possibly be responsible for the apoptosis resistance. On the basis of this notion, several inhibitors of Bcl-2 family anti-apoptotic proteins or Bcl-2 antagonists were synthesized and introduced for treatment of CLL. These therapies (gossypol, AT-101, ABT-737) that target specifically Bcl-2 family pro-survival proteins have demonstrated to bypass survival advantage and induce apoptosis in CLL cells [6].
While these studies reveal the significance of one or more anti-apoptotic members in diversified microenvironments, a complete representation of Bcl-2 family members in context to microenvironment has never been established. In this effort, for the first time, using microarray technique, we screened the molecular profile of mRNA and protein expression of all Bcl-2 family members in the context of bone marrow microenvironment. Using CLL primary cells and Nktert stromal cells (a representative stromal cell line of bone marrow stromal microenvironment), we compared total of 18 genes at mRNA and protein levels before and after co-culturing with stromal cells. Overall, our study demonstrated a significant increase in global RNA synthesis in cells co-cultured on stroma (Figure 2). In addition, our data showed an increase in the mRNA levels of all Bcl-2 family anti-apoptotic proteins in CLL cells co-cultured on stroma (Figure 3). This goes in-line with the observations reported by Buggins et al, who demonstrated that CLL cells receive specific survival signals in presence of endothelial cells that is mediated through the activation of NF-κB and the induction of downstream target genes [43]. Pro-apoptotic proteins Bik (P=0.047) and Puma (P=0.072) decreased slightly at 24 hrs while Mcl-1 increased. In contrary, no changes in Bax transcript or protein levels were observed (Figure 4 and 6) suggesting that rather than total protein levels, functional modifications such as oligomerization of Bax protein may be significant in promoting apoptosis [44]. Of note, the presence of single nucleotide polymorphism in promoter region of Bax gene together with lower Bax protein expression projected overall shorter survival in CLL [45]. Collectively, our data show that down-regulation of pro-apoptotic proteins at early time points and up-regulation of anti-apoptotic proteins at later time points may partly impart to the survival advantage to CLL cells.
Another factor to be considered is duration of co-cultures. Studies showed that the longer incubation time (up to 7 days) virtually increased the expressions of Bcl-2, Bcl-xL and Mcl-1 in CLL [43]. However, longer incubations may possibly modulate the biology of CLL cells, where other factors such as nurse-like cells might intervene and contribute to the apoptosis resistance. For this reason we chose shorter incubation times (maximum of day 3) to assess the effects of mimicking the bone marrow microenvironment. During 24 hr incubations, our data showed moderate to significant increase in almost all transcripts of both anti- and pro-apoptotic members of Bcl-2 family, but not all of them showed similar trend of increase in protein expressions (Figure 3–6). Additionally, there was an early response in pro-apoptotic proteins either decreased (2/8) or stably expressed (5/8) and late response in anti-apoptotic proteins (4/5 increased) in co-cultured cells.
The balance of pro- and anti-apoptotic proteins’ expression decides the fate of CLL cells. In the current investigation, we quantitated changes in mRNA and protein level of Bcl-2 family proteins, but did not evaluate mechanism of these changes. Changes at transcript and protein levels, reflect the impact on gene transcription and protein translation; however, posttranslational modifications may partly be responsible for turnover of proteins (reviewed in [46]). These modifications need to be evaluated to identify comprehensive mechanism of modulation of proteins.
In summary, our study demonstrates a variable pattern in all members of Bcl-2 family proteins in CLL lymphocytes co-cultured with stroma, proposing a substantial evidence for the significance of Bcl-2 family members in apoptosis resistance with respect to bone microenvironment. Further investigations using diverse microenvironments may provide the prospect of developing therapeutic strategies aimed at inducing apoptosis in CLL cells.
Acknowledgments
This work was supported in part by grants PO1CA081534 (CLL Consortium (to V.G. and W.G.W), CLL-Global Research Foundation (to K.B.) and P30-16672 Cancer Center Support Grant from the National Cancer Institute.
