Good afternoon. Thank you Dr Rotondo for your generous introduction – I couldn’t have done better myself.
Welcome to all. It is indeed an honor to give the 81st Scudder Oration. I consider being chosen by my peers to present this oration one of the highlights of my career.
Appropriately, the orator frequently uses this opportunity to thank those who have contributed critically to their careers. While I started to make a collage, there were too many people that I owe for my career and my success. Thus, I revamped and wish to acknowledge a few select people from my “surgical family.”
As all of us, particularly in academic medicine, know, we have a crucial medical family in addition to our true family. My surgical family begins with my father-mentor, C. James Carrico, [Fig. 1A] as he’s the one that convinced me to go to Seattle from Dallas with him and Dr. G. Tom Shires. He is also the one that convinced me that Trauma was the only valid career for me as an aspiring academic surgeon. He was a kind, loving, generous, supportive sponsor and mentor throughout my early career, and all those who know him agree that he was indeed a phenomenal individual.
Figure 1.
Maier Surgical Family: (A) C James Carrico, MD; (B) Donald D Trunkey, MD; (C) David B Hoyt, MD; (D) Ernest E Moore, MD; (E) Gregory J. Jurkovich, MD; and (F) Carlos A Pellegrini, MD.
Following closely is a man who has been part of my career ever since the first day – he is the always smiling but dominant leader, Donald D. Trunkey. [Fig. 1B] In addition, I was given the honor of being awarded the Jane and Donald D. Trunkey Chair in Trauma at the University of Washington. It is indeed a unique privilege to hold a Chair in my own specialty named after the “Father of Modern Trauma Care”. I am blessed to have had this happen, and to have had Don as a mentor and supporter throughout my career, as he has for so many.
Many of you may not know about this next relationship – this is my “twin”, David Hoyt. [Fig. 1C] You may or may not know where that comes from, but just Saturday night at the Presidential Dinner, a visitor who had just met both of us came up to me at the end of the dinner and said “That’s really great, that they gave you the accolades Dr. Hoyt!”, and then he looked at my badge and said “Oh, you’re not Dr. Hoyt.” This occurs frequently. Ever since David nudged me out of my research bench spot at Scripps Research Foundation in La Jolla and took my place, we have been very close friends and our academic careers have paralleled each other through numerous organizations of mutual interest, maintaining a wonderful collegial friendship throughout our careers.
The next is the ever-smiling Dr. Eugene Moore. [Fig. 1D] He’s the big brother in the relationship. We grouse at each other and even “dis” each other, but we always walk away friends, and Gene has always been my test bed. He’s never been afraid to tell me that I’m wrong, and I am one of the few to tell him the same. We’ve maintained a long-lasting brotherly relationship. Since Gene is the much older of the two brothers, he has been a true resource and supporter for me.
Jerry Jurkovich spent 20 years as my confidant and colleague – the person who developed, in large part, the Trauma Center in Seattle. [Fig. 1E] Jerry was always there supporting, working, and building. We had a great run together. It was a wonderful period of co-existence and collaborative working together.
And then in my surgical family, I couldn’t skip this gentleman. Carlos Pellegrini, my Chairman, who, as you all know, has always displayed the outstanding qualities, including great emotional intelligence, that justly warrant his Presidency of the American College of Surgeons. [Fig. 1F] But, in addition, his leadership as a Chairman, always there to support, and yet innately knowing how to give space for me to develop my own faculty and my own program. As Dr. Moore has said many times, he and I have had two of the best jobs in the country, BUT are totally dependent on who the Chairman is, and I’ve been fortunate for the bulk of my career to have Dr. Pellegrini there as my leader and supporter and he’s been phenomenal.
There’s an adage that to see forward you have to stand on the shoulders of others, and this is the group of shoulders I stand on every day – this is the General Surgery faculty at Harborview Medical Center. [Fig. 2] Every one of them a superb surgeon first, and then, in addition, each a research funded and superb scientist and/or educator. They have been my support base, my colleagues and friends for many years, and have elevated me to achieve my goals.
Figure 2.
General Surgery, Trauma, Burns and Surgical Critical Care Faculty, Harborview Medical Center, University of Washington.
Lastly is my true family. The true family that’s stuck it out with me - they’ve been my bedrock, the reason I’ve achieved what I’ve been able to achieve, and I’ll never be able to repay them for everything they’ve done and sacrificed for me. On the left is my son John Michael, my wife Lauren and my daughter Anna. [Fig. 3] They’ve been the love of my life, and I can never thank them enough.
Figure 3.
Maier Family: John Michael, Lauren, and Anna Maier.
I chose my topic to fit with the theme of the 100th year of the American College of Surgeons. I thought a perfect fit would be the evolution and progress in resuscitation of the patient in circulatory shock, which has mirrored closely the development of the American College of Surgeons as it took on the charge of education, quality of clinical care and improvement in training for the benefit of the surgical patient. Simultaneously, trauma care was evolving as a true scientific field, and has moved in parallel with the College, and so I thought– a worthy match.
Up until the turn of the 20th century, these quotes I borrowed from Dr. Norm Rich highlight trauma as the surgical standard. If you wanted to be a surgeon, Hippocrates recognized in ≈400 BC the best way to be a surgeon was to follow an army. Still two thousand years later, Ambroise Paré noted that the only people who really benefit from war are young surgeons. And, that military core still continues today. However, a critical transformation of surgery into an academic specialty occurred at the same point in time as the College was founded in 1913. At that time, led by our famous predecessors, such as William Halsted, our approach to surgery was undergoing dramatic change. It no longer was a tour de force of a unique procedure in the operating room that was frequently never repeated. Halsted involved the basic tenants of scientific investigation into the field of surgery.1 He integrated the other basic sciences, including pathology and physiology, and from that point on surgery was becoming a true science, which paralleled closely the advances that were being made and propagated by the American College of Surgeons.
