Abstract
The goal of this study was to assess the long-term biocompatibility of single-wall carbon nanotubes (SWNTs) for tissue engineering of articular cartilage. We hypothesized that SWNT nanocomposite scaffolds in cartilage tissue engineering can provide an improved molecular-sized substrate for stimulation of chondrocyte growth, as well as structural reinforcement of the scaffold's mechanical properties. The effect of SWNT surface functionalization (-COOH or -PEG) on chondrocyte viability and biochemical matrix deposition was examined in two-dimensional cultures, in three-dimensional (3D) pellet cultures, and in a 3D nanocomposite scaffold consisting of hydrogels+SWNTs. Outcome measures included cell viability, histological and SEM evaluation, GAG biochemical content, compressive and tensile biomechanical properties, and gene expression quantification, including extracellular matrix (ECM) markers aggrecan (Agc), collagen-1 (Col1a1), collagen-2 (Col2a1), collagen-10 (Col10a1), surface adhesion proteins fibronectin (Fn), CD44 antigen (CD44), and tumor marker (Tp53). Our findings indicate that chondrocytes tolerate functionalized SWNTs well, with minimal toxicity of cells in 3D culture systems (pellet and nanocomposite constructs). Both SWNT-PEG and SWNT-COOH groups increased the GAG content in nanocomposites relative to control. The compressive biomechanical properties of cell-laden SWNT-COOH nanocomposites were significantly elevated relative to control. Increases in the tensile modulus and ultimate stress were observed, indicative of a tensile reinforcement of the nanocomposite scaffolds. Surface coating of SWNTs with -COOH also resulted in increased Col2a1 and Fn gene expression throughout the culture in nanocomposite constructs, indicative of increased chondrocyte metabolic activity. In contrast, surface coating of SWNTs with a neutral -PEG moiety had no significant effect on Col2a1 or Fn gene expression, suggesting that the charged nature of the -COOH surface functionalization may promote ECM expression in this culture system. The results of this study indicate that SWNTs exhibit a unique potential for cartilage tissue engineering, where functionalization with bioactive molecules may provide an improved substrate for stimulation of cellular growth and repair.
Introduction
Advances in biomaterial development and applications are critical for the continued improvement of regenerative medicine technologies aimed at repairing musculoskeletal damage.1–3 Carbon nanotubes are an attractive component of tissue engineering materials, specifically for controlling the production or delivery of tissue-inducing substances such as growth factors, peptides, or DNA.4,5 Single-wall carbon nanotubes (SWNTs) are rolled cylindrical tubes of carbon atoms that can be tens of microns in length and as small as 1–2 nm in diameter. SWNTs possess unique mechanical and physical properties for studying cell–material interactions such as surface functionalization, structural flexibility, and the ability to interact with or penetrate into cell membranes.6
A common strategy in tissue engineering is surface functionalization, where the surface of a nanomaterial is chemically modified, adding functional groups that enhance the physical or biological performance of the material. SWNTs are inherently hydrophobic when utilized in the as-produced form, a condition that can be overcome with surface functionalization. Acid reflux of SWNTs and other nanotubes has been widely used to attach carboxyl groups to their surfaces (e.g., SWNT-COOH).7–10 These carboxylated nanotubes can be further derivatized to covalently link the SWNTs with other polymers (e.g., PEG), effectively increasing their solubility and biocompatibility.7–9
Many biomaterials have been used for cartilage tissue engineering applications, as previously summarized.2,3,11,12 Whereas hydrogels are an attractive option because of their biocompatibility, capacity to incorporate chemical cues, and innate hydrated structure, their mechanical properties are often inferior to that of native cartilage. Fiber-reinforced biomaterials, such as woven and electrospun scaffolds, have been developed and possess biomechanical properties more similar to those of native tissues.13–17 Mechanical reinforcement of gels with nanotubes has also been examined in tissue engineering of bone,18–21 although not in cartilage engineering applications. We hypothesized that SWNT nanocomposite scaffolds in cartilage tissue engineering can provide an improved molecular-sized substrate for stimulation of cellular growth, as well as structural reinforcement of the scaffold's mechanical properties.
The goal of this study was to assess the long-term biocompatibility of SWNTs for chondrocyte growth and tissue engineering of articular cartilage. The effects of SWNTs on chondrocytes were examined in two-dimensional (2D) cultures, in three-dimensional (3D) pellet cultures, and in nanocomposite scaffolds consisting of hydrogels+SWNTs. We examined the effects of SWNT surface functionalization with polyethlyne glycol (-PEG) or carboxyl groups (-COOH) on chondrocyte growth. We hypothesized that chemical surface functionalization with -COOH will promote cartilaginous matrix expression and deposition to a greater level than -PEG functionalization, resulting in a biomimetic microenvironment for enhanced cartilage regeneration. Gene expression of extracellular matrix (ECM) markers, surface adhesion proteins (fibronectin, CD44) that mediate cell–matrix interactions,22–24 and tumor marker (Tp53) were examined in this culture system. Our findings indicate that SWNT-COOH promotes the expression of ECM proteins and the biomechanical properties of cell-laden nanocomposite scaffolds grown over 5 weeks in culture, in a mechanism potentially mediated by increased surface adhesion protein expression in SWNT nanocomposite scaffolds.
Materials and Methods
Purified SWNTs functionalized with terminal carboxylic acid (SWNT-COOH) or covalent polyethylene glycol (SWNT-PEG) (Carbon Solutions, Inc.) were UV-sterilized, suspended in DI water, and sonicated for ∼3 h to arrive at a stable, homogenous suspension at room temperature. Chondrocytes were isolated from juvenile (1–3 days old) bovine knee articular cartilage from local abattoirs. Full-thickness cartilage was dissected from the femoral condyles, and digested in complete media (DMEM, 4.5 g/L D-Glucose, 110 mg/L sodium pyruvate (Gibco)+10% FBS+1% antibiotic/antimycotic [Gibco]) supplemented with pronase (2 mg/mL; Sigma, 1 h), then collagenase type-IA (0.5 mg/mL; Sigma, 4 h) at 37°C. The resulting cell suspension was filtered through 70-μm cell strainers (BD).
