Abstract
Establishment of mixed chimerism is an ideal approach to induce donor-specific tolerance while expanding its potential in various clinical settings. Despite the developments in partial conditioning regimens, improvements are still needed in reducing toxicity and bone marrow transplantation-related complications. Recently, cell-based therapies, including mesenchymal stem cells (MSCs), have been incorporated in establishing noncytoreductive mixed chimerism protocols; however, its efficacy is only partial and shows reversed immunosuppressive properties. This study demonstrates a novel approach to induce mixed chimerism and tolerance through combinatory cell-based immune modulation (CCIM) of MSCs and regulatory T cells (Tregs). We hypothesize that the interaction between these cells may lead to greater inhibition of host immune responses. Compared with single cell therapy, CCIM induced a higher engraftment rate and robust donor-specific tolerance to skin allografts across full major histocompatibility complex barriers. These regulatory effects were associated with inhibition of natural killer cell cytotoxic activity, CD4+IL-17+ cells, memory B cells, plasma cells, and immunoglobulin production levels along with increased frequencies of CD4+Foxp3+ cells, IL-10-producing mature B cells, and myeloid-derived suppressor cells. Furthermore, CCIM was able to regulate mortality in a graft-versus-host disease model through reciprocal regulation of Treg/Th17. Taken together, we suggest CCIM as a clinically applicable strategy for facilitating the induction of mixed chimerism and permanent tolerance.
Introduction
Ever since the establishment of tolerance to organ allografts through hematopoietic stem cell transplantation (HSCT), HSCT has been widely used to induce donor-specific tolerance [1]. However, it is limited by major obstacles of conventional allogeneic bone marrow transplantation (BMT), including conditioning-related toxicities, graft-versus-host disease (GVHD), and limitations in the number of HLA-identical donors [2]. In addition, the use of immunosuppressive drugs to prevent allograft rejection is associated with direct toxicities and increased opportunistic infections.
Recent studies have shown that nonmyeloablative pre-conditioning can induce mixed chimerism and establish tolerance toward transplanted donor tissue while overcoming transplant-related morbidity and mortality. Mixed chimerism is a state in which donor and host hematopoietic cells coexist, with the proportion of donor cells ranging from 1% to 100% [3]. Many studies have attempted to establish mixed chimerism through cytoreductive and immunosuppressive agents across major histocompatibility complex (MHC) barriers with the aim of facilitating engraftment and minimizing the risk of GVHD in both T-cell-depleted (TCD) bone marrow (BM) and total BMT. Despite the advancements in partial conditioning regimens, less toxic mixed chimerism regimens still need improvement.
The goal of establishing noncytoreductive mixed chimerism protocols to induce transplantation tolerance is reflected by several studies that incorporate cell therapy [3–6]. Mesenchymal stem cells (MSCs) are self-renewing, multipotent progenitor cells with multilineage potential to differentiate into other cell types of mesodermal origin [7]. Recent studies of the anti-GVHD effects of MSCs, supportive effects on hematopoietic engraftment, and immunomodulatory properties have led to the increasing use of MSCs in mixed chimerism protocols. Several clinical trials have also indicated that the co-infusion of human MSCs supports the engraftment of hematopoietic stem cells in BM [8,9]. However, the immunomodulatory effects of MSCs in vivo are controversial, and the underlying molecular mechanisms in allograft transplantation models remain unknown. Regulatory T cells (Tregs) that express the transcription factor Foxp3 play a critical role in controlling autoimmune responses and in the maintenance of peripheral tolerance [10]. Recently, they have been approved for peripheral tolerance maintenance and long-term graft acceptance [11]. However, therapy with Tregs is limited by their short survival time and their plasticity toward effector T cells under inflammatory conditions [12]. Studies have shown that the main immunosuppressive mechanism of MSCs is the induction of Tregs [8,13,14] and that the interaction between these two cell types in vivo elicits a potent inhibitory response.
Based on these reports, we hypothesized that there would be a benefit to combining MSCs and Tregs for cell therapy. We, therefore, investigated the effects of combinatory cell-based immune modulation (CCIM) of MSCs and Tregs with a low-intensity conditioning regimen to induce tolerance to organ transplants in recipients of an MHC-mismatched transplantation model through persistent mixed chimerism. CCIM treatment induced stable and durable mixed chimerism and subsequent donor-specific tolerance to allografts without the occurrence of GVHD compared with cyclophosphamide (CY). These therapeutic effects by CCIM involved the control of both natural killer (NK) cell activity and effector T/B cell homeostasis. These results suggest that CCIM with MSCs and Tregs in the early post-transplant period might provide a potential strategy for facilitating the induction of mixed chimerism and permanent allograft tolerance.
Materials and Methods
Animals
Eight-week-old female BALB/c mice (recipients, H-2d), C57BL/6 mice (donors, H-2b) were purchased from OrientBio. Animal care and euthanasia protocols were approved by the Animal Care and Use Committee of the Catholic University of Korea.
Isolation and culture of MSCs
Human adipose tissue-derived MSCs were isolated in the laboratory of Dr. Ra (Stem Cell Research Center, RNL Bio Co, Korea) [15,16]. The phenotypes of MSCs were determined by staining with CD31, CD45, HLA-ABC, HLA-DR, CD29, CD34, CD73, CD90, and CD105 antibodies (BD Biosciences).
Preparation of Tregs
To obtain Tregs, CD4+ T cells isolated from recipients were cultured with anti-CD3, anti-CD28, and human recombinant TGF-β for 3 days. To significantly enrich the population of Tregs, CD4+ T cells were stained with CD4 and CD25 antibodies, and CD4+CD25+ T cells were sorted to obtain a ∼95% pure CD4+CD25+ population.
NK cell activity
NK cells were enriched using NK cell isolation kit as recommended by the manufacturer (Milltenyi Biotec) and seeded in 96-well plates for use as effector cells. NK cells were incubated with MSCs and/or Tregs for 4 days. NK cell cytotoxicity was measured using a standard 4-h 51Cr release assay at 37°C. YAC-1 tumor cells were used as target cells. Spontaneous release was assessed in wells that contained labeled target cells alone, and maximum 51Cr release was assessed by addition of 5% Triton X-100 (Sigma-Aldrich). Specific cytotoxicity was calculated as follows: 51Cr release (%)=100×(cpm experimental−cpm spontaneous release)/(cpm maximum release−cpm spontaneous release).
BMT protocol
Recipient mice underwent total-body irradiation (TBI) at a dose of 150 cGy 3 days before BMT, followed by intravenous infusion of 3×107 donor BM cells on day−2 and CY (100 mg/kg) on day−1. On the day of BMT, recipient mice received TCD-BM cells or total BM cells. On days+1 and +3 after BMT, they received CCIM of Tregs (2×106) and MSCs (2×106) (Table 1). To establish GVHD, recipients were lethally irradiated with 800 cGy 1 day before they were injected with 5×106 donor BM cells and 5×106 donor spleen cells on day 0. On days+1 and +10, recipient mice received single cells or a combination of MSC and Treg. All animals were monitored for clinical signs and mean serial weight measurements. The clinical GVHD score was assessed weekly by weight loss, posture, activity, fur texture, and skin integrity.
Table 1.
