Abstract
The adipokine, leptin, regulates blood glucose and the insulin secretory function of beta cells, while also modulating immune cell function. We hypothesized that the dual effects of leptin may prevent or suppress the autoreactive destruction of beta cells in a virally induced rodent model of type 1 diabetes. Nearly 100% of weanling BBDR rats treated with the combination of an innate immune system activator, polyinosinic:polycytidilic acid (pIC), and Kilham rat virus (KRV), become diabetic within a predictable timeframe. We utilized this model to test the efficacy of leptin in preventing diabetes onset, remitting new onset disease, and preventing autoimmune recurrence in diabetic rats transplanted with syngeneic islet grafts. High doses of leptin delivered via an adenovirus vector (AdLeptin) or alzet pump prevented diabetes in > 90% of rats treated with pIC+KRV. The serum hyperleptinemia generated by this treatment was associated with decreased body weight, decreased non-fasting serum insulin levels, and lack of islet insulitis in leptin treated rats. In new onset diabetics, hyperleptinemia prevented rapid weight loss and diabetic ketoacidosis (DKA), and temporarily restored euglycemia. Leptin treatment also prolonged the survival of syngeneic islets transplanted into diabetic BBDR rats. In diverse therapeutic settings we found leptin treatment to have significant beneficial effects in modulating virally induced diabetes. These findings merit further evaluation of leptin as a potential adjunct therapeutic agent for treatment of human type 1 diabetes.
Keywords: Type 1 diabetes, Leptin, BB rat, Virus, Islet transplantation
INTRODUCTION
Type 1 diabetes mellitus is an autoimmune disorder characterized by insulitis, an inflammatory infiltration of pancreatic islets, and selective destruction of islet beta cells. Of various spontaneous and inducible rodent models for this disease [1, 2, 3, 4], one of the most highly penetrant is the virus inducible BBDR rat that harbors a genetically susceptible MHC haplotype, but has a phenotypically normal immune system [5]. Although these animals never become diabetic spontaneously, treatment with the combination of an innate immune system activator, polyinosinic:polycytidilic acid (pIC), and a virus, Kilham rat virus (KRV), precipitates diabetes in nearly 100% of rats within 11–21 days [6]. The diabetic rats develop insulitis, hyperglycemia and, in the absence of exogenous insulin therapy, rapidly progress to diabetic ketoacidosis (DKA) within days of disease onset [7].
Much of the research in this and other autoimmune models of type 1 diabetes has focused primarily on dysregulation of the immune system [4, 8], but metabolic dysregulation may also contribute to pathogenesis. Consistent with this idea, a recent study found that treatment with the adipokine, leptin, can normalize the uncontrolled diabetes of both autoimmune NOD mice and rats treated with beta cell cytotoxic chemicals, despite lack of detectable insulin in the plasma or pancreas [9]. Leptin, a 16 kDa protein hormone secreted primarily by adipocytes, plays a key role in regulating a wide range of biological responses including insulin secretion, energy homeostasis, neuroendocrine function, lymphopoiesis, angiogenesis, bone formation and reproduction [10, 11, 12]. In humans, leptin deficiency results in severe morbidity, hypogonadism, underdevelopment of the immune system and a generalized state of immune suppression [13]. Similarly, leptin deficient Lepob mice are immunodeficient, with reduced splenic and thymic weight, and lymphopenia [12, 13]. These mice also suffer from a syndrome of obesity, diabetes, infertility, hypothyroidism, hypercorticoidism, low sympathetic activity, and impaired thermoregulation [13]. In contrast, elevated serum leptin levels (hyperleptinemia) can inhibit glucose stimulated insulin secretion from beta cells, decrease beta cell triglyceride content, and decrease beta cell apoptosis in type 2 diabetes prone rodents [14, 15, 16, 17].