Two-dimensional culture
Primary chondrocytes were plated in high-confluence culture (1×106 cells/cm2) to minimize cell spreading and dedifferentiation in 96-well plates, and cultured in DMEM+10% FBS+1× penicillin/streptomycin up to 14 days. Cells were exposed to fresh media (3× weekly) containing one of the following treatments: (1) Control, (2) Sonicated Media (SM), (3) 0.1 mg/mL SWNT-COOH, (4) 0.01 mg/mL SWNT-COOH, (5) 0.1 mg/mL SWNT-PEG, and (6) 0.01 mg/mL SWNT-PEG. Chondrocyte viability was determined by Live/Dead fluorescence microplate assay (Molecular Probes), at days 0, 3, 7, and 14, and reported as% live cells, according to the manufacturer's protocol. Percentage viability (% live cells) in SWNT groups was determined by analysis of viability in each well relative to a corresponding killed group. Cells in one well per group were treated with 0.25% digitonin to measure viability in the killed group. Percentage viability is reported normalized to an untreated control group.
Pellet culture
Primary chondrocytes were grown in micromass cultures±SWNTs at the same concentrations (0.01 and 0.1 mg/mL) and surface functionalization (SWNT-PEG and SWNT-COOH) as described in 2D culture above. Mixtures of chondrocytes (2×105) and SWNTs in suspension were centrifuged to create a pellet. All pellets were cultured in chondrogenic media (high glucose DMEM supplemented with 10 ng/mL TGF-β3, 0.1 μM dexamethasone, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, 1× insulin- transferrin-selenous acid, and 1× penicillin/streptomycin) in 5% CO2 and 37°C. Fresh media were replaced every 3 days, and pellets were maintained in culture for up to 14 days.
Hydrogel cultures
SWNT solutions were added to molten agarose (Type VII; Sigma Aldrich), and the mixtures were sonicated for 30 min. Primary chondrocytes in suspension were mixed with the agarose/SWNT and cast between two glass plates. The mixture was allowed to gel at room temperature for 20 min and cylindrical disks (ø5 mm/∼1.7 mm thick) were then prepared using a biopsy punch. Cylindrical constructs were prepared from three treatment groups: (1) Control (no SWNTs), (2) 0.1 mg/mL SWNT-PEG, and (3) 0.1 mg/mL SWNT-COOH, with a final cell seeding density of 10×106 cells/mL and 2% agarose content. Disks were cultured in chondrogenic media for up to 35 days.
Gene expression
Gene expression of ECM genes (aggrecan, collagen-1, collagen-2, and collagen-10), surface adhesion proteins (fibronectin, CD44), and tumor marker (p53) levels was determined by quantitative RT-PCR from chondrocytes cultured in hydrogel nanocomposite scaffolds. Total RNA was isolated from hydrogel nanocomposite constructs using the RNEasy kit (Qiagen), where the lysis buffer was supplemented with β-mercaptoethanol (1%) and 2M sodium acetate (0.66%). Homogenization was performed with QIAShredder (Qiagen) according to the manufacturer's protocol. About 0.5–1 μg of RNA was reverse-transcribed into cDNA using the QuantiTect Reverse Transcription kit (Qiagen), which includes genomic DNA elimination. Proprietary primer and TaqMan(TM) probe sets were purchased from Applied Biosystems (Bt03212196_m1, Agc1; Bt03225343_g1, Col1a1; Bt03251852_m1, Col2a1; Bt03215581_m1, Col10a1; Bt03223217_mL, Tp53; Bt00415008_mL, Fn1; Bt03211311_g1, Vtn; Bt03212356_m1, CD44; and Bt03224615_g1, Pp1a). qPCR cycling conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 3 s, then 30 s at 60°C. qPCR results were calculated relative to internal control cyclophilin (Pp1a) using the 2−ΔCt method.25
Histology
Pellets and agarose disks were fixed in 10% buffered formalin and then processed for standard paraffin embedding, sectioning (10 μm), and staining. Sections were stained with Alcian blue (proteoglycan) or Picrosirius red (collagen). Sample images were acquired with a Leica DM5000B light microscope and ImageProPlus software.
Scanning electron microscopy
Samples were rinsed in the serum-free DMEM and fixed with 2% glutaraldehyde in a 0.1 M cacodlyate buffer. Samples were dehydrated with serial ethanol washes, treated in a critical point dryer, mounted for sputter coating (gold), and imaged with a Hitachi S800 scanning electron microscope.
Biomechanical characterization
The biomechanical properties of cylindrical constructs (n=3–4) were measured under unconfined compression using an Instron testing frame (equipped with 50N load cell). Samples were tested in a PBS bath where three stress relaxation compressions were applied (5% strain per ramp), and the Young's modulus (EY) was determined as the slope of the stress–strain curve obtained at equilibrium after each ramp. The dynamic modulus (G*) was computed as the ratio of peak stress to peak strain during cyclic loading applied at 0.1 Hz, and averaged over five loading cycles. Samples were preserved at −20°C after mechanical testing and utilized for biochemical content measurements. Tensile properties of acellular hydrogel±0.1 mg/mL SWNT-COOH were measured at 25°C on dog-bone shaped tensile samples (12 mm wide×2.4 mm thick×60 mm gauge length, n=4). Samples were clamped between 2 hydraulic tensile grips with 200 grit sand paper used at interface to prevent slipping. Sample dimensions were measured with digital calipers, and samples were moistened with PBS during the tensile test. Samples were loaded in uniaxial tension at the rate of 0.15 mm/s until failure. The ramp tensile modulus was calculated from the linear region of the stress–strain curve. Tensile failure strain and ultimate stress (maximum stress) and toughness (integrated area under the stress–strain curve) were calculated for each sample.