Experimental Group
| Group | Donor | Recipient | TBI, Gy | DST,×107 | CY, mg/kg | BM/TCD-BM | CY, 50 mg/kg | MSC, 2×106 | Treg, 2×106 | Chimera | Rejection | GVHD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | ||||||||||||
| A | C57BL/6 | BALB/c | 1.5 | 3 | 100 | TCD-BM 3×107 | − | − | − | 0/20 | 20/20 | 0/20 |
| B | C57BL/6 | BALB/c | 1.5 | 3 | 100 | TCD-BM 3×107 | − | − | − | 2/5 | 3/5 | 0/5 |
| C | C57BL/6 | BALB/c | 1.5 | 3 | 100 | TCD-BM 3×107 | − | − | + | 1/5 | 4/5 | 0/5 |
| D | C57BL/6 | BALB/c | 1.5 | 3 | 100 | TCD-BM 3×107 | − | + | + | 17/20 | 3/20 | 0/20 |
| Experiment 2 | ||||||||||||
| A | C57BL/6 | BALB/c | 1.5 | 3 | 100 | BM 2.5×107 | + | − | − | 3/7 | 4/7 | 0/7 |
| B | C57BL/6 | BALB/c | 1.5 | 3 | 100 | BM 2.5×107 | − | + | + | 6/7 | 1/7 | 0/7 |
| C | C57BL/6 | BALB/c | 1.5 | 3 | 100 | BM 2.5×107 | − | − | − | 7/7 | 0/7 | 7/7 |
BM, bone marrow; CY, cyclophosphamide; DST, donor-specific cell transfusion; GVHD, graft-versus-host disease; MSC, mesenchymal stem cell; TBI, total-body irradiation; TCD, T-cell-depleted; Treg, regulatory T cells.
Flow cytometric analysis
The antibodies used for flow cytometric analyses are as follows. We performed staining using antibodies against H-2b, H-2d, CD4, CD8, CD11b, CD11C, B220, CD49b, CD44, CD62L, cytotoxic T-lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced tumour necrosis factor receptor (GITR), programmed death-1 (PD-1), CD103, intercellular adhesion molecule-1 (ICAM-1), inducible costimulator (ICOS), propidium iodide (PI), and Annexin V. All cells were analyzed on an FACSCalibur (BD Biosciences).
Skin transplantation
To assess immune function in vivo, full-thickness skin grafts were transplanted from allogeneic mice and syngeneic mice to the dorsa of the recipient mice. The skin grafts (1.0×1.5 cm) were prepared, and the subcutaneous fat and microvessels were carefully removed. A piece of full-thickness skin was removed from the recipient mice at the same site as the donor skin graft. The skin graft was fixed with equal sutures along its edge and kept under pressure using bundled cotton.
Histopathological analysis
Tissues were fixed in 10% buffered formalin, and tissue sections were prepared. Sections were stained with hematoxylin and eosin and subjected to neutrophil staining for histopathological analysis. The GHVD scoring system for each parameter denoted 0 as normal, 0.5 as focal and rare, 1 as focal and mild, 2 as diffuse and mild, 3 as diffuse and moderate, and 4 as diffuse and severe. Skin structure damage and loss, as well as mononuclear infiltration in the subcutaneous tissue, were characterized.
Reverse transcription-polymerase chain reaction analysis
Total RNA was reverse transcribed into cDNA using a transcription kit (Applied Biosystems). The resulting cDNA was amplified by PCR using IL-1β sense (5′-GCC CAT CCC TGT GAC TCA T-3′) and antisense (5′-AGG CCA CAG GTA TTT TGT CG-3′) primers, IL-6 sense (5′-AGT TGC CTT GGG ACT GA-3′) and antisense (5′-TCC ACG ATT TCC CAG AGA AC-3′) primers, IL-21 sense (5′-CGC AAG ATT CCT GAG GAT CCG AGA AG-3′) and antisense (5′-GCA TTC GTG AGC GTC TAT AGT GTC-3′) primers, and IL-33 sense (5′-GAA GAT CCC AAC AGA CC-3′) and antisense (5′-TTC CGG AGG CGA GAC GTC AC-3′) primers. PCR products were separated on a 1.5% agarose gel and stained with ethidium bromide.
Measurement of IgG titers
Serum levels of IgG, IgG1, and IgG2a antibodies were measured using a commercially available ELISA kit (Bethyl Laboratories).
Statistical analysis
A comparison of numerical data between three groups was performed with nonparametric Mann–Whitney tests. Statistical analysis was performed using SPSS 10.0 for Windows (SPSS). P values<0.05 were considered significant. Data are presented as the mean±SD.
Results
Phenotypes of TGF-β-induced Tregs and culture-expanded human adipose tissue-derived MSCs
High-quality Tregs (CD4+CD25+ population) were induced under culture Treg conditions. The Tregs induced under these conditions were positive for CTLA-1, PD-1, GITR, ICAM-1, CD44, ICOS, CD62L, and Foxp3 (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd). MSCs represent an attractive and ethical cell source for stem cell therapy due to their ability to mediate potent immunosuppressive and immunoregulatory effects in an MHC-independent way [17,18]. For phenotypic characterization of MSCs, culture-expanded cells were prepared from humans, and surface protein expression at passage 3 was examined by flow cytometry. The cells were negative for CD31, CD34, CD45, and HLA-DR, but they were positive for CD29, CD44, CD73, CD90, CD105, and HLA-ABC expression (Supplementary Fig. S1B). To investigate whether human MSCs have interactive effects on mouse immune cells, we observed the immunoregulatory capacity of human MSCs to induce Treg cells in vitro from mouse activated CD4+ T cells. We showed that human MSCs were able to markedly expand Foxp3+ Treg cells in the Th0- or Treg-induced conditions. These results suggest that human MSCs could positively act on mouse regulatory cells (Supplementary Fig. S2A, B).
Regulation of NK cell activity by administration of MSCs and Tregs
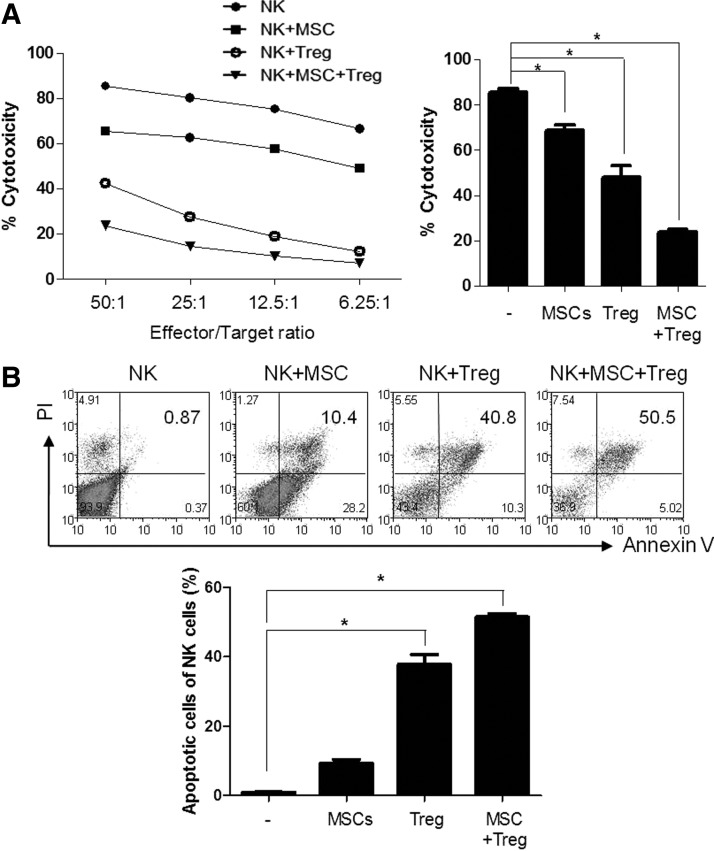
The available nonmyeloablative mixed chimerism is difficult to obtain engraftment because of the increase in NK cell activity in the remaining host immunity. Previous studies have shown that inhibition of NK cell activity in anti-ASGM1-treated mice was successful in mixed chimerism, but they were limited by the fact that no truly human-specific NK markers exist [19]. We evaluated the capacity of MSCs, Tregs, and the combination of Tregs and MSCs to suppress NK cell activity in vitro. MSCs and Tregs were separately able to suppress NK cytotoxicity. Moreover, the combination of MSCs and Tregs synergistically inhibited NK cytotoxicity (Fig. 1A). We also assessed the apoptosis and necrosis of NK cells. The combination of Tregs and MSCs promoted greater necrosis (Fig. 1B). These results suggest that CCIM with MSCs and Tregs can effectively suppress NK cell-mediated BM rejection.