Although many of the initial reports on leptin focused on its metabolic and endocrine effects, more recent studies demonstrate that leptin can also modulate immune responses. In vitro studies revealed that T and B cells, macrophages and hematopoietic cells express the leptin receptor, and leptin can directly affect the function of those cells [18, 19]. Leptin has been reported to stimulate the proliferation of T cells in culture, promote Th1 responses, and tends to have a proinflammatory role in certain experimental models of autoimmune diseases. For example, in the NOD mouse model of type 1 diabetes, intraperitoneal administration of recombinant leptin to young female NOD mice accelerated the autoimmune destruction of pancreatic beta cells and significantly increased interferon-γ production in peripheral T lymphocytes [20]. In contrast, leptin has been found to exert protective anti-inflammatory effects during activation of innate immune responses or acute inflammation [18]. In an induced mouse model of arthritis, the TLR2 ligand, zymosan A, triggers a local activation of the innate immune system and local inflammation following intra-articular injection. In ob/ob and db/db mice that lack leptin or its receptor, respectively, zymosan A caused an increased acute phase response and delayed resolution of the inflammatory process compared to wild type littermates [21, 22]. These data are in agreement with another innate immune mediated model of arthritis, in which S. aureus induces a septic arthritis which is significantly more severe in ob/ob and db/db mice compared to wild type [23].
The capacity of leptin to differentially modulate innate and adaptive immunity, as well as regulate the endocrine system, warrants its further investigation in other models of autoimmunity, especially those which may have a strong innate immune component. Here we investigated whether serum hyperleptinemia may 1) prevent an autoimmune response against the beta cell in the pIC+KRV virally induced BBDR rat model of type 1 diabetes, 2) rescue diabetic rats from ketoacidosis, and 3) prevent autoimmune recurrence in syngeneic islets transplanted into diabetic BBDR rats. We found leptin to impart significant therapeutic benefits when applied at disparate stages of virus induced diabetes, including at onset of diabetes and in the context of islet transplantation, clinically important phases for targeted therapeutic intervention.
MATERIALS AND METHODS
Animals
Viral antibody free BBDR/Wor rats were obtained from Biomedical Research Models, Inc. (Worcester, MA). Animals were certified to be free of a panel of viruses, including KRV. All animals were housed in a viral antibody free facility in microisolator cages and provided with water and commercial chow ad libitum. BBDR rats 21–25 days of age of either sex were used and maintained in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996) and guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Viruses
KRV (UMass isolate) was propagated in NRK cells grown in Dulbecco’s minimal essential medium (DMEM) [6]. Recombinant adenovirus containing rat leptin cDNA (AdLeptin) or an irrelevant gene, β-galactosidase (AdBetagal), was prepared as previously described [24]. Adenoviral vectors were injected i.v. at a dose of 1x1010 infectious units (IU) in 0.5 ml PBS per 100g body weight.
Reagents
pIC was purchased from Sigma (St. Louis, MO), dissolved in Dulbecco’s PBS (1 mg/ml), sterile filtered, and stored at −20°C until used. The concentration of contaminating endotoxin was determined commercially (Charles River Endosafe, Charleston, SC) and was uniformly < 10 units/mg. Lyophilized rat recombinant leptin (rrLeptin) was purchased from R&D Systems (Minneapolis, MN) and stored at −20°C. Lyophilized protein was reconstituted with sterile 20 mM Tris HCl, pH 8 to a concentration of 2.5 mg/ml and stored at 4°C. Alzet pump models 2001 and 2ML2 were purchased from the Durect Corporation (Cupertino, CA),loaded according to manufacturer’s instructions with either a 2.5 mg/ml solution of rrLeptin or vehicle (20 mM Tris HCl, pH 8), and inserted subcutaneously. Based on the rrleptin concentration, we determined that the 2001 and 2ML2 model pumps would deliver 60µg and 300µg of rrLeptin per day, respectively. Two model 2001 pumps per rat were used for diabetes protection studies and one model 2ML2 pump per rat was used in diabetes remission studies.