Biochemistry
Agarose samples (n=5–8) were thawed, weighed wet, and lyophilized overnight. Sample dry weight was collected and samples were digested overnight at 60°C in 2% papain (Sigma Aldrich) in a buffer composed of 0.1 M sodium acetate, 0.05 M EDTA, and 10 mM Cystine HCL. The glycosaminoglycan (GAG) content in the sample digest was measured using the Blyscan assay, according to the manufacturer's protocol against a standard of chondroitin-4 sulfate. The GAG content is reported as % of tissue wet weight.
Statistical analysis
Statistical analysis was performed using STATISTICA (StatSoft, Inc.), unless otherwise noted. Cellular viability in 2D culture was analyzed with 2-way ANOVA, with culture duration and treatment groups taken as independent variables, and the HSD post hoc test. The GAG content of nanocomposite scaffolds was analyzed with 2-way ANOVA, with culture duration and SWNT functionalization as independent variables, and the unequal N HSD post hoc test. Compressive biomechanical properties were analyzed with 2-way ANOVA and the LSD post hoc test. The tensile properties of acellular nanocomposite scaffolds were analyzed with a t-test using Excel. qPCR results were analyzed by 2-way ANOVA and the Sidak multiple comparisons test using GraphdPad Prism. Significance was considered for p<0.05.
Results
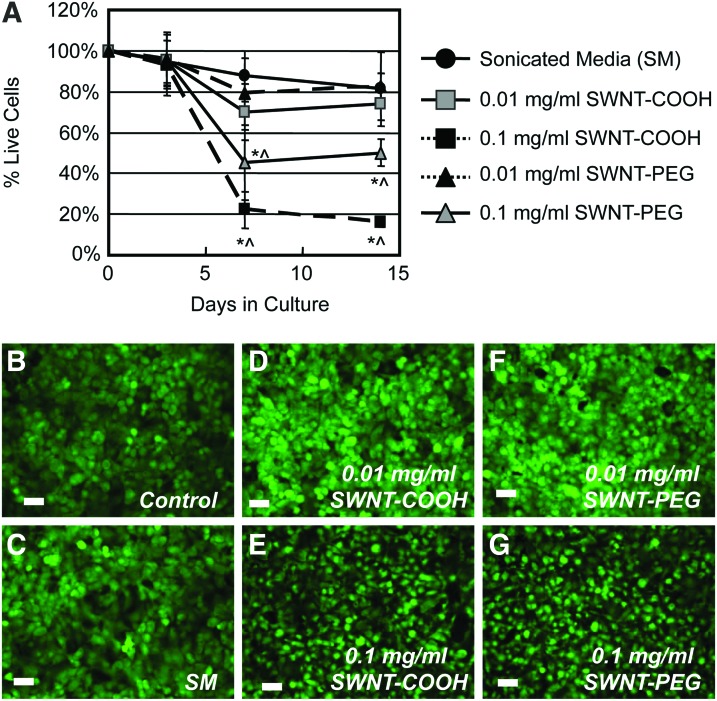
In 2D cultures, chondrocytes maintained comparable viability in all groups up to day 3 (D3) (Fig. 1). Cells cultured in SM resulted in a minor loss of viability, demonstrating 82%±18% viability at D14 (p>0.3). The addition of SWNTs at 0.01 mg/mL did not significantly alter cell viability over the course of the experiment, and chondrocytes exhibited comparable viability for both surface functionalization at D14 (SWNT-PEG: 83%±6%; SWNT-COOH: 76%±9%; p>0.8). At the higher concentration of SWNT (0.1 mg/mL), cell viability decreased significantly between D3 and D7, such that 45%±13% (p<0.001) and 22%±8% (p<0.001) of cells were viable in the presence of SWNT-PEG and SWNT-COOH, respectively (Fig. 1). By D14, no additional loss of cells was seen for either functional group at 0.1 mg/mL (p>0.5). The viability of chondrocytes cultured in the 0.1 mg/mL SWNT groups was significantly lower than their respective surface functionalization groups at 0.01 mg/mL at both D7 and D14 (SWNT-PEG: p<0.01, SWNT-COOH: p<0.0005, Fig. 1). No significant difference was observed between the 0.01 mg/mL SWNT groups and SM groups.
FIG. 1.
(A) Time-dependent viability (% live cells) of chondrocytes in 2D culture after 3, 7, or 14 days of coculture with functionalized SWNTs (-COOH or -PEG) at 0.01 or 0.1 mg/mL (n=5 per group; *p<0.05 versus SM and 0.1 mg/mL group of corresponding functionalization, within time point. ^p<0.05 versus D3 within group). (B-G) Representative live (green) images of chondrocytes in 2D culture at D7 (scale bar=40 μm). 2D, two-dimensional; SWNTs, single-wall carbon nanotubes. Color images available online at www.liebertpub.com/tea
Cells±SWNTs cultured in pellet cultures were analyzed for biochemical deposition of GAG and collagen using standard histological analyses. At D14, control pellets exhibited dense deposition of sulfated GAG and collagen (Fig. 2). Alcian blue staining of pellets cultured with SWNTs exhibited increased cell density relative to control at both 0.01 and 0.1 mg/mL concentrations, although the GAG staining intensity across groups was comparable (Fig. 2). Sections of pellets cultured with SWNT-PEG at both 0.01 and 0.1 mg/mL concentrations exhibited decreased Picrosirius red staining relative to the control group (Fig. 2), indicative of lower collagen deposition. Cell pellets cultured with 0.1 mg/mL SWNT-COOH exhibited comparable collagen deposition relative to control, although the 0.01 mg/mL SWNT-COOH group appeared to have lower collagen deposition than control (Fig. 2). SEM analysis demonstrated comparable cell morphology in pellets cultured±SWNTs (Fig. 2).
