Summary
The first hematopoietic stem cells (HSCs) that engraft irradiated adult mice arise in the aortagonad-mesonephros (AGM) on embryonic day 11.5 (E11.5). However, at this stage there is a discrepancy between the apparent frequency of HSCs suggested by imaging and their rarity when measured by limiting dilution transplant. We have attempted to reconcile this difference using neonatal recipients, which are more permissive for embryonic HSC engraftment. We found that embryonic HSCs from E9.5 and E10.5 preferentially engrafted neonates, whereas developmentally mature, definitive HSCs from E14.5 fetal liver (FL) or adult bone marrow (BM) more robustly engrafted adults. Neonatal engraftment was enhanced after treating adult BM-derived HSCs with interferon. Adult BM-derived HSCs preferentially homed to the liver in neonatal mice yet showed balanced homing to the liver and spleen in adults. These findings emphasize the functional differences between nascent and mature definitive HSCs.
Introduction
According to the classical definition, a definitive HSC reconstitutes multi-lineage hematopoiesis long-term in irradiated adult primary transplant recipients, and can be serially passed into secondary recipients, indicating the capacity for both self-renewal and multi-lineage differentiation (Becker et al., 1963; Siminovitch et al., 1963; Till and Mc, 1961; Wu et al., 1968). Under that definition, the first transplantable murine HSC emerges in vivo in the AGM at E11.5 (de Bruijn et al., 2000; Kumaravelu et al., 2002; Medvinsky and Dzierzak, 1996; Muller et al., 1994; Taylor et al., 2010). HSCs then migrate to the FL and rapidly divide to build up the stem cell pool. Ultimately, HSCs populate the developing BM where they become quiescent once homeostasis is reached in the post-natal period. Interaction with specific niches during development allows embryonic HSCs to mature into adult HSCs (Schofield, 1978; Wineman et al., 1996).
The surface antigen profile of the engrafting cell at E11.5, as identified by transplantation, is VE-Cadherin + CD45+ CD34− cKitlow Sca1+ CD31low (Taoudi et al., 2008; Taoudi et al., 2005). Imaging techniques coupled with the cell surface profile of the engrafting cell have provided insight into the emergence of the definitive HSC (Boisset et al., 2010; Kissa and Herbomel, 2010; Taoudi and Medvinsky, 2007; Yokomizo and Dzierzak, 2010). Imaging has revealed hundreds of c-Kit+ clusters in E11.5 AGM, implying a discrepancy in the quantity of potential HSCs identified by imaging relative to the frequency defined by limiting dilution transplantation in adult recipients.
Analysis of emerging hematopoietic cells in the AGM at E10.5 shows 1.7 Sca1+ c-Kit+ CD31+ CD41+ cells per embryo (Boisset et al., 2010). Transplantation has revealed that E10.5 AGM cells are capable of rare long-term multi-lineage repopulation of wild-type adult recipients (3% of transplanted animals) and more robust repopulation in immunodeficient adult recipients, indicating that the host environment plays a critical role in detection of nascent HSCs (Bertrand et al., 2005; Muller et al., 1994; North et al., 2002). Interestingly, VE-Cadherin+ CD45− cells from E10.5 AGM robustly reconstitute wild-type adult recipients after 4 days of ex vivo culture, further suggesting that there are cells in the AGM at E10.5 with enhanced potential to engraft if cultured under proper conditions (Rybtsov et al., 2011).
Before E10.5, the tissues of the para-aortic splanchnopleura (PSp) contain progenitors that are rimed to give rise to the definitive HSC, as per the classical definition (Cumano et al., 2000; Cumano et al., 2001; Muller et al., 1994). A population from E9.5 PSp has been reported to engraft immunodeficient adult recipients and wild-type neonatal recipients (Kieusseian et al., 2012; Mizuochi et al., 2012; Yoder et al., 1997a). Neonatal engraftment has also been observed from E9.0 yolk sac (Yoder et al., 1996; Yoder et al., 1997b), suggesting that the neonate may be more permissive for engraftment of early embryonic HSCs. Interestingly, we also found that definitive adult HSCs engraft less robustly in the neonate relative to the adult. In this study we quantified nascent definitive HSCs in the earliest intraembryonic tissues to date and identified the context in which HSCs from different developmental stages will engraft.