Diabetes Induction Protocol
BBDR/Wor rats were injected intraperitoneally (i.p.) with pIC (1µg/g body weight) on three consecutive days starting at 21–25 days of age (day −3, −2, and −1). On the fourth day (day 0), rats received a single i.p. dose of 1×107 PFUs of KRV in PBS. Rats were tested for glycosuria (test strips, Clinistix, Bayer, Elkhart, IN) three to four times weekly starting at 7 days post virus infection. Diabetes was confirmed by documenting two consecutive plasma glucose concentrations > 250 mg/dL (Accu-Chek Aviva, Roche Diagnostics, Indianapolis, IN). In some experiments, diabetic rats were provided insulin pellets to prevent diabetic ketoacidosis (DKA) until the end of the observation period (40–50 days).
Islet Transplantation
Pancreatic islets were isolated by collagenase digestion, hand-picked, and counted as previously described [25, 26]. Rats undergoing islet transplantation were anesthetized and syngeneic islets (10 islets/g body weight) were transplanted under the left kidney capsule [25, 26].
Real Time PCR
Total RNA was isolated from INS-1 832/13 cells using RNeasy Mini Kit (Qiagen, Valencia, CA), then reverse-transcribed with Oligo-dT primer using 1µg of total RNA. For the thermal cycle reaction, the iQ5 system (BioRad, Hercules, CA) was used at 95°C for 10 min, 40 cycles at 95°C for 10 sec, and at 55°C for 30 sec. The relative amount of each transcript was calculated by a standard curve of cycle thresholds for serial dilutions of cDNA sample and normalized to the amount of GAPDH. PCR was done in triplicate using Power SYBR Green PCR Master Mix (Applied Biosystems) and the following sets of primers: rat GAPDH, AAGATGGTGAAGGTCGGTGTG and GAAGGCAGCCCTGGTAACC; rat actin, GCAAATGCTTCTAGGCGGAC and AAGAAAGGGTGTAAAACGCAGC; rat insulin 1, GTCCTCTGGGAGCCCAAG and ACAGAGCCTCCACCAGG; rat insulin 2, ATCCTCTGGGAGCCCCGC and AGAGAGCTTCCACCAAG.
Histopathology of Pancreata
Pancreata and islet engrafted kidney samples were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), insulin, and glucagon. Insulitis was scored as follows: 0, no infiltration; 1+, peri-islet infiltration only; 2+, infiltration of some islets; 3+, infiltration of most islets.
Radioimmunoassay
Leptin radioimmunoassay was performed as previously described [9]. Insulin radioimmunoassay was performed by Millipore (Billerica, MA).
Statistics
Diabetes or DKA-free survival among groups was compared using Kaplan and Meier analysis with the log rank statistic. Comparisons of means of two groups used the student’s T test and comparisons of three or more means used one-way ANOVA with Bonferroni test for a posteriori contrasts (GraphPad Prism 4.0, San Diego, CA). P values < 0.05 were considered statistically significant.