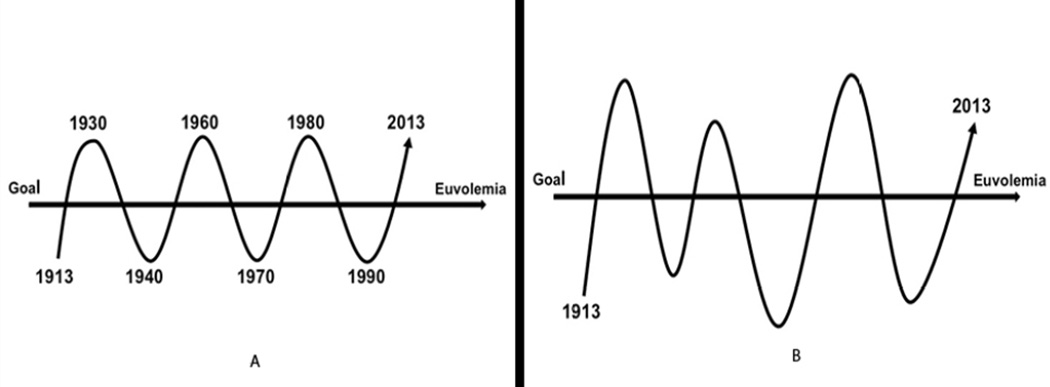
Dr. Ken Maddox, in his Master Surgeon Lecture at the 2013 Annual Meeting of the American Association for the Surgery of Trauma (AAST), postulated that over the last 100 years our approach in medicine to major problems, such as hypovolemic shock, seems to follow a rhythmic sine wave pattern similar to a pendulum moving back and forth as we go too far, then too little, then too far – in our search for euvolemia. [Fig. 4A] And by “euvolemia”, I do not mean the “normal” volume, but rather the “ideal” volume to treat the disease at that point in time. There may be significantly different endpoints, as illustrated by damage control resuscitation and a relative hypovolemia. However, while superficially our approach to hypovolemia over the decades may seem like a rhythmic sine wave narrowing to the ideal, I would propose that our prior approach to the patient in shock appears much closer to the response of other complex biologic systems, similar to the responses of the patients that we treat. Complex biologic processes are driven by, not a predictable controlled sine wave, but rather as described by chaos theory. [Fig. 4B] Chaos theory involves the behavior of dynamic, biologic systems that are highly sensitive to initial conditions, and while we acknowledge this reality, we’ve not succeeded in truly comprehending, interrogating, and integrating chaos-based approaches into a cohesive treatment plan, and which has greatly impeded advances in the care of our patients. Empirically, we recognize associations. For example, patients become coagulopathic and they die. We conclude that obviously the coagulopathy caused the death. Maybe. Possibly. Probably. But in many patients, maybe not. A major tenet of chaos theory is that while the present determines the future, the approximate present does not approximate the future. And, “we”, as traditional linear-thinking scientists, have trouble dissociating processes and comprehending non-linear impact. I would re-formulate the concept as “small early changes can lead to major frequently unpredictable differences in outcome.” I would propose this as my major theme and take home message from this presentation. We need to focus on this construct as we attempt to prove causality to better optimize our care for the patient in shock.
Figure 4.
Resuscitation volume proposals to reach euvolemia in traumatic shock: (A) biologic sine wave oscillating above and below the mean (euvolemia) goal; (B) chaos theory approach to complex systems biology of euvolemia.
The frequent wars, which the United States incurs at regular intervals, have provided a wealth of knowledge and insight in to the care of the injured patient. The first, just after the College was formed, was World War I. Walter B. Cannon, a physiologist from Boston, in 1918 wrote a series of articles in JAMA that concluded, in part, after empiric observations of battlefront conditions, “Control the hemorrhage and resuscitate with intravenous fluids (blood if you have it)”, … was the appropriate approach for penetrating trauma. He also made the comment “if the pressure is raised before the surgeon can check bleeding, you are going to lose sorely needed blood.”2 This concept, as we now know, was lost until recently when it was re-confirmed by Dr. Maddox in the mid 1990’s - a concept that was first recognized in 1918. Frequently, in our rush to adopt new ideas, we have been unable to maintain sight of old lessons. We repeatedly seem to lose our way.
Another unique insight that Cannon proposed in 1918 was that “shock is a loss of homeostasis. And without homeostasis the patient does not survive.” In this concept, Cannon was identifying a future major component of the chaos theory that he derived from empiric observations in 1918 on the battlefield. He went on to hypothesize that the loss of homeostasis was due to a neurologic dysfunction, which was subsequently discredited. But, as Dr. Frank Lewis presented in his Fitz Lecture three weeks ago at the Annual Meeting of the AAST, the net balance of the organism trying to preserve microvascular flow, primarily during sepsis, in the face of hypovolemia leads to hypotension and redistribution of blood flow. This is not really neurologic dysfunction, but rather an attempt to preserve survival. And Dr. Lewis posed the question – should the hypotensive state be pharmacologically reversed, or is it protective? A secondary conclusion by Dr. Cannon was that secondary (or persistent) shock was caused by toxins released by the injury. Again, a theory discredited and lost until the presence and impact of DAMPS (Damage Associated Molecular Patterns) released from injured tissue was “re-discovered” by P. Matzinger in the early 1990’s.3 The DAMPS are known to activate the proinflammatory innate immune system and cause diffuse organ injury. In addition, the amount of DAMPS released correlate directly with the extent of tissue damage, and helps to explain the greater impact of similar levels of hypotension when related to blunt tissue damage compared to penetrating trauma with limited tissue damage. And lastly, Cannon noted a biphasic coagulopathic response in the severely injured. There was a very early hypercoagulable phase, followed by a hypocoagulable phase even without resuscitation. Again, a concept recognized in 1918 that was lost until very recently, and in the last 10 years has become the primary focus of research in trauma resuscitation – 100 years later.