Results
Quantification of robust reconstitution of neonates with early embryonic tissues
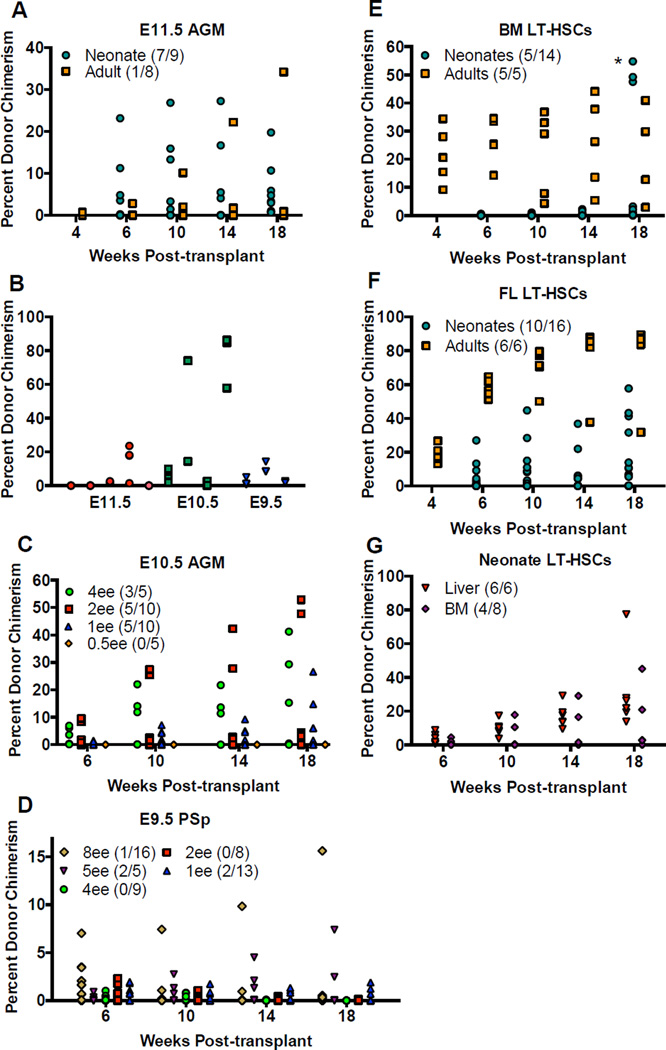
We first compared engraftment of neonates and adults with cells from E11.5 AGM, the earliest population reported to engraft wild-type adult recipients. Neonatal recipients were more robustly engrafted than adult recipients with similar conditioning. Whereas 7/9 neonatal recipients were engrafted with 1ee, at most 3/6 adult recipients receiving sub-lethal irradiation and no helper cells were engrafted at low levels (Figure 1 A and Table S1). Three of 11 neonates transplanted with 0.25 embryo equivalents (ee) showed long-term engraftment (Figure S1 A, B). Secondary transplants demonstrated donor HSC self-renewal when taken from primary animals with multi-lineage reconstitution (Figure 1 B), but three primary recipients with long-lived myeloid biased lineage skewing did not engraft secondary recipients, and thus were not engrafted with a self-renewing HSC (Figure S1 B). We also compared neonatal engraftment with E11.5 AGM to the current gold standard of lethally irradiated adult recipients receiving helper cells. Only 1/8 adult mice was engrafted (Table S1). Before quantitative analysis, these data suggest the neonatal transplant assay is a more permissive functional test for early embryonic HSCs than the gold standard assay.
Figure 1. Neonates are only more permissive for engraftment of early embryonic HSCs.
A) Adult (square) and neonatal (circle) recipients were both transplanted with 1ee of whole E11.5 AGM. Also see Figure S2. B) Secondary transplants were carried out for primary recipients engrafted with early embryonic HSCs. Neonatal recipients were transplanted with limiting doses of C) whole E10.5 AGM and D) whole E9.5 PSp. Also see Figure S1. E) Neonatal (circle) and adult (square) recipients were transplanted with 100 adult BM LT-HSCs. *While in 3 neonatal recipients we detected a robust signal in the peripheral blood at a single time point of 18 weeks in one experiment, substantially lower donor chimerism was observed in these mice at the next time point of 23 weeks, and no other experiments showed this phenomenon, leading us to conclude that these data may have been a spurious experimental artifact. F) Neonatal (circle) and adult (square) recipients were transplanted with 100 E14.5 FL LT-HSCs. G) Neonatal recipients were transplanted with 50 neonatal liver LT-HSCs (triangle) and 50 neonatal BM LT-HSCs (diamond). The numbers in the graph legends reflect the number of animals engrafted over the number of animals transplanted.
VE-Cadherin+ CD45+ cells from E11.5 AGM have been reported to engraft adult recipients (Taoudi et al., 2005). Similarly, we found that the VE-Cadherin+ CD45+ but not the VE-Cadherin + CD45− compartment engrafted neonates, thus demonstrating that neonates and adults are engrafted with the same cells from E11.5 AGM (Figure S2 B). However, the predicted repopulating cell frequency was higher in neonatal than adult recipients. Using the neonatal transplant model, limiting dilution analysis (LDA) predicted 1 repopulating cell in 0.935 ee, thus quantifying the sensitivity of the neonatal system for engraftment of embryonic HSCs (Table 1). In our hands using the adult transplant model with 300,000 helper cells, LDA predicted 1 repopulating cell in 5.7 ee of E11.5 AGM, whereas the published frequency using adult recipients is approximately 1 per ee (Kumaravelu et al., 2002) (Table 1). When we replicated the conditions from Kumaravelu et al. using 20,000 helper cells, the calculated frequency was 1 repopulating cell in 0.538 ee (Table 1). Thus, our experimental quantifications reveal sensitivity in the adult transplant model to helper dose and no preference of HSCs at E11.5 for neonatal recipients.