FIG. 1.
Natural killer (NK) activity regulation by combinatory cell-based immune modulation (CCIM) with mesenchymal stem cells (MSCs) and regulatory T cells (Tregs). (A) Cytolytic activity of NK cells was evaluated by chromium release assay of labeled target Yac-1 cells in the presence of different ratios of effector cells (NK cells). NK cells isolated from BALB/c mice were cultured with irradiated MSCs or Tregs or MSCs plus Tregs before incubation with 51Cr-labeled Yac-1 tumor targets. Results of the chromium release assay are represented as the means±SEM of triplicate wells from one representative of three experiments. (B) Incubation of NK cells with MSCs or Tregs or MSCs plus Tregs for 48 h. After staining of cells with annexin V-propidium iodide (PI), apoptotic cells (annexin V+/PI− and annexin V+/PI+ cells) were analyzed as a dot plot using a flow cytometer. The numbers in the quadrants of each plot indicate the percentage of annexin-positive (apoptotic) NK cells. The figures are representative of three replicates. *P<0.05.
CCIM with MSCs and Tregs in early post-transplant period facilitates the induction of mixed chimerism by host NK-cell inhibition
Previous studies have shown that conditioning based on donor-specific cell transfusion (DST) and subsequent selective depletion of activated donor-reactive cells by CY facilitate allo-engraftment, and that post-transplantation low-dose CY administration leads to the induction of chimerism without GVHD [20]. However, more than one cycle of CY administration during BMT can cause life-threatening adverse effects due to drug toxicity. Therefore, we hypothesized that cell therapy used in vivo as a part of a BMT regimen has the potential to be an alternative to CY treatment.
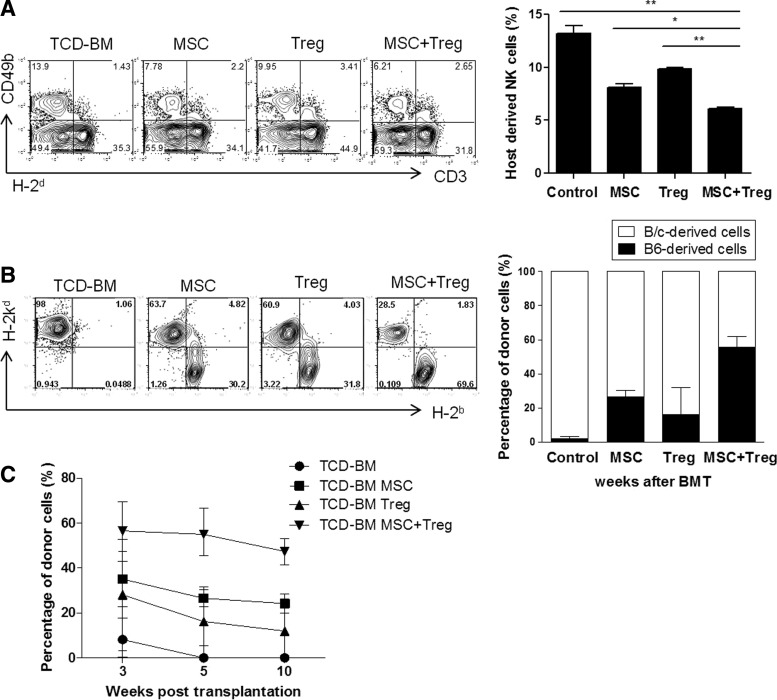
Table 1 summarizes the results of two experiments involving the transplantation of MHC-incompatible marrow after nonmyeloablative conditioning. In the first experiment, BALB/c mice underwent TBI on day−3, followed by intravenous infusion of BM cells on day−2 and CY on day−1 before BMT. On the day of BMT, they received TCD-BM cells from C57BL/6 mice (Table 1). TCD-BMT control mice that did not receive any cell therapy showed failure of engraftment. The mice treated with MSCs or Tregs alone developed mixed chimerism (40% and 20%, respectively). Mixed chimeras that received CCIM exhibited a higher rate (85%). Next, we examined the effect of MSCs and Tregs on host NK-cell function. At 7 days post-transplantation, the NK cell population was significantly depleted in the combination cell therapy group compared with the control or single cell therapy groups. The bars show the percentages of host-derived CD3-CD49b+ cells in each group (Fig. 2A). We also evaluated the percentage of donor cells in each group of mice at 35 days after BMT (Fig. 2B). The percentage of cells of donor origin in peripheral blood from recipients treated with MSCs or Tregs alone showed low levels of chimerism (20%–35%). Recipients of both MSCs and Tregs showed substantial levels of macrochimerism (55.76%±11.03%). The kinetics of chimerism induction and maintenance were assessed in each group (Fig. 2C). There was no significant difference in the chimerism level in these groups during the monitoring period. At 12 months post-transplantation, we analyzed multi-lineage donor chimerism in the recipients that received CCIM (Supplementary Fig. S3A). Relative ratios of cells of donor origin in the spleen were 15%±9.1% for CD4+ cells, 20.5%±2.3% for CD8+ cells, 35.7%±16.2% for B220 cells, 18.4%±6.5% for NK cells, 67.3%±19% for CD11c cells, 53.5%±3.7% for CD11b cells, and 44%±1% for Gr-1 cells, suggesting the engraftment of pluripotent hematopoietic stem cells. Chimerism in peripheral blood was correlated with chimerism in lymphoid organs from BMT recipients treated with CCIM (Supplementary Fig. S3B). These results demonstrate that stable engraftment and long-lasting multi-lineage chimerism is associated with CCIM-mediated NK-cell suppression.
FIG. 2.
CCIM with MSCs and Tregs in the early post-transplant period induces stable hematopoietic chimerism without graft-versus-host disease (GVHD) in full major histocompatibility complex (MHC)-mismatched murine models. On day 0, recipients received 3×107 T-cell-depleted (TCD) bone marrow (BM) cells from MHC-mismatched C57BL/6 (H-2b) donors. On days+1 and +3 after bone marrow transplantation (BMT), recipients received 2×106 MSCs, 2×106 Treg cells, or 2×106 MSCs plus 2×106 Treg cells. (A) The splenocytes was collected at 7 days after BM transplantation to detect host-derived NK cells by staining with anti-H-2d, anti-CD3, and anti-CD49b. (B) To obtain peripheral blood mononuclear cells (PBMCs), leukocytes isolated from recipients of these all groups were stained with MHC class I (H-2b and H-2d) at 5 weeks post-transplantation. The bars show the ratios of C57BL6-originated cells (■) and BALB/c-originated cells (□). (C) Percentages of donor cells (H-2d) in recipients in each group were assessed by flow cytometry. Data are shown as the mean±SEM; results are representative of four independent experiments. *P<0.05; **P<0.01.