FIG. 2.
Histological evaluation of chondrocytes grown in pellet culture with functionalized SWNTs (-COOH or -PEG) at 0.01 or 0.1 mg/mL at day 14. (A–E) Alcian blue staining, indicative of proteoglycan deposition; (F–J) Picrosirius red staining, indicative of collagen deposition (scale bar=50 μm); (K–M) SEM evaluation of chondrocytes in pellet culture in control or SWNT groups (at 0.1 mg/mL). Color images available online at www.liebertpub.com/tea
Based on the deposition of a cartilaginous ECM in chondrocyte pellets cultured in 0.1 mg/mL SWNTs, we created nanocomposite constructs of agarose hydrogel±SWNTs seeded with chondrocytes at this concentration. Morphology and viability of chondrocytes in SWNT nanocomposite constructs were evaluated (Fig. 3). At D7 in culture, chondrocytes exhibited high viability in SWNT-COOH and SWNT-PEG nanocomposites that was similar to the viability exhibited in the controls (Fig. 3). In addition, the DNA content of chondrocytes cultured in nanocomposites was found to be similar to or greater than that measured in the control group up to D35 (data not shown), further suggesting that functionalization promoted cellular growth.
FIG. 3.
Representative images of construct morphology (A–C), live (D–F), dead (G–I), and composite live/dead (J–L) staining at day 7 of chondrocytes cultured in hydrogel-SWNT nanocomposite scaffolds (scale bar=50 μm). No significant change in construct morphology or cell viability was observed at D7 in culture in nanocomposite scaffolds at 0.01% w/v (0.1 mg/mL) SWNTs. Color images available online at www.liebertpub.com/tea
Time-dependent analyses of the gene expression of chondrocytes cultured in nanocomposite constructs were performed using qPCR. Aggrecan (Agc1) expression, an integral component of cartilage ECM, was transient, reaching peak expression at D14 and D21 in culture in control samples. At D14 and D21, Agc1 expression was significantly diminished in the SWNT-PEG group relative to control (p<0.05 and p<0.01, respectively, Fig. 4). No significant differences in Agc1 expression were observed between control and SWNT-COOH groups. Collagen-2 (Col2a1) expression, the primary collagen found in articular cartilage ECM, was significantly elevated at D14, D28, and D35 in SWNT-COOH relative to control (p<0.05), with no significant upregulation observed in the SWNT-PEG group. Collagen-10 (Col10a1), a marker of chondrocyte hypertrophy, expression diminished with time in culture across all groups, with SWNT-COOH exhibiting elevated expression relative to control at early time points (D0 and D7, p<0.001). No significant differences were seen in Col10a1 expression between groups at other time points. Expression of collagen-1 (Col1a1), a marker of dedifferentiation of chondrogenic phenotype, increased slightly over time in culture in all groups, although these increases were not statistically different between groups (p>0.05; Fig. 4). The expression of two surface adhesion proteins, fibronectin (Fn1) and CD44 antigen (CD44), was assessed in the SWNT composite samples over time in culture. Chondrocytes cultured in SWNT-COOH exhibited significantly elevated Fn1 expression at multiple time points (D7 and D28, p<0.01) in -COOH groups, while at D14, Fn1 expression in SWNT-PEG was significantly greater than in control (p<0.05). CD44 expression increased over time in culture in all groups between D14 and D35, although levels of CD44 were greatest in the control group (Fig. 4). CD44 expression levels in both SWNT groups were significantly lower than in the control group at D28 and D35 (p<0.01, Fig. 4). Expression for the tumor marker Tp53 was found to be unchanged among groups over the duration of the culture (Fig. 4), suggesting that the presence of SWNTs is not likely to promote oncogenic transformation.
FIG. 4.
Quantitative PCR gene expression of chondrocytes cultured in Control (no SWNTs), 0.1 mg/mL SWNT-COOH, or 0.1 mg/mL SWNT-PEG nanocomposite scaffolds up to 35 days in culture. Expression of (a) Agc, (b) Col2a1, (c) Col10a1, (d) Col1a1, (e) Fn, (f) CD44, and (g) Tp53 was quantified (n=3 per group, *p<0.05 versus control, **p<0.01 versus control, ***p<0.001 versus control).
The GAG biochemical content of SWNT hydrogel composites was also assessed (Fig. 5A). The GAG content significantly increased in culture up to D28 and D35 in control and SWNT groups, respectively (Fig. 5A, p<0.001). The SWNT-COOH group exhibited a significantly higher GAG content relative to controls on D21, D28, and D35 (p<0.001). The GAG content in the SWNT-PEG group was significantly greater than in the control group at D28 and D35 (p<0.01). At D21, the GAG content of SWNT-COOH group was significantly greater than the SWNT-PEG group (p<0.001, Fig. 5A).
FIG. 5.
(A) Glycosaminoglycan (GAG) content (% of wet weight) and (B) compressive material properties (Young's modulus) of nanocomposite scaffolds composed of control (no SWNTs) or functionalized SWNT group (-COOH or -PEG) at 0.1 mg/mL up to 35 days in culture (n=4–6 per group). ^p<0.05 versus day 0 within group; *p<0.05 versus control at corresponding time point; +p<0.05 versus PEG at corresponding time point.