Table 1. Early embryonic but not adult-like HSCs prefer neonatal recipients.
LDA of reconstitution of neonatal and adult recipients with adult BM LT-HSCs, E14.5 FL LT-HSCs, whole E11.5 AGM, whole E10.5 AGM, and whole E9.5 PSp. The upper and lower limits provide the 95% confidence interval for the repopulating cell frequency. The goodness of fit tests how well the data fit the single-hit model that one repopulating cell will give a positive signal. A value of 1 suggests a good fit, less than 1 suggests heterogeneity in the donor cell population, and greater than 1 suggests a multi-hit model where recipients are hypersensitive to the given dose of donor cells. P-values are between neonatal and adult recipients transplanted with the same donor population and were calculated by the LDA algorithm. ND – not determined. Also see Table S1.
| Donor | Recipient | Helper Cell Dose |
Estimated Repopulating Cell Frequency |
Frequency Upper Limit |
Frequency Lower Limit |
Fit Single- hit Model |
p-value |
|---|---|---|---|---|---|---|---|
| BM LT-HSCs | Adult | 300,000 | 1/13.1 cells | 1/6.19 | 1/27.7 | 0.96 | 3.33E–8 |
| Neonate | None | 1/177 cells | 1/100 | 1/313 | 1.2 | ||
| FL LT-HSCs | Adult | 300,000 | 1/25.7 cells | 1/13.8 | 1/47.6 | 0.55 | 3.07E–4 |
| Neonate | None | 1/114 cells | 1/66.9 | 1/195 | 0.91 | ||
| E11.5 AGM | Adult | 20,000 | 1/0.538 ee | 1/0.24 | 1/1.2 | −4.46 | |
| 300,000 | 1/5.7 ee | 1/2.16 | 1/15 | 2.6 | 5.12E– 5 | ||
| Neonate | None | 1/0.935 ee | 1/0.624 | 1/1.4 | 0.74 | ||
| E10.5 AGM | Adult | ND | ND | ND | ND | ND | |
| Neonate | None | 1/2.84 ee | 1/1.6 | 1/5.02 | 0.71 | ||
| E9.5 PSp | Adult | ND | ND | ND | ND | ND | |
| Neonate | None | 1/44.8 ee | 1/18.4 | 1/109 | −0.212 |
We then evaluated the engraftment potential of newly specified HSCs from earlier stages of ontogeny. Neonates showed robust, long-term, multi-lineage reconstitution from 4 ee to 1 ee of unfractionated E10.5 AGM (30–39 somites) (Figure 1 C and Figure S1 C). At 18 weeks post-transplant, 3/5 recipients receiving 4 ee, 5/10 receiving 2 ee, and 5/10 receiving 1 ee showed multi-lineage engraftment with an average of 28%, 22%, ad 11% donor contribution, respectively. No neonates were engrafted with 0.5 ee. In comparison, no engraftment was observed in adult recipients receiving up to 7 ee with as few as 20,000 helper cells (Table S1). Secondary transplants demonstrated self-renewal capacity of the repopulating donor cells from E10.5 AGM (Figure 1 B). LDA predicted 1 repopulating cell in 2.84 ee of E10.5 AGM when using the neonatal transplant model (Table 1), indicating that the repopulating cell is less abundant in the AGM at E10.5 than it is at E11.5. These data establish that whole E10.5 AGM robustly reconstitutes neonatal but not adult recipients, and demonstrate the existence of an HSC capable of long-term multi-lineage reconstitution in the AGM of some embryos as early as E10.5.
When we examined reconstitution potential from even earlier in ontogeny, we detected long-term multi-lineage donor chimerism from unfractionated E9.5 PSp (16–26 somites) transplanted into neonates in doses from 8 ee to 1 ee (Figure 1 D and Figure S1 D). At 18 weeks post-transplant, 1/16 recipients receiving 8 ee, 2/5 receiving 5 ee, and 2/13 receiving 1 ee were engrafted at an average of 15%, 5%, and 2%, respectively. Curiously, mice engrafted with E9.5 PSp showed an early B-cell skewing, which was not as pronounced in neonates engrafted with E10.5 AGM and not seen with E11.5 AGM (Figure S1 D). LDA predicted 1 repopulating cell in 44.8 ee of E9.5 PSp (Table 1). Secondary transplants confirmed that the engrafting cell from E9.5 PSp was truly a self-renewing stem cell (Figure 1 B). These findings provide additional conclusive evidence for the existence of an intraembryonic repopulating cell as early as E9.5 in some embryos.