CCIM with MSCs and Tregs modulate balance of Treg and Th17 after nonmyeloablative conditioning of BMT
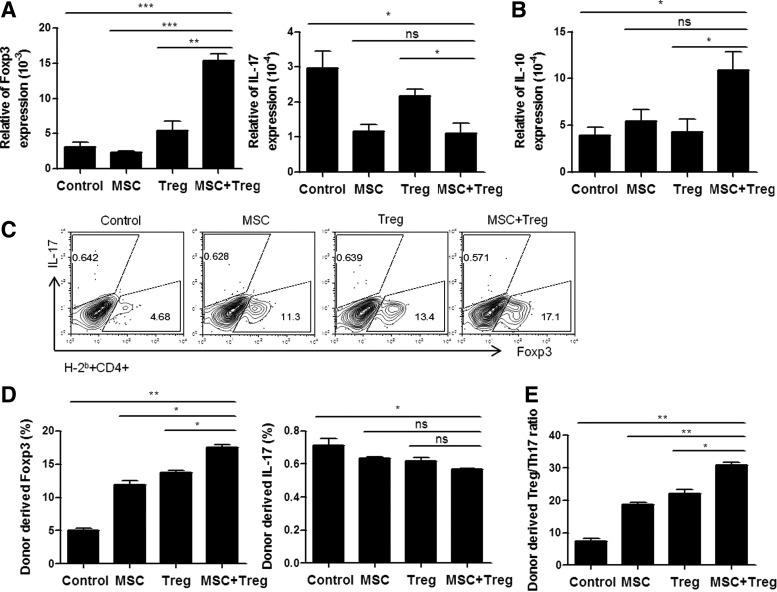
The balance between Treg and Th17 cells plays an important role in regulating BM graft rejection and GVHD [21]. To investigate the in vivo effect of CCIM with MSCs and Tregs on helper T cells, we compared the subsets of T cells in nonmyeloablative conditioning of BMT mice. The mRNA expression of Foxp3 was increased, whereas IL-17 expression was reduced in the splenocytes combined with MSCs and Tregs treatment (Fig. 3A). We also observed an increase in mRNA expression of IL-10 (Fig. 3B). Next, we analyzed cytokine expression at 7 days after BMT. When we analyzed the FACS results, instead of detecting donor-derived cells, we first gated H-2b and then examined the change in helper T cells to determine the change solely in the donor cells. The results showed that treatment with CCIM resulted in a higher increase in donor-derived Foxp3 expression levels. In contrast, levels of donor-derived IL-17 cytokines were lower (Fig. 3C). However, the level of IL-17 cells was not statistically significant in each group. The bars show the percentages of donor-derived CD4+Foxp3+ Treg cells and CD4+IL-17+ Th17 cells (Fig. 3D). The Treg/Th17 cell ratio was significantly higher in spleens of the CCIM group as compared with those in each single cell therapy group (Fig. 3E). These results demonstrate that the therapeutic effects of CCIM involve reciprocal regulation of the endogenous Treg and Th17 population.
FIG. 3.
Immunological effects of CCIM after nonmyeloablative conditioning of BMT. The CCIM with MSCs and Tregs results in a significant reduction in Th17 cells, but enhancement in Treg cells. Expression level of transcription factor mRNA is measured in the spleen cells by real-time PCR at 1 week post-transplantation. (A) Expression of IL-17 was decreased and Foxp3 and (B) IL-10 were increased in CCIM treatment groups than in single cell therapy groups. (C, D) The following endogenous T-cell differentiation subsets in the all groups were identified by FACS analysis with antibodies: endogenous Treg cells (H-2b+CD4+Foxp3+) and endogenous Th17 cells (H-2b+CD4+IL-17+). (E) Data are presented as the ratio of Treg/Th17 among CD4+ T cells, which was calculated as the ratio of IL-17+ CD4+ T cells divided by the percentage of Foxp3+ CD4+ T cells. The purity of all cell subsets was >95% as determined by fluorescence-activated cell sorting (FACS) analysis. Data are shown as the mean±SEM; results are representative of four independent experiments. *P<0.05; **P<0.01; ***P<0.001.
CCIM with MSCs and Tregs in early post-transplant period leads to chimerism without induction of GVHD
The earlier experiments demonstrate that co-infusion of MSCs and Tregs is more effective than MSCs or Tregs alone for promoting alloengraftment. The second experiment, unlike the first, used T-cell nondepleted whole BM (2.5×107), which has a risk of inducing uncontrollable fatal GVHD. We compared post-transplantation CY administration and CCIM in our BMT models. The clinical GVHD score was assessed weekly after BMT, as described in the Materials and Methods section. As shown in Table 1, recipients that received CY showed a low rate of engraftment (40%), but no GHVD. Recipients that received CCIM achieved high rates of engraftment (85%) without GVHD. When they did not receive any therapy, complete chimerism was established in all seven recipients; however, recipients in this group were euthanized due to severe GVHD. On the other hand, recipients that were co-transplanted with whole BM and CCIM developed donor-dominant chimerism and improved the incidence of GVHD.
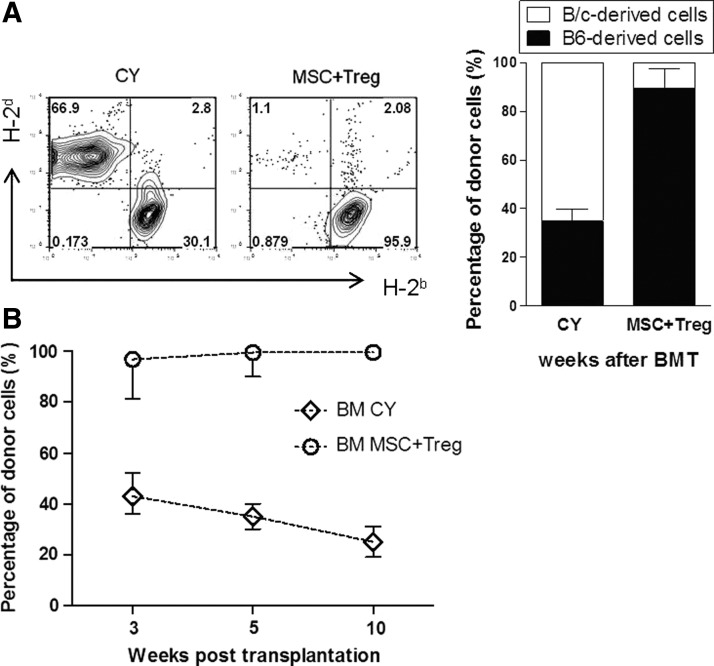
We also determined the percentage of donor cells in each group of mice at 35 days after BMT (Fig. 4A). The percentage of cells of donor origin in peripheral blood from recipients treated with CY showed low levels of mixed chimerism (30.1%±9.9%). The recipients that received both MSCs and Tregs showed donor-dominant mixed chimerism (95.9%±4.4%). Post-transplantation, chimerism levels in both groups persisted during the monitoring period (Fig. 4B). At 12 months post-transplantation, we assessed multi-lineage donor chimerism in recipients that received CCIM (Supplementary Fig. S4A). Relative ratios of cells of donor origin among spleen cells were more than 95%–99% for CD4+ ells, CD8+ cells, B220+ cells, NK+ cells, CD11c+ cells, CD11b+ cells, and Gr-1+ cells. Chimerism in peripheral blood was correlated with chimerism in lymphoid organs from BMT recipients treated with MSCs plus Tregs (Supplementary Fig. S4B). These results demonstrate that post-transplantation CCIM is more effective than post-transplantation CY in terms of promoting allo-engraftment with no incidence of GVHD.