Significant temporal increases in the compressive biomechanical properties of the controls and SWNT-COOH composites were exhibited over time in culture (p<0.03; Fig. 5B). EY increased to 30±10 kPa by D21 in the control group (p<0.05 vs. D0). The presence of 0.1 mg/mL SWNT-COOH increased EY by 78%, reaching 53±8 kPa at D21 (p<0.05 vs. D0). The presence of SWNT-PEG demonstrated transient effects on EY, with no significant increases over control (Fig. 5A). The dynamic modulus, G*, of samples in the SWNT-COOH group was found to increase by 37% and 48% relative to control groups at D21 and D35, respectively (D21; control: 0.25±0.08 MPa, SWNT-COOH: 0.36±0.11 MPa, p<0.005). The tensile properties of acellular agarose-SWNT composites were measured. The addition of 0.1 mg/mL SWNT-COOH increased the tensile modulus by ∼30% (p<0.001) and increased the ultimate stress by ∼40% (p<0.05, Table 1). No significant differences in the tensile failure strain or toughness of the composite were observed relative to controls (Table 1).
Table 1.
Tensile Mechanical Properties of Acellular Nanocomposite Hydrogel±Functionalized SWNTs at 0.1 mg/ml
| Modulus (kPa) | Ultimate stress (kPa) | Failure strain (%) | Toughness (kPa) | |
|---|---|---|---|---|
| Hydrogel | 79.9±1.78 | 7.99±0.63 | 11.27±2.9 | 0.52±0.23 |
| Hydrogel+SWNT-COOH | 103.6±2.8 | 11.21±1.67 | 10.5±1.7 | 0.52±0.18 |
| p-value | 0.0002* | 0.035* | 0.721 | 0.978 |
Significant increases in the tensile modulus and ultimate stress were observed due to addition of SWNT-COOH (*p<0.05). No significant changes in the failure strain or toughness of samples were observed in the SWNT group versus control (p>0.5).
SWNTs, single-walled carbon nanotubes.
Discussion
The goal of this study was to evaluate the long-term biocompatibility of SWNTs in chondrocyte cultures for potential use as a reinforcement material in hydrogel composites for cartilage tissue engineering. Our findings indicate that chondrocytes have a high tolerance for -COOH and -PEG functionalized SWNTs, with minimal observed cytotoxicity in 3D culture systems (pellet and nanocomposite constructs). In addition, functionalized SWNTs were found to promote the expression of ECM proteins found in articular cartilage (at gene and protein levels) as well as improve the mechanical properties of cell-laden constructs grown over 5 weeks in culture.
In 2D cultures, chondrocytes experienced a dose-dependent loss in viability in the presence of SWNT-COOH or SWNT-PEG (Fig. 1), where low concentrations of SWNTs were as benign as SM alone (Fig. 1), but higher concentrations reduced viability of chondrocytes two- to fourfold by D7. However, the behavior of chondrocytes in 2D can only be limitedly applicable to longer cultures, due to the propensity of chondrocytes to dedifferentiate in this environment.26 Moreover, in 2D cultures, SWNTs may diffuse throughout the medium, increasing the potential for endocytosis by cells, a phenomenon observed in other cell types.27,28 Nevertheless, cell death was minimized by altering both the SWNT dose and surface functionalization, enabling greater than 80% viability up to D14 in 2D culture. In 3D cultures, chondrocytes tolerated SWNTs at higher concentrations than in 2D cultures, possibly because the nanotubes are entrapped within the extracellular (pellet) or hydrogel (composite) matrix, limiting their ability to be absorbed by the cells. In addition, no marked changes in the expression of oncogene Tp53 were seen in 3D culture in SWNT groups relative to control, further confirming their biocompatibility for cartilage tissue engineering applications.
The 3D culture duration (35 days) represents a significantly longer culture time than previous reports examining the effects of other types of nanotubes on the cellular responses of musculoskeletal cells.29,30 The presence of SWNTs enhanced the deposition of GAGs in nanocomposites, and cell pellets containing SWNT-COOH exhibited comparable collagen deposition relative to control cultures. These findings suggest that the presence of SWNTs in 3D allows chondrocytes to maintain their ECM phenotype.
The presence of SWNT-COOH increased the compressive biomechanical properties of nanocomposite scaffolds, with temporal enhancement in EY and G* relative to control, indicating functional enhancement of tissue-engineered constructs. The significant increases in compressive EY observed at D21 and D35 in culture are likely due to increased GAG and other matrix deposition and their interactions with SWNT-COOH, rather than due to SWNT reinforcement of the hydrogel alone in compression. No significant change in the compressive EY was observed in SWNT groups relative to control at D0, before contribution of de novo ECM deposition (Fig. 5B). We did not measure changes in the tensile properties of SWNT composite hydrogels over the duration of the culture. However, our findings on acellular samples suggest that an increase in the tensile modulus and ultimate stress is indicative of a tensile reinforcement of the nanocomposite scaffolds. These findings are consistent with previous studies demonstrating that single-wall or multiwall carbon nanotubes introduced into synthetic biopolymers can improve the material properties and strength of nanocomposite scaffolds.19,31 We hypothesize that the greater induction of de novo ECM deposition in the SWNT-COOH composite group may result in better tensile properties relative to the SWNT-PEG or control groups, over time in culture, although future studies are needed to confirm this hypothesis.