Neonates are not more supportive for engraftment of adult-like HSCs
We next determined if the neonate is a more permissive recipient for all HSCs or only those from the early embryo. LT-HSCs (Lineage− Sca1+ c-Kit+ CD150+ CD48−) from adult BM (Kiel et al., 2005) were transplanted into neonatal and adult recipients in doses ranging from 100 cells to 10 cells (Figure 1 E and Table S1). Surprisingly, adult donor LT-HSCs failed to give rise to robust engraftment in neonatal recipients monitored for up to 23 weeks. Adult recipients were robustly engrafted, whereas engraftment in neonatal recipients was minimal. At 18 weeks post-transplant, all adult recipients that received 100 cells and 3 of 5 animals that received 10 cells showed multi-lineage engraftment. LDA from adult recipients predicted 1 repopulating cell in 13.1 LT-HSCs from adult BM, which is less frequent than published reports, which used 200,000 helper cells (Kiel et al., 2005). While considering low-level engraftment of neonates, LDA predicted 1 repopulating cell in 177 LT-HSCs (Table 1).
We mined published microarray data of HSCs from E11.5 AGM and adult BM to better understand why HSCs from different developmental stages might prefer different transplant hosts (McKinney-Freeman et al., 2012). Modules from the microarray data point to key differences in cytokine-cytokine receptor interaction, transcription, metabolism, the toll-like receptor signaling pathway, and membrane composition. With 324 differentially expressed genes encoding membrane proteins, we hypothesized HSCs from various points in ontogeny may home to unique niches in different hematopoietic tissues. Interestingly, all examined genes known to be involved in homing (cxcr4, cd44, connexin-43, cd49d, lfa-1, and psgl1) showed consistent expression between E11.5 AGM HSCs and adult BM HSCs.
To determine if a mature definitive HSC population from the FL could engraft neonates, we transplanted cells with the same surface antigen profile (Lineage− Sca1+ c-Kit+ CD150+ CD48−) from E14.5 FL into neonatal and adult recipients in doses ranging from 100 cells to 10 cells (Figure 1 F and Table S1). Adult recipients had high level engraftment at all doses, whereas neonatal engraftment was only robust with 100 donor cells. At 18 weeks post-transplant, 10/16 neonatal recipients that received 100 cells showed balanced multi-lineage reconstitution with an average of 22.7% donor contribution; whereas only 1/8 neonates transplanted with 50 cells, 2/6 transplanted with 25 cells, and 1/8 transplanted with 10 cells were engrafted. LDA from neonatal recipients predicted 1 repopulating cell in 114 LT-HSCs; whereas from adult recipients LDA predicted 1 repopulating cell in 25.7 LT-HSCs (Table 1). Thus, while E14.5 FL LT-HSCs engrafted neonates more readily than adult BM LT-HSCs, both classes of definitive LT-HSCs from FL and adult BM engrafted better in irradiated adults than in neonates.
To further examine the differences between HSCs residing in the liver versus the BM, we transplanted LT-HSCs from neonatal BM and neonatal liver into neonates. At 18 weeks post-transplant, we observed a difference in the engraftment potential; more animals were engrafted at higher levels with neonatal liver LT-HSCs (Figures 1 G and Table S1). These data demonstrate a decrease in neonatal engraftment potential in HSCs residing in the BM, whether neonatal or adult.