Alfred Blalock, in the 1930s, modernized Shock Research by defining through experimentation the cause of shock as due to loss of intravascular volume.4 Shock was concluded to be not due to toxins as commonly held at the time, but rather circulatory collapse from lack of volume– and fluid therapy was required and sufficient for survival from shock. Another important observation he made in the 1930s, that was lost for many years, was that blood pressure is an inadequate and unreliable monitor of intravascular fluid volume. Of note, not so long ago, Dr. Shoemaker “reinvented” this as a new concept. As is frequently true in medicine, if we could only retain what we once learn, in this case in the 1930s, and heed the implications for therapy, we would advance care far more quickly, and potentially with fewer excesses leading to iatrogenic injury.
After the development of blood banking in 1937 at Cook County Hospital, and other surgical resuscitation advances in World War II, we moved into the Korean Conflict with the ability to give blood transfusions for the first time at the battlefront, and simultaneously had rapid access to the injured due to the development of helicopter transport and air superiority. Curtis Artz and William Fitts, Jr. made the observation during the Korean conflict that the severely injured soldier required both return of shed blood PLUS crystalloid for improved survival.5 If you merely return the blood that the patient lost, you have a sick and under-resuscitated patient. This empiric observation led, in part, to the subsequent seminal studies by G. Tom Shires. His analyses of fluid spaces and distribution, in the injured either animal model or human patient, demonstrated a significant extracellular fluid deficit after severe hemorrhagic shock due to loss of cellular membrane energy gradients.6 To restore and maintain adequate perfusion and prevent further cellular injury, replacement of blood plus crystalloid to replete the compensatory loss in extracellular fluid, i.e. “third-spacing”, was necessary.
And thus, the concept of third spacing was elucidated. Simultaneously, we had increasingly rapid access to the wounded warrior, along with access to blood and resuscitation fluids. And suddenly, we learned we had to give extra fluid for improved survival; however, we didn’t know how much, so, as usual, we went to excess and gave a lot, which appears to be a basic force of human nature – “if some is good, more is better.” The concept of third spacing increased our understanding of the shock condition, but also produced an argument and rationale to support fluid overload, which was frequently and increasingly achieved.
In Vietnam, air superiority again permitted early, rapid and aggressive fluid resuscitation. Literally overnight, rapid control of bleeding, return of lost blood and large supplements of crystalloid were able to essentially eliminate AKI (Acute Kidney Injury), which was a major cause of death in the Korean Conflict. Conversely, the elimination of AKI was simultaneous with the development of a “new disease”, originally coined “Da Nang lung”, and subsequently defined as Acute Respiratory Distress Syndrome (ARDS).7 Within a 5-year period, we converted our primary cause of late military death from death due to renal failure to death due to ARDS; all due to well-intentioned implementation of our improved fluid resuscitation knowledge and techniques. Of note, Dr. R. L. Simmons, a critical surgical researcher, was stationed in Vietnam in 1968, and reported an early highly hypercoagulable consumptive state followed by a severe multifactorial coagulopathy in these severely injured and aggressively resuscitated patients.8 Another case of déjà vu was to follow.
In support of these empiric military observations, William Blaisdell and others at San Francisco General Hospital published an early pathophysiologic paper on ARDS demonstrating an associated, and proposed causal, massive microembolization in ARDS following severe trauma and hypovolemic shock.9 The pulmonary vasculature of the patient with ARDS demonstrated a microvasculature loaded with microemboli. Correlating with Dr. Simmons’s and other’s observations, massive microvascular thrombosis rapidly occurs with hypovolemia and ischemia, followed by an aggressive volume resuscitation that flushes these microemboli into the next large microvascular bed – the lung - further contributing to ischemic microvascular injury, capillary leak and subsequent flooding of the alveoli, resulting in ARDS. Blaisdell created a model for ARDS in large mammals, which required cross clamping of the aorta, hypotension and injecting thrombin to cause enhanced thrombosis – recreating ARDS as seen in humans. Relevant to current discussions in the field of trauma-induced hemorrhage control, is the proposal to control massive lower body bleeding in the injured patient by inserting a percutaneous intraaortic balloon to occlude the aorta of the bleeding hypovolemic patient while simultaneously giving tranexamic acid to enhance thrombosis. We may have forgotten about Dr. Blaisdell’s work, but sounds very similar to the model used to replicate ARDS in animals.