Our findings also point to a bimodal reinforcement of both the compressive and tensile properties of hydrogels. Changes in the ratio of tensile to compressive properties of articular cartilage (and other multiphasic materials such as hydrogels) may enhance the fluid load support, reduce frictional properties, and consequently bolster the load-bearing capability of native cartilage or tissue engineering hydrogel materials.32,33 Although the mechanical properties of the SWNT-hydrogel composites remain below the target properties of native articular cartilage,34,35 our findings suggest that this nanocomposite material may bolster the mechanical properties of agarose hydrogels, while maintaining optimal density and porosity needed to maintain cellular viability and promote cartilaginous growth.36,37
Surface coating of SWNTs was found to regulate the biological responses of chondrocytes in 3D culture. The carboxyl group (COOH) is a charged surface functional group, which partly mimics the negative charges found in PGs of cartilage (e.g., sulfate and carboxylic groups). Surface coating with -COOH resulted in increased Col2a1 and Fn gene expression throughout the culture in nanocomposite scaffolds, indicative of increased chondrocyte metabolic activity. In contrast, surface coating with neutral -PEG moiety had no significant effect on Col2a1 or Fn gene expression, suggesting that the charged nature of the -COOH surface functionalization may promote ECM expression in this culture system. Increased expression may be a direct effect of the chemical surface functionalization or may be mediated by enhanced chemoelectrophysical regulation of cells within a charged ECM, which may induce protein regulation through resulting osmotic gradients or streaming potentials.38,39
Regulation of Col2a1 and Fn1 expression in the -COOH surface functionalized group may be occurring through altered cell–matrix interactions in this model system. For example, Col2 and Fn act as ligands for βl integrins in chondrocytes.23,40–42 β1 integrins are responsible for chondrocyte adhesion to cartilage matrix and are involved in mechanosensing and load-related changes in Agc1 and matrix metalloproteinases.43,44 Although unexamined in this study, we hypothesize that -COOH surface functionalization may alter integrin expression or binding, potentially leading to altered ligand expression or cell–matrix interactions. Alternately, differential integrin activation (via α4b1, α5b1, or αvb3) by Fn domains may contribute to cell survival, differentiation, or matrix remodeling.45–50 Thus, the observations demonstrated within may suggest a mechanism wherein Fn or its fragments function in an autocrine manner to promote the enhanced GAG content and EY observed in the -COOH surface functionalized group. Further work is required to demonstrate whether altered Fn1 expression in -COOH surface functionalized groups concomitantly influences cognate integrin expression and whether this provides an instructive signal toward chondrogenic phenotype and matrix production.
Interestingly, Agc1 gene expression was not differentially regulated in the -COOH versus -PEG groups (Fig. 4), and composite constructs exhibited comparable GAG content in both functionalization groups (Fig. 5A), suggesting that surface functionalization with -COOH did not upregulate all protein synthesis uniformly. A few studies have examined the effects of surface functionalization of nanotubes with peptides or proteins on cellular responses in 2D and 3D (e.g.,10,51,52). Webster and colleagues have developed an RGDSK (Arg-Gly-Asp-Ser-Lys) modified rosette nanotube hydrogel composite for bone and cartilage repair.30,53 They reported that modified nanotube hydrogels improve osteoblast and chondrocyte function and improve cellular adhesion compared with hydrogel controls. Our findings are consistent and expand upon these studies, by highlighting the contribution of both surface chemistry and biomimetic nanoscale properties toward generating a cell favorable environment in nanocomposite materials.30,53,54
The expression of CD44, a receptor for chondrocyte binding with hyaluronan (HA), was found to significantly increase in the control group over culture duration. This increase may be associated with the observed increase in GAG content, typically associated with increasing HA deposition with time in culture. In nanocomposite scaffolds, CD44 expression was significantly diminished relative to control, independently of the surface functionalization. Whereas GAG levels did increase over the duration of the culture in SWNT groups, this increase was not associated with increased CD44 expression. This may be due to potentially lower ratios of HA relative to other GAGs in this group. Alternatively, CD44 expression may be inhibited due to the ultrastructural characteristics of the SWNT-hydrogel nanocomposite. Previous studies have shown that chondrocytes respond to biomaterials early after implantation by altering the expression of CD44 and βl integrins, as well as ultrastructural characteristics.55 Whereas chondrocytes and mesenchymal stem cells maintain CD44 expression when cultured on HA-based scaffolds,56–58 it is unclear whether CD44 expression in chondrocytes is maintained in non-HA-based nano- or micromaterials.59,60
In conclusion, functionalized SWNTs promoted expression of a chondrogenic ECM and improved the biomechanical properties of cell-laden nanocomposites grown over 5 weeks in culture. Surface coating of SWNTs with -COOH, but not with -PEG, resulted in increased Col2a1 and Fn expression throughout the culture in nanocomposite scaffolds, suggesting that the charged nature of the -COOH surface functionalization may be regulating the chondrocyte metabolic activity in this culture system. The results of this study indicate that SWNTs exhibit a unique potential for cartilage tissue engineering, where functionalization with bioactive molecules may provide an improved substrate for stimulation of cellular growth and regeneration. Our findings complement the body of literature demonstrating the efficacy of functionalized SWNT nanocomposite scaffolds for repair, growth, and/or regeneration of injured musculoskeletal tissues.20,21,61
Acknowledgments