RESULTS
AdLeptin Protects BBDR Rats from Type 1 Diabetes
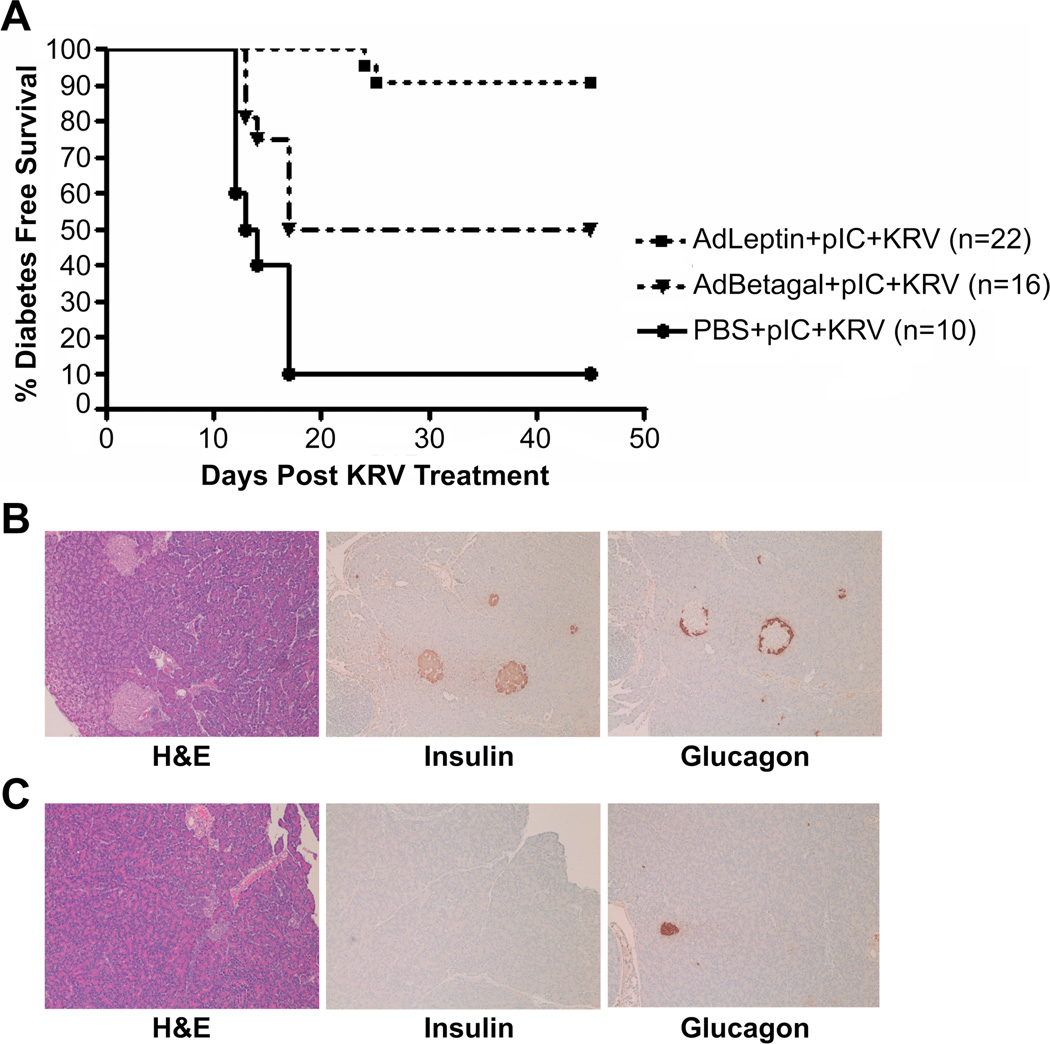
To determine whether hyperleptinemia could prevent virus induced autoimmune diabetes, we treated 21–25 days old weanling BBDR rats with a single i.v. dose of 1x1010 IU/100g body weight of either AdLeptin, AdBetagal, or the volume equivalent of PBS, followed by the pIC+KRV induction protocol. Additional control groups included rats treated with AdLeptin, AdBetagal or PBS without the pIC+KRV induction treatment; none developed diabetes during the study period (data not shown). At 45 days after KRV infection, there was a significantly greater diabetes-free survival in the AdLeptin pretreated rats (> 90%), compared with AdBetagal (50%), or PBS pretreated rats (10%) (Figure 1A). Histologic sections of pancreas recovered from rats 45 days after KRV infection demonstrated that islets of diabetes-free AdLeptin protected rats were intact with no or minimal insulitis, and positive insulin and glucagon staining by immunohistochemistry (Figure 1B). In contrast, islets from diabetic AdBetagal or PBS treated rats maintained on insulin to the end of the observation period appeared as involuted, end-stage islets exhibiting glucagon-containing but, as expected no insulin-containing cells (Figure 1C). As expected, there was little to no insulitis in the islets of diabetic AdBetagal or PBS pretreated rats.