Later, in the 1980s, William Shoemaker and colleagues in Los Angeles confirmed that Blalock was indeed correct 50 years earlier. Blood pressure is not a good monitor of intravascular volume. In fact urine output and many other common physiologic parameters are also not good monitors. Many hypovolemic patients have return of urine output and blood pressure with resuscitation but are still under-resuscitated.10 This relative hypovolemia produces a cyclic vasoconstriction and microcirculatory stasis followed by intermittent reperfusion and injury, which ultimately drives multiple organ failure. With each cycle, organs are deprived of adequate oxygen and a proposed oxygen debt develops. While progressive ischemia is detrimental, it is unclear how a biologic system can “remember” the extent of oxygen debt that will need to be repaid. An ischemic cell is not able to track the number of oxygen molecules missing or deficient. Thus, while no doubt the longer inadequate oxygen delivery exists the worse the impact on cellular survival, it was unproven that excess oxygen molecules provided by subsequent resuscitation are necessary to make up a deficit; or does the excess oxygen contribute potentially to the reperfusion injury? The protocol derived from Shoemaker’s work, proposed aggressive resuscitation to drive an over supply of oxygen to “repay” any oxygen deprivation that had occurred and prevent organ failure. Resuscitation was driven to supranormal levels of oxygen delivery to greater than 600ml per minute.11 Initial cohort studies seemed to support that patients did better with supranormal O2 delivery. The side effects of massive tissue swelling, including intestines, lungs and CNS, due to excessive resuscitation were thought necessary to save these critically ill patients. A new international society was created to study the side effects of this massive resuscitation paradigm, including intraabdominal hypertension (IAH) and the abdominal compartment syndrome (ACS) – what turned out to be primarily an iatrogenic disease complex.
Subsequently, a formal randomized controlled trial (RCT) was performed to assess causality.12 This study, and others, demonstrated that supranormal oxygen delivery did not save lives, but actually doubled mortality. The old adage that “the enemy of good is better”, had been fulfilled. While the excess delivery of O2 to treat oxygen debt sounded good in theory, in practice it did not produce the benefits as we thought it would. In fact, numerous complications, including intraabdominal hypertension (IAH), the abdominal compartment syndrome (ACS), multiple organ failure (MOF) and death, approximately double with this aggressive fluid resuscitation protocol.
At the beginning of the 1990s, increasing data supported that we were grossly over-resuscitating hemorrhagic shock, with excessive crystalloid volumes. At this time, Mattox and colleagues in Houston performed a pre-hospital RCT that avoided all pre-hospital resuscitation despite a significant degree of hypotension in young males with penetrating torso trauma.13 This study demonstrated that mortality and patient length-of-stay were both significantly improved in those unresuscitated and left hypotensive with NO preoperative IV fluids compared to routine pre-hospital volume resuscitation for hypotension. Thus, they confirmed that you not only do not have to be aggressively resuscitated – but in fact, patients with penetrating torso trauma do better without fluid, as previously advocated by Dr. Cannon in 1918 during WWI.
Unfortunately, we humans also have an inherent tendency to extrapolate beyond the findings and supporting data. Mattox demonstrated that predominantly young males, shot in the torso, in Houston, do better with RAPID surgery and without pre-operative IV fluids. That is what the study tested. What was extrapolated was a concept of preferential significant hypotensive resuscitation in all trauma. The adoption of medical knowledge must be done carefully, and data applied appropriately to the correct patient population to avoid undue extrapolation, similar to the recent over-utilization of beta-blockers perioperatively. Several pre-hospital systems have been reported anecdotally as interpreting the data as an indication to return to the 17th century practice of bloodletting, with blood loss in the field until the patient becomes hypotensive to ensure an optimal outcome. However, I would argue that was NOT the conclusion of the study, and this extrapolation will lead to poorer outcomes rather than advance care.
A study from Gene Moore and the Denver group in the early 1990s defined the causes of death from trauma.14 [Table 1] While acute and ongoing blood loss is the primary cause for death in the early initial resuscitation phase, it still accounted for only 55% - an almost equal number die from severe traumatic brain injury. And, as you would expect, at later time periods, very few deaths are due to hemorrhage. The average trauma patient in a Level I Trauma Center is not the patient in the study by Mattox – but rather the multiply blunt-injured patient who is most likely to die from traumatic brain injury or multiple organ failure (MOF). And, while we can do little to reverse the initial brain injury, secondary injury to the brain, primarily due to hypotension, creates a much larger deficit, with a worsened functional outcome and increased mortality. Dr. Chesnut, from our institution, has confirmed that brief episodes of hypotension after brain injury dramatically worsened the outcome.15 If patients with a head injury are hypotensive in the field and not given fluid, they have very poor outcomes from their brain injury. This is further supported by a Denver study that identified three major independent factors that predict poor outcome and multiple organ failure. While we can not change the age of the patient, the one major predictive factor that can be treated is ongoing significant hypoperfusion, i.e. hypotension.
Table 1.
Why Do Trauma Patients Die?
| Acute (<48 h) | Early (48 h–7 d) | Late (>7 d) | |
|---|---|---|---|
| Brain injury | 40% | 64% | 39% |
| Blood loss | 55% | 9% | 0% |
| MOFS | 1% | 18% | 61% |
(Adapted with permission from data from The Journal of Trauma and Acute Care Surgery, Sauaia A, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193.)
The Glue Grant, a multi-institutional trial funded over a 10-year period by NIH/NIGMS, collected detailed physiologic data on massively blunt injured patients who were hypotensive and/or acidotic and received a blood transfusion for ongoing blood loss. This dataset is the most complete serial physiologic data that have been collected from trauma patients. Using this repository, Jason Sperry and colleagues compared patients hypotensive in the field given, on average, two liters of Ringers lactate to patients with persistent hypotension.16 The patients left hypotensive with inadequate resuscitation after blunt trauma had a markedly lower survival compared to those who were hypotensive and received the two liter bolus of Ringers lactate as recommended by ATLS.
Not surprisingly, the results duplicate the conclusion of Tom Shires in the 1960’s, that the hypotensive trauma patient benefits from a limited volume of crystalloid during transport. What he did not propose was that the normotensive patient in the field undergo aggressive resuscitation. In fact, as shown by the Sperry analysis, there is a reverse effect on outcome.