FIG. 4.
CCIM with MSCs and Tregs in the early post-transplant period induced immune tolerance without post-transplantation cyclophosphamide (CY) conditioning. On day 0, they received 2.5×107 BM cells from MHC-mismatched C57BL/6 (H-2b) donors. On day+1 after BMT, recipients received CY or 2×106 MSCs plus 2×106 Treg cells. (A) To obtain PBMCs, leukocytes isolated from recipients of these all groups were stained with MHC class I (H-2b and H-2d) at 5 weeks post-transplantation. The bars show the ratios of C57BL6-originated cells (■) and BALB/c-originated cells (□). (B) Percentages of donor cells (H-2d) in recipients in each group were assessed by flow cytometry. Data are shown as the mean±SEM; results are representative of three independent experiments.
Skin graft tolerance induced by CCIM with MSCs and Tregs
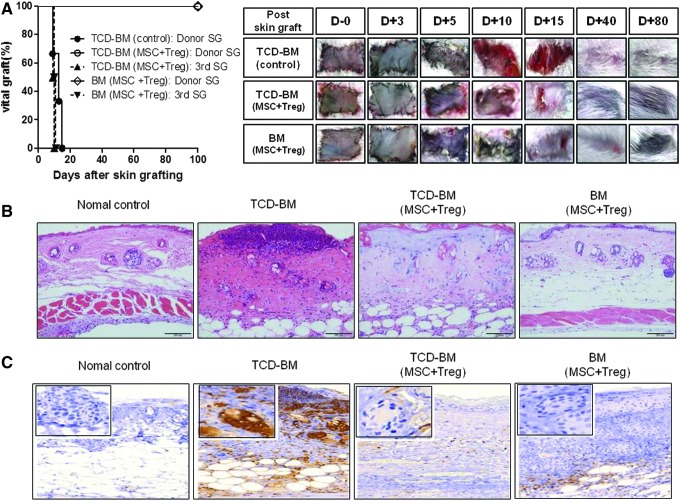
To assess specific transplantation tolerance through mixed chimerism by CCIM, we grafted donor and third-party skin to recipients with established mixed chimerism. As expected, the recipients (both TCD-BM and total BM models) that received MSC plus Tregs accepted donor skin from C57BL/6 mice (survival>300 days) but rejected third-party skin from C3H mice. In contrast, the untreated control group rejected donor grafts (Fig. 5A).
FIG. 5.
Allograft tolerance in recipients co-infused with MSCs and Tregs. (A) After T-cell depletion BMT, the groups treated with or without MSCs plus Tregs (BALB/c) were transplanted with skin grafts (SG) from C57BL/6 (H-2b) mice and C3H (H-2k). The untreated MSCs plus Tregs control group (●) rejected the donor graft. These grafts were significantly contracted for wound remodeling at 12 days after skin grafting. The mixed chimerism group co-infused with MSCs and Tregs accepted donor skin from C57BL/6 mice (○) but rejected third-party skin from C3H mice (▲).In addition, the full chimerism group that exhibited no GVHD at all survived, and their hair grew during the early phase (◊). The third-party skin grafts from C3H mice were rejected at average 10 days post-transplantation (▼). (B) Skin allografts were removed from recipients at 7 days post-transplantation for histological analysis through hematoxylin and eosin staining. Original magnification ×200. (C) Immunohistochemistry staining for neutrophils in the SG (original magnification ×200). Rejection of grafts was correlated with the degree of the inflammatory response, as evidenced by markedly increased neutrophil numbers. Data represent the pool of two independent experiments.
Histological examination of the vascularized skin grafts confirmed rejection in the untreated controls, with evidence of epidermal and dermal necrosis and extensive cell infiltration (Fig. 5B). In contrast, normal skin architecture with limited cell infiltration was observed in long-surviving skin grafts of hosts co-infused with MSCs and Tregs. Rejection of grafts was correlated with the degree of the inflammatory response, as evidenced by markedly increased neutrophil numbers (Fig. 5C). However, the recipients (both TCD-BM and total BM models) that received MSCs and Tregs showed a remarkable reduction in neutrophil numbers in histology. Together, these findings suggest that CCIM can prolong the survival of donor-derived skin grafts.
Comparison of the immunomodulatory properties between CY and CCIM with MSCs and Tregs in early post-transplant period
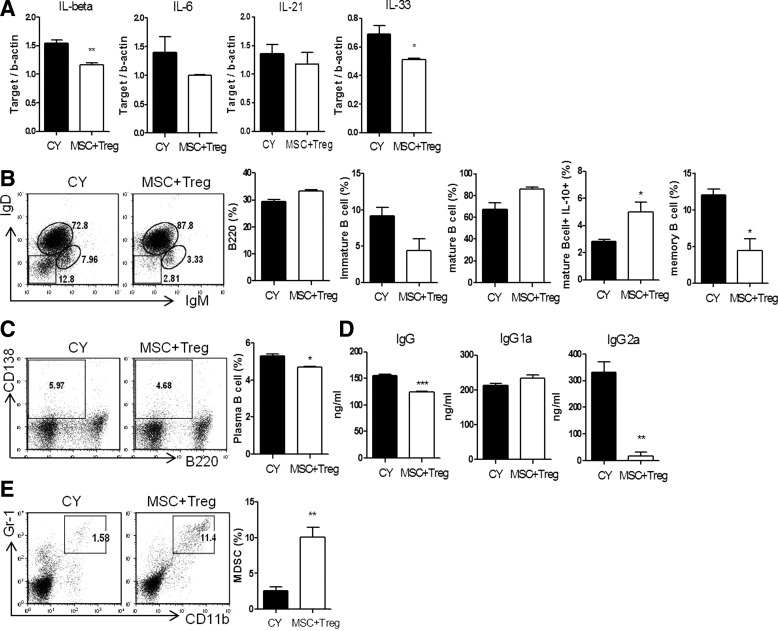
Recent clinical studies have shown that administration of low-dose CY has beneficial immunomodulatory effects, preventing GVHD in BMT [22] and minimizing chances of organ rejection [23]. To investigate the influence of the combination of MSCs and Treg on immunological function, we compared the frequencies of immune cells in BMT mice treated with CCIM or low-dose CY. There was no significant difference between the T-helper cell profiles of the two groups (data not shown). However, levels of proinflammatory cytokines in recipients which received CCIM were lower than in those that received CY (Fig. 6A). In addition, we showed that recipients which received CCIM had an increased portion of mature/IL-10-producing mature B cells, and smaller proportions of immature B cells and memory B cells, than mice treated with low-dose CY post-transplantation (Fig. 6B). We also observed decreases in plasma cell frequencies in recipients that received CCIM compared with those treated with CY (Fig. 6C). Serum IgG and IgG2a levels were found to be significantly lower in recipients that received CCIM than in those treated with CY (Fig. 6D). Interestingly, the frequency of myeloid-derived suppressor cells was increased by about 10-fold in mice treated with CCIM (Fig. 6E). Furthermore, these regulatory effects by CCIM show more synergistic than single therapy (Supplementary Fig. S5). We found that the infusion of MSCs and Tregs in the BMT model can regulate B-cell development and antibody production, suggesting that CCIM might more effectively control GVHD after HSCT compared with CY.