Improving neonatal engraftment potential of adult BM HSCs
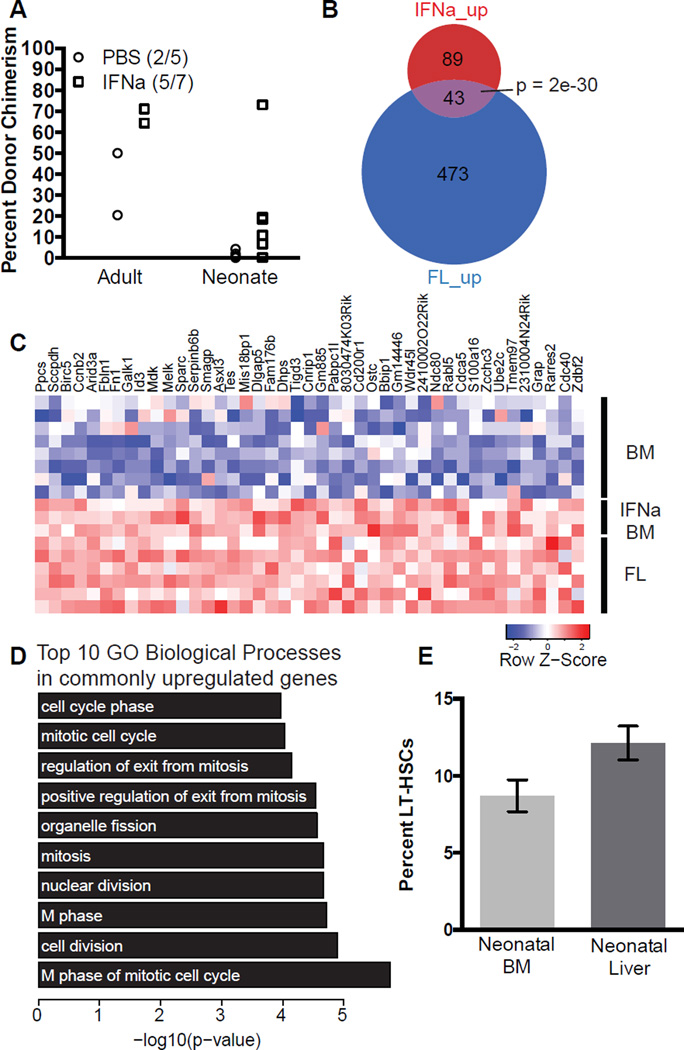
We observed more robust engraftment in neonates transplanted with FL LT-HSCs compared to BM LT-HSCs. FL LT-HSCs are rapidly cycling and expanding in the developing embryo, while adult BM LT-HSCs are typically quiescent (Bowie et al., 2007b; Cheshier et al., 1999; Harrison et al., 1997; Morrison et al., 1995). Thus we tested whether proliferation status could influence the potential for definitive adult-like HSCs to engraft neonates. To drive adult-like HSCs into the cell cycle, we treated adult mice with interferon-α (IFNα) and 5-FU (Essers et al., 2009; Randall and Weissman, 1997; Sato et al., 2009; Venezia et al., 2004). Cycling HSCs were then isolated and transplanted into neonatal recipients (Figure S3 A). HSCs exposed to IFNα were able to engraft neonates and adults more robustly than PBS treated controls (Figure 2 A). When HSCs were exposed to 5-FU, which has less effect on quiescent HSCs than IFNα (Lerner and Harrison, 1990), we observed no enhancement of neonatal engraftment (Figure S3 B). Further analysis of FL HSCs and BM HSCs treated with IFNα and 5-FU through published microarrays revealed 43 up-regulated genes common between FL HSCs and IFNα treated HSCs (Figure 2 B and C). The gene-ontogeny categories of the commonly up-regulated genes are biological processes essential for proliferation (Figure 2 D). Gene-set enrichment analysis showed 5-FU treated HSCs do not up-regulate the same genes as IFNα treated HSCs or FL HSC (Figure S3 C), suggesting IFNα and 5-FU have discrepant effects on HSCs, which may explain the difference in neonatal engraftment potential. Adult BM HSCs exposed to G-CSF, another treatment associated with HSC mobilization and activation of HSC cycling, likewise up-regulates the commonly up-regulated genes in FL and IFNα treated HSCs (Figure S3 D). We also observed that more neonatal liver LT-HSCs are proliferating than neonatal BM LT-HSCs, suggesting that neonatal engraftment potential as well as proliferation decreases around birth in HSCs that have migrated to the BM (Figure 2 E). Collectively, these data suggest that an active cell cycle enhances neonatal engraftment but that additional elements are also at work.
Figure 2. Cycling adult LT-HSCs engraft neonates.
A) Neonatal and adult recipients were transplanted with 100 BM LT-HSCs from adult mice treated with IFNα (square) or PBS (circle). Data shown are at 18 weeks post-transplantation. Using Fisher’s exact test to compare the percent of animals engrafted, the p-value is 0.5581, whereas using student’s t-test to compare the average percent engraftment, the p-value is 0.1730. B) Depiction of overlap of up-regulated genes in IFNα-treated bone-marrow HSCs (Essers et al. 2007) and FL HSCs (McKinney-Freeman et al., 2012) when compared to untreated bone-marrow HSCs. Significance of overlap was determined via the hypergeometric test. C) List of commonly up-regulated genes. D) Top 10 gene-ontology biological processes in commonly upregulated genes from panel B. E) Quantification of FACS analysis of Ki-67+ LT-HSCs in neonatal liver and BM. Data shown is the mean ± SEM of three experimental replicates with pooled samples. Paired t-test value = 0.065. Also see Figure S3.