While normotensive patients who received no resuscitation volume have improved survival, those normotensive patients who receive two liters of Ringers lactate have a significantly increased mortality. The lesson re-learned is to treat patients for the disease they have. If patients are hypotensive, limited fluid resuscitation is good – if the patient is normo-tensive, drowning with salt water has never been advocated as beneficial for their outcome. And yet, somehow we have pursued that end point.
Based on these studies and others by many of the investigators that are present here, and based on the original military observations from the 1920s, hypotension should not be aggressively corrected until surgical control of bleeding is accomplished.17 However, limited resuscitation is still required for optimal care. Current military observations, in the austere environments of Afghanistan and Iraq with frequently delayed access to definitive care, have established the presence of a radial pulse as an appropriate surrogate for a systolic blood pressure of 90, and is a simple monitor to apply for resuscitation in both the pre-hospital phase in the austere environment and the rural civilian setting.
Presently, we have adopted what currently appears to be reasonable concepts of damage control resuscitation, or controlled volume resuscitation.18 This approach also conforms well with the most recent military observations; that severe trauma-induced hemorrhagic shock and hypoperfusion alone is a main cause of a rapid-onset coagulopathy. And, while it is aggravated by dilution of coagulation components with resuscitation, the tissue injury (release of DAMPS) and the hypovolemia-induced ischemia are primary causes of the coagulopathy, rather than the traditionally accepted dilutional etiology. MacLeod first reported that major injury is associated with a non-dilutional coagulopathy.19 Brohi and colleagues, and others since, have demonstrated that the hypotension and associated ischemia of the endothelial cell increases activated protein C, suppression of thrombin with ongoing low level thrombin activation, with a simultaneous increase in fibrinolysis, weakening clot formation and a progressive coagulopathy, which contributes to uncontrollable bleeding and increased mortality.20
Extensive confirmation of this pathophysiology has been provided by the young wounded warrior in the Mideast too frequently due to multiple extremity amputations from an IED blast. There is massive blood loss, with extensive tissue injury, and a rapidly developing coagulopathy, even without resuscitation. Similar findings have been documented in the severely injured civilian population.21 For each ISS category, patients with a concomitant coagulopathy have a dramatically increased mortality.22 To complement these findings, Dr. Holcomb and colleagues, in Iraq and Afghanistan, made the empiric observations that as they gave more plasma to treat the coagulopathy and the ratio of FFP to pRBCs increased, the mortality from acute hemorrhagic death decreased dramatically.23,24,25,26 The obvious conclusion was FFP helped replace what was lost – whole blood – and not only RBCs, and is good for the massively injured. But unresolved is the supplemental conclusion of the benefit of an increased ratio of FFP to pRBC, which may be more than mere volume of increased FFP, but also may be a surrogate for FFP given early in the resuscitation. Thus, the benefit may not be a direct relationship to the amount of FFP given, but rather the timing of the FFP.
Trending transfusion patterns over eight years of the Glue Grant, in patients with massive transfusion, we were concerned that there was no apparent change in our ratio of FFP to pRBCs. We acknowledge that we are resistant to change, but this seemed excessive in light of the data. So we looked at patients in the sub-massive transfusion category – that did not reach a massive transfusion level of blood.27 At the later resuscitation time points, both early in the study and late in the study period, there was minimal to no difference in the FFP:pRBC ratios. However, what did change over the eight years, was that FFP was started much earlier after hospital arrival. The absolute amount was not significant, but it was given earlier, within six hours. By 24 hours, there was no difference in our ultimate FFP:pRBC ratios. But the consequence was dramatic. Even though the average ISS increased over the eight years of the study, the incidence of massive transfusion decreased by half. A remarkable effect since the amount of FFP given was limited and would not be expected to correct a significant coagulopathy. The apparent effect appears to be prevention. It is best not to wait for a coagulopathy to develop, but to give FFP very early in the course of treatment and prevent the coagulopathy.28 As with many diseases, early intervention (such as improved ventilator management early in the course of acute lung injury, or ALI) has much greater effect, rather than treating an established disease process (ARDS). Chaos theory proposes that early small interventions produce large end results.
Similar to the use of tranexamic acid to prevent coagulopathy, benefit requires infusion within three hours of injury to produce a measurable effect. Again using the Glue Grant dataset, FFP given early versus later in the resuscitation demonstrates that the entire benefit of improved survival comes from the FFP given in the first six hours. After the first six hours, amount of additional FFP has no demonstrable impact on improving outcome. The benefit in survival occurs when the increased ratio of FFP:pRBC is achieved within the first six hours.
Interestingly, the impact on outcome is similar or even better when platelets are transfused early in the severely blunt-injured patient.28 So treatment may not require FFP; platelets alone may suffice if given early in the course of resuscitation. Similarly, in Europe, there are ongoing trials testing a potential similar protective effect in preventing coagulopathy through early infusions of fibrinogen concentrates as a way to minimize potential FFP toxicity, yet effectively treat early coagulopathy. Currently, short of fresh warm whole blood, the ideal blood component is not known, but increasingly data show that early utilization counts the most.
In fact, to further this concept, a study, presented at the annual AAST meeting, investigated a small number of Glue Grant patients who received a blood transfusion prior to hospital arrival, either at a transferring hospital or during helicopter transport within the 6-hour post-injury window.29 The patients received no FFP, other blood component or treatment for coagulopathy. However, the improvement in outcome between transfused patients and a propensity-matched cohort was dramatic. There was an 80% reduction in mortality in those receiving pRBC prior to arrival. As the intervention moves closer to the time of the insult, the apparently larger the impact is on outcome. In this case, in addition to volume, presumably the primary impact was provision of oxygen-carrying capacity to these patients, potentially reversing microvascular and endothelial ischemia. Again, from a complex systems biology approach, small, early perturbations in the disease course create significant changes in outcome. And, thus, we may avoid chasing advanced disease, which frequently fails. There may not be a need to totally correct the dysfunction, and run the risk of over-treating and overcorrecting.