FIG. 6.
Differences in differentiation and functions of T, B cells and myeloid-derived suppressor cells (MDSCs) in hematopoietic chimeras between the CY and MSC plus Treg-treated groups. Splenocytes were assessed at 6 weeks after allogeneic transplantation. (A) Total mRNA was extracted from spleen cells and subjected to quantitative real-time PCR analysis of the indicated genes. Data represent the relative amount of target mRNA normalized to β-actin. (B) The following B cell differentiation subsets in the CY and MSCs plus Tregs groups were identified by FACS analysis with antibodies: B cells (B220), immature B cells (IgM+IgD−B220+), mature B cells (IgM+IgD+B220+), IL-10-expressing B cells (mature IL-10+B220+), and memory B cells (IgM−IgD−B220+). (C) The proportions of plasma cells (CD138+B220+) and (E) MDSCs (CD11b+Gr-1+) were determined by FACS as indicated. (D) Serum levels of total IgG, IgG1, and IgG2a were measured by ELISA. Data are shown as the mean±SEM; results are representative of three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
Inhibition of GVHD by administration of MSCs and Tregs
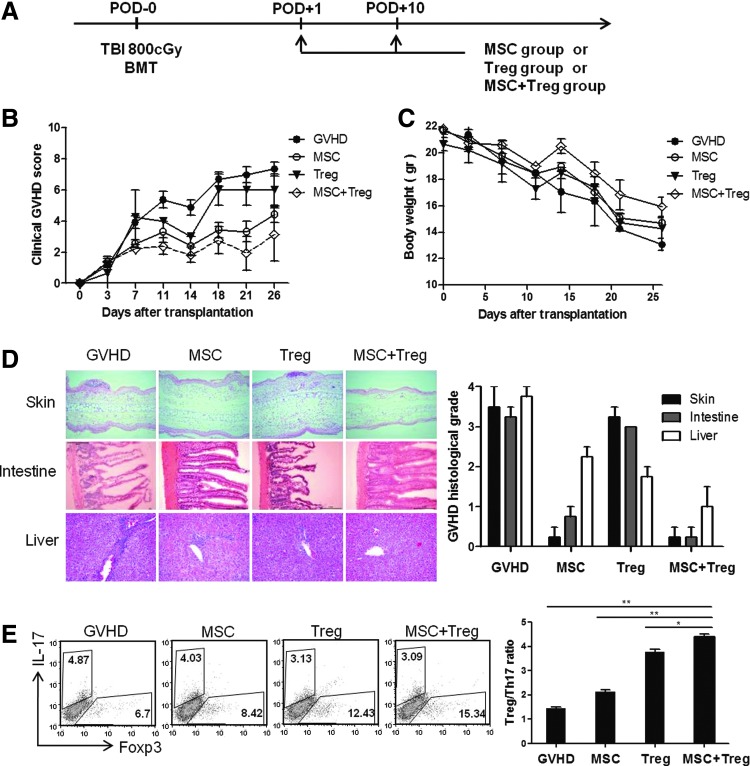
To assess the therapeutic effect of MSCs and Tregs on GVHD, we established an acute GVHD model following complete MHC-mismatched HSCT (Fig. 7A). Co-infusion of MSCs and Tregs was able to prevent GVHD lethality in recipients and reduced weight loss significantly more effectively compared with the MSCs alone and GVHD control groups (Fig. 7B, C). We suggest that MSCs and Tregs contributed synergistically to the alleviation of GVHD induced by T cells. Histological analysis of skin, liver, and large intestine tissues from GVHD control group mice showed scattered eosinophilic degradation and slight vacuolation of individual epidermal cells (Fig. 7D). The CCIM group revealed a remarkable reduction in histopathology. Histological grading of GVHD of the large intestine showed effacement and blunting of the villous architecture, mucous cell depletion, and sloughing of epithelial cells, with patchy mucosal ulceration. Liver samples in the GVHD group showed lobular inflammation, with more lymphocytes infiltrating liver cell plates compared with the CCIM group. These data indicate the positive effect of CCIM on GVHD severity. To investigate the in vivo cellular mechanism of CCIM in the acute GVHD model, we analyzed T-cell subsets at 2 weeks after BMT. The percentages of total CD4+ T, Th17 cells in spleens decreased in the CCIM-treated mice, as compared with the untreated control group. On the other hand, the percentage of Foxp3+ Tregs increased in the CCIM-treated group (Fig. 6E). These data suggest that the therapeutic effects of CCIM were associated with regulation of the Th17 and Treg cell balance.
FIG. 7.
Reduction of GVHD severity by post-transplantation infusion of MSCs and Tregs. (A) Lethally irradiated (800 cGy) BALB/c (H-2d) mice received 5×106 whole BM cells and 5×106 spleen cells from C57BL/6 (H-2b) donors on day 0. On days+1 and +10, mice in the untreated group (●, 1×106, n=6), the MSC group (○, 1×106, n=6), the Treg group (▼, 1×106, n=6), and the group co-infused with MSCs and Tregs (◊, 1×106, n=6) received transplants. (B) All animals were monitored for clinical signs and (C) mean serial weight measurements. (D) Histological GVHD scores at 2 weeks after transplantation were evaluated in skin, intestine, and liver tissues. (E) The splenocytes were stained with anti-CD4 antibody followed by intracellular Foxp3 and IL-17 antibodies and examined by flow cytometry. Treg/Th17 ratio in spleen. Data are shown as the mean±SEM; results are representative of three independent experiments. *P<0.05; **P<0.01.
Discussion
Establishing donor-specific transplant tolerance is an efficient approach that is used for preventing chronic rejection and for avoiding side effects related to long-term immunosuppressive drug treatment. However, clinical transplantation is limited by the lack of a feasible method that is devoid of a cytoreductive condition regimen. Achieving mixed chimerism via BMT is a beneficial approach that can help patients better preserve their own immunocompetence and reduce the risk of GVHD [24]. The creation of mixed chimerism with BMT based on T-cell eradication [25], co-stimulation blockade [26], MSCs [4], or Treg cell therapy [3,5] has been reported to successfully induce tolerance to allografts. However, these protocols required cytoreductive conditioning and/or a high dose of BM cells for stable mixed chimerism.
Studies show that cell therapy is more effective and has fewer side effects than common drugs, which makes it a particularly good therapeutic approach for drug-resistant patients [27]. Numerous cell therapies have been considered for clinical application, but little is known about their mechanism of action in vivo. Immunomodulation by MSCs is mediated by cell–cell contact and the release of soluble factors, such as TGF-β, IL-10, hepatocyte growth factor (HGF), prostaglandin E2 (PGE2), and indoleamine 2,3-dioxygenase (IDO) [7]. Although MSCs have potent immunoregulatory effects, they are short lived and found in organs for approximately 24 h after an intravenous injection [28]. CD4+Foxp3+ Treg cells possess various immunomodulatory capacities. Their mechanism of action can be mediated by suppressive molecules, such as CD25, CTLA-4, GITR, and TGF-β, involving adhesion molecules ICAM-1, CD103, PD-1, CD44, and ICOS [10]. However, the main drawback of Tregs is that they are short-lived cells with a rapid turnover and need continuous antigen stimulation for their maintenance [29,30]. In addition, Tregs have the potential to convert back to effector T cells [12,31]. Therefore, there are still limitations to the therapeutic capacities of MSCs and Tregs when used separately.