LT-HSCs home to different recipient tissues in neonates versus adults
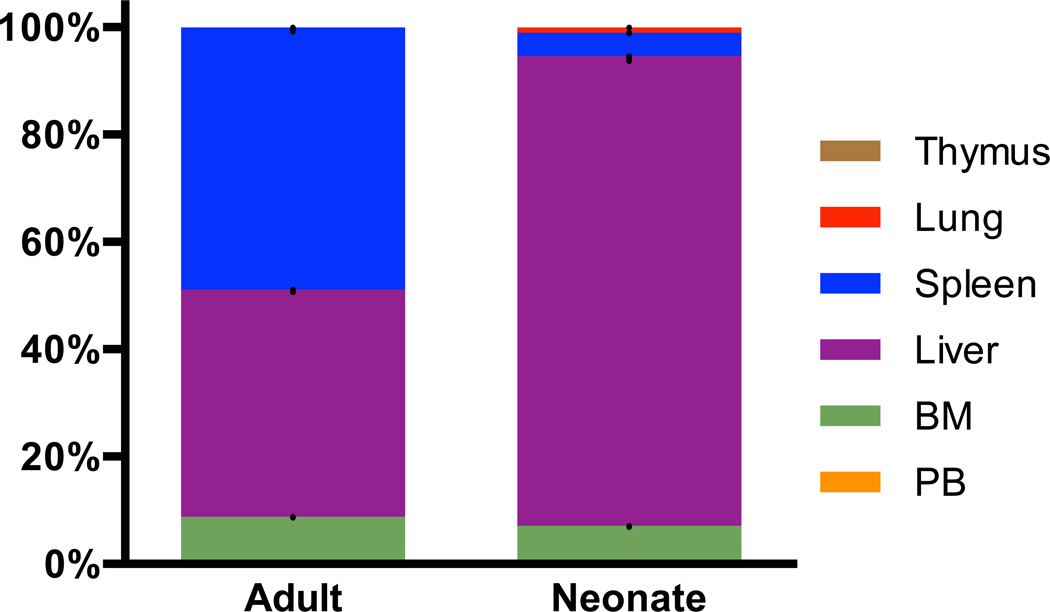
We also hypothesized that HSCs from different developmental stages home to different hematopoietic tissues in neonates and adults, which likely has a significant impact on long-term engraftability. We transplanted LT-HSCs from adult BM into neonatal or adult recipients, and after 15 hours recovered and dissociated multiple tissues and surveyed for donor cells by flow cytometry. We chose to examine the recipients 15 hours post-injection to assess homing prior to proliferation of the donor cells (Driessen et al., 2003; Hendrikx et al., 1996; Nilsson et al., 1997). At 15 hours post-transplant, we detected comparable numbers of donor cells in both neonates and adults, indicating that a lack of short-term donor cell survival could not account for the failure of adult LT-HSCs to engraft in neonates. In adult recipients 42% of injected cells were detected in the liver, 48% in the spleen, and 8.7% in the marrow of the leg bones. In neonatal recipients, 88% of injected cells were detected in the liver, 4.3% in the spleen, and 6.9% in the marrow of the leg bones. Very few or no donor cells were found in the thymus, peripheral blood, or lungs of either adults or neonates (Figure 3). These data demonstrate that definitive adult-like HSCs home to different tissues in neonates relative to adult recipients, which may underscore the difference observed in long-term engraftment. We also assayed the homing of VE-Cadherin+ CD45+ cells from E11.5 AGM but were unable to detect any donor cells at 15 hours post-transplant. This suggests few early embryonic HSCs survive 15 hours post-transplants, and those that survive to contribute to reconstitution are too few to detect.
Figure 3. Homing of adult LT-HSCs differs in adult and neonatal recipients.
Percentages of GFP+ CD45.2+ donor adult BM LT-HSCs detected in six tissues in adult and neonatal recipients 15 hours post-transplantation. Data shown is the mean ± SEM, representing a total of 5 – 6 mice per condition with data collected in experimental duplicate for adults and triplicate for neonates. Liver and spleen p-value < 0.05; lung, BM (BM), thymus, and peripheral blood (PB) p-value > 0.05 as calculated by the student’s t-test.
Discussion
Although prior literature suggests that the neonate harbors a more permissive environment for engraftment of early HSCs (Yoder and Hiatt, 1997; Yoder et al., 1997b) and microarray data of early and adult-like HSCs show differentially expressed genes with various functional roles (McKinney-Freeman et al., 2012), there have been no prior reports directly comparing and quantifying the repopulating cell frequency of HSCs from multiple points during ontogeny in both neonatal and adult recipients. Here we highlight the importance of the recipient in determination of engraftment outcomes, and identify several perplexing differences between the capacity of nascent embryonic and definitive adult-like HSCs to engraft either neonates or adults. Specifically, we show that nascent embryonic HSCs are better suited to engraftment in neonates; conversely, definitive adult-like HSCs, whether harvested from FL or adult BM, more efficiently reconstitute adult hosts. We used the neonatal transplant model to better understand the engraftment requirements of adult HSCs and identified two factors that may modulate neonatal engraftment potential, differential proliferative activity and homing potential. The neonatal transplant assay provides a tool to further our understanding of the factors required for survival, maturation, and function of embryonic HSCs and can be utilized to functionally examine nascent HSC-like cells derived from pluripotent stem cells.