In a recent study of perioperative cardiac surgery patients, blood transfusions in this propensity-matched cohort analysis behave as a classic toxin in a dose response manner. For each unit of blood transfused perioperatively, there is a step-wise increase in mortality.30 The only identifiable difference between these well-matched cohorts was blood transfusion. Unnecessary banked-blood transfusions in large quantities are detrimental. We have learned from numerous ICU studies that patients receiving blood transfusions unnecessarily to reach an arbitrary hemoglobin level had significantly increased mortality compared to patients receiving “damage control” transfusion for Hgb < 7 only.31 If we move away from trying to “normalize” all parameters, patients may do better.32
Using data generated by Dr. Holcomb and colleagues from military experience in the Middle East, the death rate identified retrospectively from acute hemorrhage upon giving FFP, and increasing the ratio of FFP to pRBC, dramatically decreased mortality from acute hemorrhage. However, of interest, in the patients that survive, 60% of the subsequent mortality is from multiple organ failure (MOF). While one can logically argue that to die late from MOF requires survival from the acute early hemorrhage, in contrast, one could also argue there is also a balance of benefit versus potential risk of large-volume FFP. Can we achieve the optimal benefit by giving limited FFP, but very early, rather than by giving excess FFP, wimilar to crystalloid?
In a recent study by Inaba and researchers at LA County, FFP was given to all severely injured patients for initial resuscitation in the ER.33 In a retrospective analysis, the report included patients that did not have significant blood loss, but received a variable amount of FFP. The results reveal a dose response in FFP-associated complications from 0 to 7 units of FFP; the incidence of ARDS alone increased 10-fold. This response appears to be a model for creating multiple organ failure, through the transfusion of FFP, particularly in patients not massively bleeding and thus not requiring treatment for coagulopathy. While appropriate for those acutely bleeding and at risk of coagulopathy, FFP as a resuscitation fluid may not be beneficial to those not acutely coagulopathic, similar to blood transfusions in those with adequate oxygen delivery.
The inherent dilemma and remaining major challenge with which we are faced is the holy grail of resuscitation. How do we identify which specific patient, and when and what specific therapy is needed? Not only is each injury and each injury pattern different, but also each individual responds uniquely. The Glue Grant analyzed the entire human genome in circulating leucocytes, every three or four days for 28 days, in severely blunt-injured patients to identify potential unique predictive genomic patterns.34 Early after injury, approximately 3,000 genes show greater-than two-fold changes in activity, and overall more than 70% of the entire human genomic expression pattern changes. With cancer, up to two-dozen genes may be altered, while major trauma affects 20,000 genes. An amazing “genomic storm” occurs following trauma, and then, over time, returns toward control levels of expression. However, even at 28 days, when most of these severely injured patients are being discharged, the pattern has still not returned to normal.
The overall patterns are interesting, and elucidate at the molecular level much of our current understanding of the pathophysiology of severe injury. Innate immune responses, which are the predominant pro-inflammatory agents, including multiple cytokines and chemokines, are thought to be the major cause of organ injury and multiple organ failure. The genes involved in innate immunity are predominantly and excessively up-regulated, producing the common pro-inflammatory phenotype seen in critically ill patients. But interestingly, the majority of the gene expression changes, involving two-thirds of the significantly altered genes, are suppression, not up-regulation of gene activity. And this suppression involves primarily adaptive immunity pathways. This profound suppression can well explain why patients develop multiple complications, including ventilator-associated pneumonia, wound infections and impaired healing. The normal adaptive immune response is widely and significantly suppressed. As an example; injured patients who received blood transfusions, when compared to those who did not, had major changes in the insulin-dependent pathways of cellular function, including expression of the insulin cell surface receptor, and the molecular basis for uptake of glucose. The transfused patients not only became glucose intolerant clinically, but, at the genomic level, after blood transfusion compared to non-transfusion patients, developed markedly suppressed insulin signaling and decreased ability of cellular uptake of glucose. Thus, the genomic response to transfusion elucidates the molecular basis of hyperglycemia and insulin resistance commonly seen in the critically ill.
In addition, we compared critically injured patients who had an uncomplicated course, with patients who had complications and prolonged ICU LOS. We found 63 genes with different and markedly altered gene expressions. Both groups of patients, with and without complications, have qualitatively similar changes in their genomic expression. Both undergo the “genomic storm” of severe injury, and the similarity of the initial genomic response explains why multiple attempts of assaying for specific predictive biomarkers is unsuccessful. There is significant overlap in genomic-based biomarkers between the two groups, which can’t be cleanly separated.