This work was conducted under the auspices of the US DOE by LLNL (DE-AC52-07NA27344). This study was, in part, funded by the Ernest Lawrence Fellowship (NOC), LLNL-LDRD 09-LW-072, and NSF CAREER Award 1151605. The authors would like to thank Heather Thompson for technical support.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Amini A.R., Laurencin C.T., and Nukavarapu S.P.Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40,363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher M.B., and Mauck R.L.Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Eng Part B Rev 19,1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnstone B., Alini M., Cucchiarini M., Dodge G.R., Eglin D., Guilak F., Madry H., Mata A., Mauck R.L., Semino C.E., and Stoddart M.J.Tissue engineering for articular cartilage repair - the state of the art. Eur Cells Mater 25,248. [DOI] [PubMed] [Google Scholar]
- 4.Harrison B.S., and Atala A.Carbon nanotube applications for tissue engineering. Biomaterials 28,344, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Wan A.C., and Ying J.Y.Nanomaterials for in situ cell delivery and tissue regeneration. Adv Drug Deliv Rev 62,731. [DOI] [PubMed] [Google Scholar]
- 6.Vardharajula S., Ali S.Z., Tiwari P.M., Eroglu E., Vig K., Dennis V.A., and Singh S.R.Functionalized carbon nanotubes: biomedical applications. Int J Nanomed 7,5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y.C., Broekhuis A.A., Stuart M.C.A., Landaluce T.F., Fausti D., Rudolf P., and Picchioni F.Cross-linking of multiwalled carbon nanotubes with polymeric amines. Macromolecules 41,6141, 2008 [Google Scholar]
- 8.Zhao B., Hu H., and Haddon R.C.Synthesis and properties of a water-soluble single-walled carbon nanotube-poly(m-aminobenzene sulfonic acid) graft copolymer. Adv Funct Mater 14,71, 2004 [Google Scholar]
- 9.Zhao B., Hu H., Yu A., Perea D., and Haddon R.C.Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J Am Chem Soc 127,8197, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Crouzier T., Nimmagadda A., Nollert M.U., and McFetridge P.S.Modification of single walled carbon nanotube surface chemistry to improve aqueous solubility and enhance cellular interactions. Langmuir 24,13173, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Daher R.J., Chahine N.O., Greenberg A.S., Sgaglione N.A., and Grande D.A.New methods to diagnose and treat cartilage degeneration. Nat Rev 5,599, 2009 [DOI] [PubMed] [Google Scholar]
- 12.O'Connell G.D., Lima E.G., Bian L., Chahine N.O., Albro M.B., Cook J.L., Ateshian G.A., and Hung C.T.Toward engineering a biological joint replacement. J Knee Surg 25,187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freed L.E., Engelmayr G.C., Jr., Borenstein J.T., Moutos F.T., and Guilak F.Advanced material strategies for tissue engineering scaffolds. Adv Mater 21,3410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W.J., Mauck R.L., Cooper J.A., Yuan X.N., and Tuan R.S.Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech 40,1686, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moutos F.T., Freed L.E., and Guilak F.A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater 6,162, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Li W.J., Mauck R.L., and Tuan R.S.Electrospun nanofibrous Scaffolds: production, characterization, and applications for tissue engineering and drug delivery. J Biomed Nanotechnol 1,259, 2005 [Google Scholar]
- 17.Mauck R.L., Baker B.M., Nerurkar N.L., Burdick J.A., Li W.J., Tuan R.S., and Elliott D.M.Engineering on the straight and narrow: the mechanics of nanofibrous assemblies for fiber-reinforced tissue regeneration. Tissue Eng Part B Rev 15,171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi X., Sitharaman B., Pham Q.P., Spicer P.P., Hudson J.L., Wilson L.J., Tour J.M., Raphael R.M., and Mikos A.G.In vitro cytotoxicity of single-walled carbon nanotube/biodegradable polymer nanocomposites. J Biomed Mater Res A 86,813, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Shi X.F., Hudson J.L., Spicer P.P., Tour J.M., Krishnamoorti R., and Mikos A.G.Rheological behaviour and mechanical characterization of injectable poly(propylene fumarate)/single-walled carbon nanotube composites for bone tissue engineering. Nanotechnology 16,S531, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Christenson E.M., Anseth K.S., van den Beucken L., Chan C.K., Ercan B., Jansen J.A., Laurencin C.T., Li W.J., Murugan R., Nair L.S., Ramakrishna S., Tuan R.S., Webster T.J., and Mikos A.G.Nanobiomaterial applications in orthopedics. J Orthop Res 25,11, 2007 [DOI] [PubMed] [Google Scholar]
- 21.MacDonald R.A., Laurenzi B.F., Viswanathan G., Ajayan P.M., and Stegemann J.P.Collagen-carbon nanotube composite materials as scaffolds in tissue engineering. J Biomed Mater Res A 74,489, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Knudson C.B.Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol 120,825, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loeser R.F.Integrin-mediated attachment of articular chondrocytes to extracellular matrix proteins. Arthritis Rheum 36,1103, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Mollenhauer J., Bee J.A., Lizarbe M.A., and von der Mark K.Role of anchorin CII, a 31,000-mol-wt membrane protein, in the interaction of chondrocytes with type II collagen. J Cell Biol 98,1572, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Benya P.D., and Shaffer J.D.Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30,215, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Haniu H., Saito N., Matsuda Y., Tsukahara T., Maruyama K., Usui Y., Aoki K., Takanashi S., Kobayashi S., Nomura H., Okamoto M., Shimizu M., and Kato H.Culture medium type affects endocytosis of multi-walled carbon nanotubes in BEAS-2B cells and subsequent biological response. Toxicol In Vitro 27,1679. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M., Zhou X., Iijima S., and Yudasaka M.Small-sized carbon nanohorns enabling cellular uptake control. Small 8,2524. [DOI] [PubMed] [Google Scholar]
- 29.Price R.L., Waid M.C., Haberstroh K.M., and Webster T.J.Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials 24,1877, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Bilgen B., Pareta R.A., Myles A.J., Fenniri H., Ciombor D.M., Aaron R.K., and Webster T.J.Self-assembled rosette nanotube/hydrogel composites for cartilage tissue engineering. Tissue Eng Part C Methods 16,1233. [DOI] [PubMed] [Google Scholar]