One prerequisite for the combined use of different therapies is that they should have a different mode of action and should not damage the functions of one another. Although molecular function of MSC and Tregs partially overlaps, they act on the adaptive immune system through different mechanisms and do not impair each other's functions [14,32]. Previously, studies have shown that cell therapy using either MSCs or Tregs alone could facilitate BM engraftment and prolong allograft survival after organ transplantation [6,33,34]. We found that co-treatment with MSCs and Tregs is a more potent approach to induce tolerance and stable mixed chimerism in MHC-incompatible nonmyeloablative transplantation. The use of MSCs alone in an allotransplantation setting is often controversial, because a number of environmental conditions should be satisfied for MSCS to function as immune regulators [14,35]. Recently, it has been reported that without pre-activation, the infusion of MSCs alone can induce an inflammatory response [36]. Similarly, when we infused MSCs during the early post-transplant period, MSCs failed to adjust the inflammatory cytokine levels. Interestingly, when we administered MSCs in combination with Tregs without any previous activation, IL-1β and IL-6 levels were dramatically decreased; whereas MSCs alone were not able to manage these levels (Supplementary Fig. S5). These observations suggest that co-infused Tregs may provide the additional environmental conditions for MSCs to fully function as immunesuppressors in vivo.
Furthermore, combination cell therapy of MSC and Treg displayed increased total Foxp3 expressions (Fig. 3A) with increased donor-derived Foxp3+ levels (Fig. 3D) compared with a single group. These results may indicate that in addition to donor-derived Tregs, the infused host-derived Tregs increased; however, further studies are needed to observe the changes of adoptively transferred Treg cells caused by combination and a single MSC injection in vivo. We also observed that the levels of IL-10 (Fig. 3B) and TGF-β (data not shown) increased in the MSC group. Studies have reported that the secretion of soluble factors such as IL-10 and TGF-β by MSCs play a key regulatory role in the maintenance of Foxp3 expression [7,14]. Thus, MSCs are able to promote the activation and expansion of Treg cells while suppressing the proliferation of the effector T cells. Although the synergistic effect of MSCs and Tregs may be attributable to the additional increase of the total Foxp3+ Treg population and stability promoted by IL-10 and TGF-β from MSCs, the underlying molecular mechanisms need be further studied.
Although T cells play a major role in rejection of MHC-mismatched BM grafts, host NK cell-mediated allograft rejection has also been well discussed [37–39]. NK cell-mediated resistance is also known as hybrid resistance and has been reported to contribute to allogeneic graft rejection in the early post-transplantation stage [40,41]. Our group previously reported that depleting host NK cells could induce successful engraftment of TCD-BM grafts in fully MHC-mismatched nonmyeloablative BMT [19]. MSCs were reported to inhibit IL-2-induced NK cell proliferation, cytotoxicity, and cytokine production [42], while Tregs suppressed NK cell functions via cell–cell interactions [43]. We also observed that the combination of MSCs and Tregs is more effective in controlling NK cell effector function and survival (Figs. 1 and 2). Thus, the engraftment of donor BM and the induction of stable donor-specific tolerance may also contribute to the inhibition of NK cell functions by CCIM.
Another important finding of our study is the significantly reduced exacerbation of GVHD by the co-infusion of MSCs and Tregs during BMT compared with mice that received no treatment. It is known that post-transplantation CY treatment can reduce the incidence and severity of GVHD in BMT [22], but CY can also increase blood pressure and has the tendency to induce diabetes, as well as life-threatening conditions such as infections and cancer. Our results showed that the mice treated with MSCs and Tregs had similar, if not higher, engraftment rates of donor BM without GVHD symptoms, as well as increased regulatory immune cell populations, suggesting that CCIM can be a suitable alternative to CY treatment. In addition to the evaluation of T-helper cell subsets and cytokine profiles, we dissected the B-cell populations affected by treatment. There is increasing evidence that B cells play a significant role in the long-term survival of allografts [44]. In the mixed chimeras, we observed significant decreases in populations of effector B cells and an increase in the frequency of IL-10-producing B cells within the mature B-cell population. These effector B cells may be detrimental to the maintenance of immune tolerance, because B cells act as potent antigen-presenting cells that promote allograft rejection [45]. Regulatory IL-10-producing B cells have been shown to play an immunomodulatory role in various models, including colitis, lupus, and arthritis models [46–49]. We suggest that CCIM can inhibit the production of antibodies against allografts during BMT. Furthermore, the involvement of B cells in the pathogenesis of acute GVHD has been shown in both animal and human studies [50]. The use of CCIM had a similar effect on B cells in the GVHD model, with decreases in the proportions of effector B cells. In addition, we showed an increase in the proportion of naïve B cells, which have been shown to stimulate naive T cells toward Treg differentiation [51]. We also showed that, in both mixed chimera and GVHD models, the use of CCIM was superior to CY in terms of its effects on B-cell populations which are beneficial for the establishment of immune tolerance.
The present study is the first that reports the use of CCIM to overcome H-2 barriers through the establishment of long-lasting mixed chimeras. However, our conditioning for BMT has limitations for clinical trials. DST in the form of whole BM followed by CY treatment before BMT requires a large amount of BM and, thus, may be difficult to apply in a clinical setting. Therefore, we need to develop a more simplified conditioning method to apply in clinics. In addition, since the experimental conditions are specific strain transplants, these findings should be validated in different MHC-mismatched mouse strain combinations. Nonetheless, we have demonstrated that the combination of MSCs and Tregs can promote engraftment and intentionally induce stable mixed chimerism in an MHC-incompatible nonmyeloablative setting by inhibiting NK activity and enhancing immunomodulatory effects. We suggest a novel and powerful tolerogenic approach of CCIM with MSCs and Tregs that can achieve mixed chimerism and tolerance while minimizing GVHD risks during organ transplantation.