Our data demonstrate robust long-term multi-lineage hematopoietic reconstitution of unmanipulated, uncultured E10.5 AGM in wild-type murine recipients, thereby establishing that definitive HSCs indeed arise within the embryo at this early stage, but a receptive host is required to observe this functionality. Others have shown that E10.5 AGM and even E9.5 PSp can engraft immunodeficient adult recipients, suggesting that genetic immunodeficiency in adult recipients enables the same permissiveness to engraftment of embryonic cells as we observe in the neonate (Bertrand et al., 2005; Cumano et al., 2001; Kieusseian et al., 2012; Levy, 2007; Marodi, 2006). Indeed, immaturity of the neonatal immune system may represent a reduced transplantation barrier for early embryonic HSCs, which express lower levels of MHC Class I molecules, and are thus susceptible to rejection by NK-mediated mechanisms (Cumano et al., 2001). Interestingly, we have also shown that the neonate is not a more permissive recipient for HSCs from all stages of ontogeny, as purified LT-HSCs from E14.5 FL and adult BM do not engraft neonates as robustly as adults. Adult-like HSCs likely respond to the neonatal environment differently than early HSCs because of the differential expression of genes involved in cytokine-cytokine receptor interaction and membrane composition. The preference of immature HSCs for engraftment in neonates may have clinical parallels in the tendency of umbilical cord blood to more robustly engraft juveniles as compared to adults, a phenomenon which may not depend entirely on cell dose (Gluckman, 2001; Laughlin et al., 2001; Wagner et al., 2002).
Our study suggests that HSC migration to the BM is coupled to limited neonatal engraftment potential. HSCs harvested from the neonatal liver re-engraft in neonates more robustly than HSCs harvested from the neonatal BM, indicating that either cell intrinsic changes that trigger migration from the liver to the BM also cause decreased neonatal engraftment, or that migration to the BM induces an alteration in HSCs that persists as the animal matures, as adult BM HSCs yield no or low level engraftment in neonates. When we surveyed homing of adult BM HSCs in neonatal and adult recipients, we found most donor cells in the liver in neonates and the liver and spleen in adults.
Although we detected similar numbers of donor cells in neonates and adults, and a comparable percent of donor cells homed to the BM in neonates and adults 15 hours post-injection, long-term engraftment of adult BM HSCs in neonates was very poor. Several potential explanations can be entertained to account for this. Coupled with our finding that FL HSCs engraft neonates more robustly than adult BM HSCs, one possible explanation is that the quiescent state of adult HSCs may not be suitable for engraftment in neonatal recipients. Concentrations of steel factor in neonatal hematopoietic niches may play a role (Bowie et al., 2007a). Recent work defines two distinct niches for cycling and quiescent HSCs in adult BM, which may be present in different ratios or non-existent in the developing neonatal BM (Kunisaki et al., 2013). Additionally, our neonatal engraftment conditions permit only sublethal doses of irradiation, whereas engraftment of adults is typically measured after lethal doses of irradiation. The sub-lethal irradiation of neonates may not generate a sufficiently strong homeostatic drive to induce substantial proliferation of the otherwise quiescent adult HSCs, especially considering the presence of competing actively cycling neonatal HSCs.
In conclusion, we have used the neonatal transplant model to functionally assay the engraftment potential of emerging early embryonic HSCs, which go undetected when using wild-type adult recipients. Future studies using the neonatal transplant model may allow us to discover new populations in hematopoietic tissues from other points in ontogeny that also engraft neonatal but not adult recipients. Additionally, neonates may provide a unique environment to functionally assay hematopoietic derivatives of pluripotent stem cells.
Experimental Procedures
Bone Marrow Transplantation
Donor and recipient cells were distinguished by CD45.1 and CD45.2. Adult recipients were either conditioned with a lethal dose of irradiation, 10 Gy total split by 3 hours, and received 3 × 105 cells from whole BM as helper or sublethally conditioned with a 3.5 Gy or 6.5 Gy dose of irradiation and did not receive helper cells. Neonatal recipients, 1 – 2 days old, were conditioned with a sublethal single dose of irradiation, 3.5 Gy, and received no helper cells.
HSC Proliferation
To induce HSC proliferation, adult mice were treated with one dose of IFNα (10,000 units given subcutaneously) 24 hours prior to HSC isolation. Alternatively, adult mice received one dose of 5-FU (150 mg kg−1) by intraperitoneal injection 5 days prior to HSC isolation.
Homing
Approximately 104 isolated cells were transplanted into neonatal and adult recipients. Transplanting purified LT-HSCs in addition to injecting a small number of cells reduces the likelihood of donor cells being trapped in the lung. At 15 hours post-injection, the lung, peripheral blood, BM from the long bones of the leg, spleen, liver, and thymus were analyzed by FACS.
Microarray Analysis
The microarray data were analyzed per standard protocol using R/Bioconductor. Data was obtained from GEO accession: GSE14361 (Essers et al., 2009), GSE1559 (Venezia et al., 2004), GSE37000 (McKinney-Freeman et al., 2012), and GSE55095 (Schuettpelz et al., 2014).
For full details, see the supplemental experimental procedures.
Supplementary Material
Highlights.