- 31.Xie F., Weiss P., Chauvet O., Le Bideau J., and Tassin J.F.Kinetic studies of a composite carbon nanotube-hydrogel for tissue engineering by rheological methods. J Mater Sci 21,1163. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan R., Kopacz M., and Ateshian G.A.Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res 22,565, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soltz M.A., and Ateshian G.A.A Conewise Linear Elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J Biomech Eng 122,576, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chahine N.O., Wang C.C., Hung C.T., and Ateshian G.A.Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J Biomech 37,1251, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C.C., Chahine N.O., Hung C.T., and Ateshian G.A.Optical determination of anisotropic material properties of bovine articular cartilage in compression. J Biomech 36,339, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D.A., and Bader D.L.The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol 31,828, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Mauck R.L., Soltz M.A., Wang C.C., Wong D.D., Chao P.H., Valhmu W.B., Hung C.T., and Ateshian G.A.Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122,252, 2000 [DOI] [PubMed] [Google Scholar]
- 38.MacDonald R.A., Voge C.M., Kariolis M., and Stegemann J.P.Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater 4,1583, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Voge C.M., Kariolis M., MacDonald R.A., and Stegemann J.P.Directional conductivity in SWNT-collagen-fibrin composite biomaterials through strain-induced matrix alignment. J Biomed Mater Res A 86,269, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Durr J., Goodman S., Potocnik A., von der Mark H., and von der Mark K.Localization of beta 1-integrins in human cartilage and their role in chondrocyte adhesion to collagen and fibronectin. Exp Cell Res 207,235, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Kurtis M.S., Tu B.P., Gaya O.A., Mollenhauer J., Knudson W., Loeser R.F., Knudson C.B., and Sah R.L.Mechanisms of chondrocyte adhesion to cartilage: role of beta1-integrins, CD44, and annexin V. J Orthop Res 19,1122, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Loeser R.F.Growth factor regulation of chondrocyte integrins. Differential effects of insulin-like growth factor 1 and transforming growth factor beta on alpha 1 beta 1 integrin expression and chondrocyte adhesion to type VI collagen. Arthritis Rheum 40,270, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Kurtis M.S., Schmidt T.A., Bugbee W.D., Loeser R.F., and Sah R.L.Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheum 48,110, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Millward-Sadler S.J., Wright M.O., Lee H., Nishida K., Caldwell H., Nuki G., and Salter D.M.Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in human articular chondrocytes. J Cell Biol 145,183, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aszodi A., Hunziker E.B., Brakebusch C., and Fassler R.Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev 17,2465, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cha M.H., Do S.H., Park G.R., Du P., Han K.C., Han D.K., and Park K.Induction of re-differentiation of passaged rat chondrocytes using a naturally obtained extracellular matrix microenvironment. Tissue Eng Part A 19,978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loeser R.F.Integrins and cell signaling in chondrocytes. Biorheology 39,119, 2002 [PubMed] [Google Scholar]
- 48.Long D.L., and Loeser R.F.p38gamma mitogen-activated protein kinase suppresses chondrocyte production of MMP-13 in response to catabolic stimulation. Osteoarthritis and cartilage/OARS, Osteoarthritis Res Soc 18,1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulai J.I., Del Carlo M., Jr., and Loeser R.F.The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum 46,1528, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Singh P., and Schwarzbauer J.E.Fibronectin and stem cell differentiation - lessons from chondrogenesis. J Cell Sci 125,3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nimmagadda A., Thurston K., Nollert M.U., and McFetridge P.S.F.Chemical modification of SWNT alters in vitro cell-SWNT interactions. J Biomed Mater Res Part A 76A,614, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Kawaguchi M., Fukushima T., Hayakawa T., Nakashima N., Inoue Y., Takeda S., Okamura K., and Taniguchi K.Preparation of carbon nanotube-alginate nanocomposite gel for tissue engineering. Dental Mater J 25,719, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Zhang L., Rakotondradany F., Myles A.J., Fenniri H., and Webster T.J.Arginine-glycine-aspartic acid modified rosette nanotube-hydrogel composites for bone tissue engineering. Biomaterials 30,1309, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Khang D., Park G.E., and Webster T.J.Enhanced chondrocyte densities on carbon nanotube composites: the combined role of nanosurface roughness and electrical stimulation. J Biomed Mater Res A 86,253, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Schagemann J.C., Kurz H., Casper M.E., Stone J.S., Dadsetan M., Yu-Long S., Mrosek E.H., Fitzsimmons J.S., O'Driscoll S.W., and Reinholz G.G.The effect of scaffold composition on the early structural characteristics of chondrocytes and expression of adhesion molecules. Biomaterials 31,2798. [DOI] [PubMed] [Google Scholar]
- 56.Chung C., and Burdick J.A.Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A 15,243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grigolo B., De Franceschi L., Roseti L., Cattini L., and Facchini A.Down regulation of degenerative cartilage molecules in chondrocytes grown on a hyaluronan-based scaffold. Biomaterials 26,5668, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Pasquinelli G., Orrico C., Foroni L., Bonafe F., Carboni M., Guarnieri C., Raimondo S., Penna C., Geuna S., Pagliaro P., Freyrie A., Stella A., Caldarera C.M., and Muscari C.Mesenchymal stem cell interaction with a non-woven hyaluronan-based scaffold suitable for tissue repair. J Anat 213,520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laroui H., Grossin L., Leonard M., Stoltz J.F., Gillet P., Netter P., and Dellacherie E.Hyaluronate-covered nanoparticles for the therapeutic targeting of cartilage. Biomacromolecules 8,3879, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Zille H., Paquet J., Henrionnet C., Scala-Bertola J., Leonard M., Six J.L., Deschamp F., Netter P., Verges J., Gillet P., and Grossin L.Evaluation of intra-articular delivery of hyaluronic acid functionalized biopolymeric nanoparticles in healthy rat knees. Biomed Mater Eng 20,235. [DOI] [PubMed] [Google Scholar]
- 61.Roman J.A., Niedzielko T.L., Haddon R.C., Parpura V., and Floyd C.L.Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J Neurotrauma 28,2349. [DOI] [PMC free article] [PubMed] [Google Scholar]