Supplementary Material
Acknowledgments
This work was supported by a grant (HI09C1555) from the Korea Healthcare Technology R&D Project, the Ministry for Health, Welfare, and Family Affairs, Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Copelan EA. (2006). Hematopoietic stem-cell transplantation. N Engl J Med 354:1813–1826 [DOI] [PubMed] [Google Scholar]
- 2.Welniak LA, Blazar BR. and Murphy WJ. (2007). Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol 25:139–170 [DOI] [PubMed] [Google Scholar]
- 3.Pilat N. and Wekerle T. (2010). Mechanistic and therapeutic role of regulatory T cells in tolerance through mixed chimerism. Curr Opin Organ Transplant 15:725–730 [DOI] [PubMed] [Google Scholar]
- 4.Asari S, Itakura S, Rawson J, Ito T, Todorov I, Nair I, Shintaku J, Liu CP, Kandeel F. and Mullen YS. (2011). Mesenchymal stem cells facilitate mixed hematopoietic chimerism induction and prevent onset of diabetes in nonobese diabetic mice. Pancreas 40:846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilat N, Baranyi U, Klaus C, Jaeckel E, Mpofu N, Wrba F, Golshayan D, Muehlbacher F. and Wekerle T. (2010). Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transplant 10:751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itakura S, Asari S, Rawson J, Ito T, Todorov I, Liu CP, Sasaki N, Kandeel F. and Mullen Y. (2007). Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant 7:336–346 [DOI] [PubMed] [Google Scholar]
- 7.Marigo I. and Dazzi F. (2011). The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol 33:593–602 [DOI] [PubMed] [Google Scholar]
- 8.Wu KH, Wu HP, Chan CK, Hwang SM, Peng CT. and Chao YH. (2012). The role of mesenchymal stem cells in hematopoietic stem cell transplantation: from bench to bedsides. Cell Transplant 22:723–729 [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Kim N. and Cho SG. (2013). The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Exp Mol Med 45:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bluestone JA. (2005). Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol 5:343–349 [DOI] [PubMed] [Google Scholar]
- 11.Tang Q, Bluestone JA. and Kang SM. (2012). CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol 4:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C. and Rudensky AY. (2010). Stability of the regulatory T cell lineage in vivo. Science 329:1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo SY, Cho KA, Jung YJ, Kim HS, Park SY, Choi YB, Hong KM, Woo SY, Seoh JY, Cho SJ. and Ryu KH. (2010). Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy 12:361–370 [DOI] [PubMed] [Google Scholar]
- 14.Burr SP, Dazzi F. and Garden OA. (2013). Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol 91:12–18 [DOI] [PubMed] [Google Scholar]
- 15.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ. and Kwon E. (2011). Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 20:1297–1308 [DOI] [PubMed] [Google Scholar]
- 16.Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, Kang SK, Ra JC. and Hong SH. (2012). Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum 64:243–253 [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal S. and Pittenger MF. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822 [DOI] [PubMed] [Google Scholar]
- 18.Grinnemo KH, Mansson A, Dellgren G, Klingberg D, Wardell E, Drvota V, Tammik C, Holgersson J, Ringden O, Sylven C. and Le Blanc K. (2004). Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg 127:1293–1300 [DOI] [PubMed] [Google Scholar]
- 19.Cho SG, Shuto Y, Soda Y, Nakazaki Y, Izawa K, Uchimaru K, Takahashi S, Tani K, Tojo A. and Asano S. (2004). Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol 32:1246–1254 [DOI] [PubMed] [Google Scholar]
- 20.Prigozhina TB, Elkin G, Khitrin S. and Slavin S. (2004). Depletion of donor-reactive cells as a new concept for improvement of mismatched bone marrow engraftment using reduced-intensity conditioning. Exp Hematol 32:1110–1117 [DOI] [PubMed] [Google Scholar]
- 21.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, et al. (2009). Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood 114:3101–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prigozhina TB, Elkin G, Khitrin S. and Slavin S. (2008). Prevention of acute graft-vs-host disease by a single low-dose cyclophosphamide injection following allogeneic bone marrow transplantation. Exp Hematol 36:1750–1759 [DOI] [PubMed] [Google Scholar]
- 23.Itescu S, Burke E, Lietz K, John R, Mancini D, Michler R, Rose E, Oz M. and Edwards N. (2002). Intravenous pulse administration of cyclophosphamide is an effective and safe treatment for sensitized cardiac allograft recipients. Circulation 105:1214–1219 [DOI] [PubMed] [Google Scholar]
- 24.Sykes M. (2001). Mixed chimerism and transplant tolerance. Immunity 14:417–424 [DOI] [PubMed] [Google Scholar]
- 25.Wekerle T, Nikolic B, Pearson DA, Swenson KG. and Sykes M. (2002). Minimal conditioning required in a murine model of T cell depletion, thymic irradiation and high-dose bone marrow transplantation for the induction of mixed chimerism and tolerance. Transpl Int 15:248–253 [DOI] [PubMed] [Google Scholar]
- 26.Graca L, Daley S, Fairchild PJ, Cobbold SP. and Waldmann H. (2006). Co-receptor and co-stimulation blockade for mixed chimerism and tolerance without myelosuppressive conditioning. BMC Immunol 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C. and Trucco M. (1992). Cell migration, chimerism, and graft acceptance. Lancet 339:1579–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH. and Hoogduijn MJ. (2012). Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC. and Akbar AN. (2006). Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest 116:2423–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritzsching B, Korporal M, Haas J, Krammer PH, Suri-Payer E. and Wildemann B. (2006). Similar sensitivity of regulatory T cells towards CD95L-mediated apoptosis in patients with multiple sclerosis and healthy individuals. J Neurol Sci 251:91–97 [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W. and Bluestone JA. (2009). Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10:1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engela AU, Baan CC, Peeters AM, Weimar W. and Hoogduijn MJ. (2013). Interaction between adipose tissue-derived mesenchymal stem cells and regulatory T-cells. Cell Transplant 22:41–54 [DOI] [PubMed] [Google Scholar]
- 33.Xia G, He J. and Leventhal JR. (2008). Ex vivo-expanded natural CD4+ CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant 8:298–306 [DOI] [PubMed] [Google Scholar]
- 34.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P. and van Meerwijk JP. (2008). Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 14:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sudres M, Norol F, Trenado A, Gregoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, et al. (2006). Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol 176:7761–7767 [DOI] [PubMed] [Google Scholar]
- 36.Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FK, Franquesa M, de Bruin RW, Betjes MG, Weimar W. and Baan CC. (2013). Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev 22:2825–2835 [DOI] [PubMed] [Google Scholar]
- 37.Murphy WJ, Kumar V. and Bennett M. (1987). Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. J Exp Med 166:1499–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Moine A. and Goldman M. (2003). Non-classical pathways of cell-mediated allograft rejection: new challenges for tolerance induction? Am J Transplant 3:101–106 [DOI] [PubMed] [Google Scholar]
- 39.Vilches C. and Parham P. (2006). Do NK-cell receptors and alloreactivity affect solid organ transplantation? Transpl Immunol 17:27–30 [DOI] [PubMed] [Google Scholar]
- 40.Murphy WJ, Kumar V. and Bennett M. (1987). Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID). Evidence that natural killer cells can mediate the specificity of marrow graft rejection. J Exp Med 165:1212–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cudkowicz G. and Bennett M. (1971). Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med 134:1513–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC. and Moretta L. (2008). Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111:1327–1333 [DOI] [PubMed] [Google Scholar]
- 43.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, et al. (2005). CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202:1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redfield RR, 3rd, Rodriguez E, Parsons R, Vivek K, Mustafa MM, Noorchashm H. and Naji A. (2011). Essential role for B cells in transplantation tolerance. Curr Opin Immunol 23:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noorchashm H, Reed AJ, Rostami SY, Mozaffari R, Zekavat G, Koeberlein B, Caton AJ. and Naji A. (2006). B cell-mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol 177:7715–7722 [DOI] [PubMed] [Google Scholar]
- 46.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D. and Anderton SM. (2002). B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3:944–950 [DOI] [PubMed] [Google Scholar]
- 47.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS. and Bhan AK. (2002). Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16:219–230 [DOI] [PubMed] [Google Scholar]
- 48.Lenert P, Brummel R, Field EH. and Ashman RF. (2005). TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol 25:29–40 [DOI] [PubMed] [Google Scholar]
- 49.Mauri C, Gray D, Mushtaq N. and Londei M. (2003). Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197:489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF. and von Bergwelt-Baildon MS. (2009). The role of B cells in the pathogenesis of graft-versus-host disease. Blood 114:4919–4927 [DOI] [PubMed] [Google Scholar]
- 51.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K. and Gunzer M. (2007). Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood 110:1519–1529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.