References
- 1.Schimmer AD, Munk-Pedersen I, Minden MD, Reed JC. Bcl-2 and apoptosis in chronic lymphocytic leukemia. Curr Treat Options Oncol. 2003;4:211–218. doi: 10.1007/s11864-003-0022-y. [DOI] [PubMed] [Google Scholar]
- 2.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitada S, Andersen J, Akar S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91:3379–3389. [PubMed] [Google Scholar]
- 4.Kitada S, Reed JC. MCL-1 promoter insertions dial-up aggressiveness of chronic leukemia. J Natl Cancer Inst. 2004;96:642–643. doi: 10.1093/jnci/djh153. [DOI] [PubMed] [Google Scholar]
- 5.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–153. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger M, Hartmann T, Krome M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 8.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 9.Petlickovski A, Laurenti L, Li X, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105:4820–4827. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 10.Balakrishnan K, Burger JA, Quiroga MP, et al. Influence of bone marrow stromal microenvironment on forodesine-induced responses in CLL primary cells. Blood. 2010;116:1083–1091. doi: 10.1182/blood-2009-10-246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 12.Pedersen IM, Kitada S, Leoni LM, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- 13.Vogler M, Butterworth M, Majid A, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 14.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 15.Del Gaizo Moore V, Letai A. BH3 profiling - Measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2012 doi: 10.1016/j.canlet.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanz L, Garcia-Marco JA, Casanova B, et al. Bcl-2 family gene modulation during spontaneous apoptosis of B-chronic lymphocytic leukemia cells. Biochem Biophys Res Commun. 2004;315:562–567. doi: 10.1016/j.bbrc.2004.01.095. [DOI] [PubMed] [Google Scholar]
- 17.McConkey DJ, Chandra J, Wright S, et al. Apoptosis sensitivity in chronic lymphocytic leukemia is determined by endogenous endonuclease content and relative expression of BCL-2 and BAX. J Immunol. 1996;156:2624–2630. [PubMed] [Google Scholar]
- 18.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 19.Ma A, Pena JC, Chang B, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci U S A. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opferman JT, Iwasaki H, Ong CC, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 21.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 22.Zhou P, Levy NB, Xie H, et al. MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood. 2001;97:3902–3909. doi: 10.1182/blood.v97.12.3902. [DOI] [PubMed] [Google Scholar]
- 23.Olsson A, Norberg M, Okvist A, et al. Upregulation of bfl-1 is a potential mechanism of chemoresistance in B-cell chronic lymphocytic leukaemia. Br J Cancer. 2007;97:769–777. doi: 10.1038/sj.bjc.6603951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Print CG, Loveland KL, Gibson L, et al. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci U S A. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepper C, Lin TT, Pratt G, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112:3807–3817. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 26.Lazaridou A, Miraxtsi C, Korantzis J, Eleftheriadis N, Christakis JI. Simultaneous detection of BCL-2 protein, trisomy 12, retinoblastoma and P53 monoallelic gene deletions in B-cell chronic lymphocytic leukemia by fluorescence in situ hybridization (FISH): relation to disease status. Leuk Lymphoma. 2000;36:503–512. doi: 10.3109/10428190009148398. [DOI] [PubMed] [Google Scholar]
- 27.Kuwana T, Mackey MR, Perkins G, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 28.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonsson B, Conti F, Ciavatta A, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 30.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinkel SS, Ong CC, Ferguson DO, et al. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 34.Bouillet P, Purton JF, Godfrey DI, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 35.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 36.Fischer SF, Bouillet P, O’Donnell K, Light A, Tarlinton DM, Strasser A. Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood. 2007;110:3978–3984. doi: 10.1182/blood-2007-05-091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranger AM, Zha J, Harada H, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton E, Pearce L, Morgan L, et al. Mimicking the tumour microenvironment: three different co-culture systems induce a similar phenotype but distinct proliferative signals in primary chronic lymphocytic leukaemia cells. Br J Haematol. 2012;158:589–599. doi: 10.1111/j.1365-2141.2012.09191.x. [DOI] [PubMed] [Google Scholar]
- 39.Davids MS, Deng J, Wiestner A, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120:3501–3509. doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willimott S, Baou M, Naresh K, Wagner SD. CD154 induces a switch in pro-survival Bcl-2 family members in chronic lymphocytic leukaemia. Br J Haematol. 2007;138:721–732. doi: 10.1111/j.1365-2141.2007.06717.x. [DOI] [PubMed] [Google Scholar]
- 41.Kay NE, Shanafelt TD, Strege AK, Lee YK, Bone ND, Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an “angiogenic switch”. Leuk Res. 2007;31:899–906. doi: 10.1016/j.leukres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buggins AG, Pepper C, Patten PE, et al. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Res. 2010;70:7523–7533. doi: 10.1158/0008-5472.CAN-10-1634. [DOI] [PubMed] [Google Scholar]
- 44.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starczynski J, Pepper C, Pratt G, et al. Common polymorphism G(-248)A in the promoter region of the bax gene results in significantly shorter survival in patients with chronic lymphocytic Leukemia once treatment is initiated. J Clin Oncol. 2005;23:1514–1521. doi: 10.1200/JCO.2005.02.192. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi V, Balakrishnan K, Chen LS. Mcl-1: the 1 in CLL. Blood. 2008;112:3538–3540. doi: 10.1182/blood-2008-07-170241. [DOI] [PubMed] [Google Scholar]