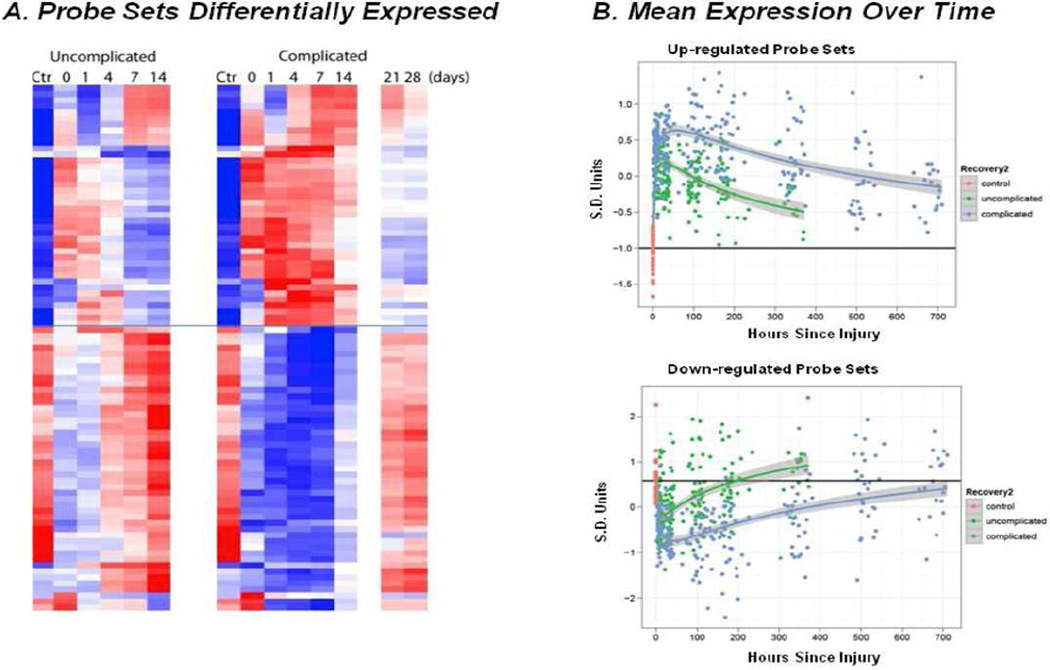
We found that the difference that predicts clinical course is not the peak in alterations, but rather patients without complications have a more rapid return to normal gene expression than those with complications. [Fig. 5] The complication-free patients are able to achieve homeostasis (again Cannon was correct in 1918), and return to a balanced functional state necessary for optimal function in a complex biologic system. The patients who can’t achieve homeostasis remain dysfunctional and develop multiple complications.34,35 This again argues and explains that, while much can be done to prevent complications, such as nosocomial infections, there will remain a sub-population defined by their genomic response with excessive suppression of immunity, and regardless of our treatments, will develop complications in the ICU setting.
Figure 5.
Differences in gene expression patterns between patients with a complicated and uncomplicated clinical recovery. (A) Probe sets differentially expressed. Heat map of gene probe sets whose expression was at least twofold different when compared with controls (CTRL) for patients with a complicated (Comp) or uncomplicated recovery (Uncomp). (B) Mean expression over time. Mean gene probe expression changes vs time course of return to normal function (homeostasis) of significantly altered genes post injury. Upper panel are upregulated primarily proinflammatory innate immunity genes and lower portion are suppressed primarily adaptive immunity genes. Patients with complicated clinical courses have a delay in return toward normal. Recovery2: red, control; green, uncomplicated; blue, complicated. (Adapted with permission from Xiao et al., 2011. Originally published in The Journal of Experimental Medicine.)
This remaining challenge is a major goal for personalized care in the critically ill; to utilize genomic responses, most likely serially over 2–3 days, to predict patient outcomes and identify patients that need aggressive, genomically defined, therapeutic targets.36 This remains our holy grail.37
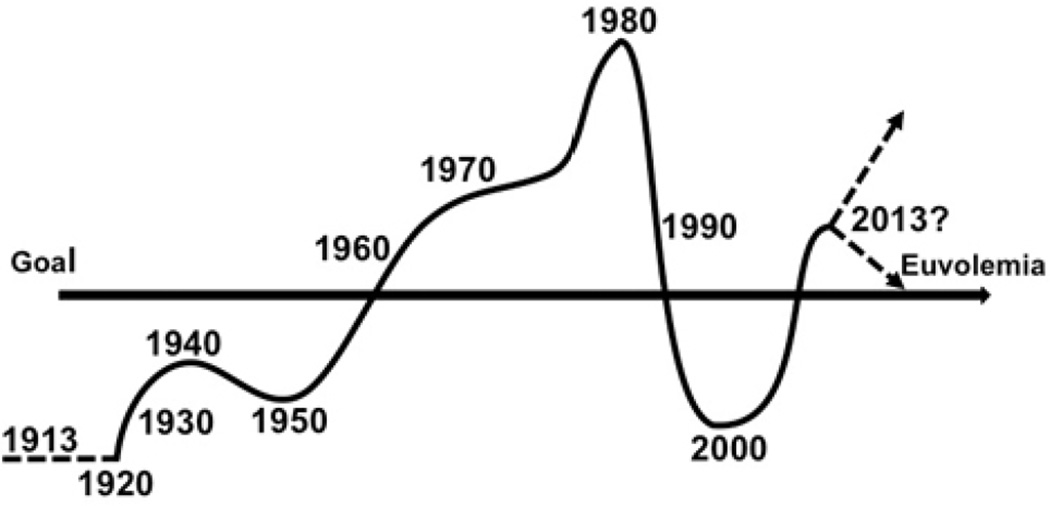
In conclusion, I believe, as a group of physicians, we have achieved chaos, as defined by our approach to volume resuscitation in our attempt to reach euvolemia. [Fig. 6] Remember, euvolemia is not, as is sometimes defined, normo-volemia, but rather the appropriate volemia for the disease and the phase being treated. Our goal is to identify which patient is not euvolemic, what is their ideal euvolemic state, and how, and with what, to achieve that goal. How will we respond to the challenge? Will we switch from “drowning with salt water” to over-treatment with blood components? Or can we better identify, quantify and specify our interventions? And optimally, as predicted by chaos theory, can we identify the patient and their needs early so that small volumes of appropriate therapy will have a major beneficial impact to achieve homeostasis and modify outcome? Can we finally fulfill the challenge to find the right patient for the right treatment at the right time?