Quantification of robust reconstitution of neonates with early embryonic tissues
Neonates are not more supportive for engraftment of adult-like HSCs
Proliferation enhances neonatal engraftment potential of adult bone marrow HSCs
LT-HSCs home to different recipient tissues in neonates versus adults
Acknowledgements
The authors would like to thank the Dana-Farber Cancer Institute flow cytometry core for assistance and F. Fahey, A. Packard, J. Dearling, and E. Snay for assistance with small animal imaging. GQD is supported by grants from the US National Institute of Diabetes and Digestive and Kidney Diseases (R24-DK092760) and the National Heart, Lung, Blood Institute Progenitor Cell Biology Consortium (UO1-HL100001); Alex’s Lemonade Stand; Harvard Stem Cell Institute; and the Doris Duke Medical Foundation. GQD is an associate member of the Broad Institute, and an investigator of the Howard Hughes Medical Institute and the Manton Center for Orphan Disease Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, Cumano A. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci U S A. 2005;102:134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Copley MR, Eaves CJ. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood. 2007a;109:5043–5048. doi: 10.1182/blood-2006-08-037770. [DOI] [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, Hoodless PA, Eaves CJ. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007b;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A, Dieterlen-Lievre F, Godin I. The splanchnopleura/AGM region is the prime site for the generation of multipotent hemopoietic precursors, in the mouse embryo. Vaccine. 2000;18:1621–1623. doi: 10.1016/s0264-410x(99)00496-x. [DOI] [PubMed] [Google Scholar]
- Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol. 2003;31:1284–1291. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Gluckman E. Hematopoietic stem-cell transplants using umbilical-cord blood. N Engl J Med. 2001;344:1860–1861. doi: 10.1056/NEJM200106143442410. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Zhong RK, Jordan CT, Lemischka IR, Astle CM. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp Hematol. 1997;25:293–297. [PubMed] [Google Scholar]
- Hendrikx PJ, Martens CM, Hagenbeek A, Keij JF, Visser JW. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24:129–140. [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kieusseian A, Brunet de la Grange P, Burlen-Defranoux O, Godin I, Cumano A. Immature hematopoietic stem cells undergo maturation in the fetal liver. Development. 2012;139:3521–3530. doi: 10.1242/dev.079210. [DOI] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, Gerson SL, Lazarus HM, Cairo M, Stevens CE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- Lerner C, Harrison DE. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990;18:114–118. [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Marodi L. Innate cellular immune responses in newborns. Clinical immunology (Orlando, Fla) 2006;118:137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- McKinney-Freeman S, Cahan P, Li H, Lacadie SA, Huang HT, Curran M, Loewer S, Naveiras O, Kathrein KL, Konantz M, et al. The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell. 2012;11:701–714. doi: 10.1016/j.stem.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Mizuochi C, Fraser ST, Biasch K, Horio Y, Kikushige Y, Tani K, Akashi K, Tavian M, Sugiyama D. Intra-aortic clusters undergo endothelial to hematopoietic phenotypic transition during early embryogenesis. PLoS One. 2012;7:e35763. doi: 10.1371/journal.pone.0035763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Dooner MS, Quesenberry PJ. Synchronized cell-cycle induction of engrafting long-term repopulating stem cells. Blood. 1997;90:4646–4650. [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- Randall TD, Weissman IL. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89:3596–3606. [PubMed] [Google Scholar]
- Rybtsov S, Sobiesiak M, Taoudi S, Souilhol C, Senserrich J, Liakhovitskaia A, Ivanovs A, Frampton J, Zhao S, Medvinsky A. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med. 2011;208:1305–1315. doi: 10.1084/jem.20102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, Woloszynek JR, Greenbaum AM, Link DC. G-CSF regulates hematopoietic stem cell activity, in part, through activation of toll-like receptor signaling. Leukemia. 2014 doi: 10.1038/leu.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L, McCulloch EA, Till JE. THE DISTRIBUTION OF COLONY-FORMING CELLS AMONG SPLEEN COLONIES. J Cell Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3:99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoudi S, Morrison AM, Inoue H, Gribi R, Ure J, Medvinsky A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132:4179–4191. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- Taylor E, Taoudi S, Medvinsky A. Hematopoietic stem cell activity in the aorta-gonad-mesonephros region enhances after mid-day 11 of mouse development. Int J Dev Biol. 2010;54:1055–1060. doi: 10.1387/ijdb.103152et. [DOI] [PubMed] [Google Scholar]
- Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation research. 1961;14:213–222. [PubMed] [Google Scholar]
- Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:e301. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- Wineman J, Moore K, Lemischka I, Muller-Sieburg C. Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood. 1996;87:4082–4090. [PubMed] [Google Scholar]
- Wu AM, Till JE, Siminovitch L, McCulloch EA. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968;127:455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Cumming JG, Hiatt K, Mukherjee P, Williams DA. A novel method of myeloablation to enhance engraftment of adult bone marrow cells in newborn mice. Biol Blood Marrow Transplant. 1996;2:59–67. [PubMed] [Google Scholar]
- Yoder MC, Hiatt K. Engraftment of embryonic hematopoietic cells in conditioned newborn recipients. Blood. 1997;89:2176–2183. [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997a;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A. 1997b;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137:3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.