Fig. 6.
Fluid resuscitation volume protocols for traumatic shock over time fulfill the chaos theory.
Thank you very much for your attention, and the honor of presenting the Scudder oration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the American College of Surgeons 99th Annual Clinical Congress Washington, DC, October 2013.
References
- 1.Rutkow I. American Surgery: An Illustrated History. 1st ed. Vol. 215 Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 2.Cannon WB, Fraser J, Cowell EM. The preventative treatment of wound shock. JAMA. 1918;70:618–621. [Google Scholar]
- 3.Matzinger P. Tolerance, danger and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 4.Blalock A. Principles of Surgical Care: shock and other problems. St. Louis: C.V. Mosby; 1940. [Google Scholar]
- 5.Artz CP, Fitts T. Replacement Therapy in Shock. Journal of Trauma. 1962;2:355–357. doi: 10.1097/00005373-196207000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Shires GT. Pathophysiology and fluid replacement in hypovolemic shock. Ann Clin Res. 1977;8:144–150. [PubMed] [Google Scholar]
- 7.Lewin I, Weil MH, Shubin H, et al. Pulmonary Failure Associated with Clinical Shock States. J Trauma. 1971;11(1):22–35. doi: 10.1097/00005373-197101000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Simmons RL, Collins JA, Heisterkamp CA, et al. Coagulation disorders in combat casualties. I. Acute changes after wounding. II. Effects of massive transfusion. III. Post-resuscitative changes. Ann Surg. 1969;169(4):455–482. doi: 10.1097/00000658-196904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaisdell FW, Lim RC, Jr, Amberg JR, et al. Pulmonary microembolism. A cause of morbidity and death after major vascular surgery. Arch Surg. 1966;93(5):776–786. doi: 10.1001/archsurg.1966.01330050080012. [DOI] [PubMed] [Google Scholar]
- 10.Shoemaker WC, Peitzman AB, Bellamy R, et al. Resuscitation from severe hemorrhage. Critical Care Medicine. 1996;24:S12–S23. [PubMed] [Google Scholar]
- 11.Shoemaker WC, Appel PL, Kram HB. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest. 1992;102:208–215. doi: 10.1378/chest.102.1.208. [DOI] [PubMed] [Google Scholar]
- 12.Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 13.Bickell WH, Wall MJ, Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 14.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Brown J, Cohen MJ, Peitzman A, Maier RV, et al. Goal directed resuscitation in the prehospital setting: a propensity adjusted analysis. J Trauma and Acute Care Surgery. 2013;74(5):1207–1212. doi: 10.1097/TA.0b013e31828c44fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eastridge BJ, Owsley J, Sebesta J, et al. Admission physiology criteria after injury on the battlefield predict medical resource utilization and patient mortality. J Trauma. 2006;61:820–823. doi: 10.1097/01.ta.0000239508.94330.7a. [DOI] [PubMed] [Google Scholar]
- 18.Gruen RL, Brohi K, Schreiber M, et al. Haemorrhage control in severely injured patients. Lancet. 2012;380:1099–1108. doi: 10.1016/S0140-6736(12)61224-0. [DOI] [PubMed] [Google Scholar]
- 19.MacLeod JB, Cohn SM, Johnson EW, et al. Trauma deaths in the first hour: are they all unsalvageable injuries? Am. J. Surg. 2007;193(2):195–199. doi: 10.1016/j.amjsurg.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2009;64:1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 21.Curry N, Hopewell S, Doree C, et al. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care. 2011;15:R92. doi: 10.1186/cc10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchesne JC, Hunt JP, Wahl G, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2009;65:272–276. doi: 10.1097/TA.0b013e31817e5166. [DOI] [PubMed] [Google Scholar]
- 23.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 24.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 25.Duchesne JC, Islam TM, Stuke L, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009;67:33–37. doi: 10.1097/TA.0b013e31819adb8e. [DOI] [PubMed] [Google Scholar]
- 26.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 27.Kautza BC, Cohen MJ, Cuschieri J, et al. Changes in massive transfusion over time: an early shift in the right direction? J Trauma Acute Care Surg. 2012;72:106–111. doi: 10.1097/TA.0b013e3182410a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JB, Cohen Mj, Minei JP, et al. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73:358–364. doi: 10.1097/TA.0b013e31825889ba. [DOI] [PubMed] [Google Scholar]
- 29.Brown JB, Cohen MJ, Minei JP, et al. The Early Bird Gets the Worm: Pre Trauma Center Blood Transfusion is Associated with Reduced Mortality and Coagulopathy in Severely Injured Blunt Trauma Patients. Paper presented at: 72nd Meeting of the American Association for the Surgery of Trauma and Clinical Congress of Acute Care Surgery; Neurological Trauma, Abdominal and Shock Resuscitation; September 2013; Session XA. San Francisco, CA: [Google Scholar]
- 30.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 31.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: anemia and blood transfusion in the critically ill – current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 32.Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 33.Inaba K, Branco BC, Rhee P, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010;210:957–965. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Sure. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuenca AG, Gentile LF, Lopez MC, et al. Development of a genomic metric that can be rapidly used to predict clinical outcome in severely injured trauma patients. Crit Care Med. 2013;41(5):1175–1185. doi: 10.1097/CCM.0b013e318277131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cryer HG, Leong K, McArthur DL, et al. Multiple organ failure: by the time you predict it, it’s already there. J Trauma. 1999;46:597–604. doi: 10.1097/00005373-199904000-00007. [DOI] [PubMed] [Google Scholar]