Figure 1.
AdLeptin protects BBDR rats from pIC+KRV induced diabetes. BBDR rats were treated with AdLeptin, AdBetagal or PBS prior to pIC+KRV treatment as described in Methods. A. Diabetes free survival of treated BBDR rats was followed for 45 days and defined as the time until the first of two elevated blood glucose levels (> 250 mg/dl) on two consecutive days. Data are pooled from two independent experiments; AdLeptin vs. AdBetagal (p=0.0026), AdLeptin vs. PBS (p < 0.005), AdBetagal vs. PBS (p=0.01). Numbers (n) of rats in each group are shown in parentheses. B. Representative pancreas sections from an AdLeptin + pIC+KRV treated rat protected from diabetes on day 45. C. Representative pancreas sections from a diabetic rat treated with PBS + pIC+KRV and maintained on insulin pellets to the end of the observation period on day 45. All sections were stained with H&E and images obtained at 10x magnification.
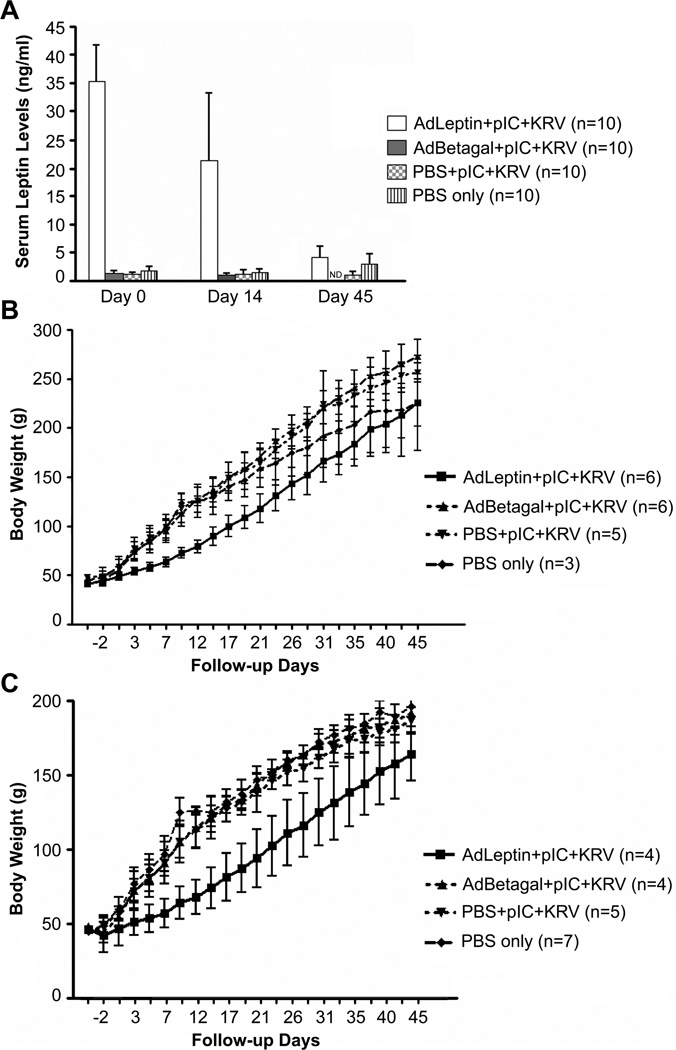
Ten randomly selected rats from each of the 3 groups treated with pIC+KRV (plus a group receiving only PBS) were also analyzed for serum leptin levels and body weight. The mean serum leptin level in the AdLeptin pretreated group was 35±6.7 ng/ml on day 0, which declined to 21±12 ng/ml by day 14, and to 4±2 ng/ml by day 45 (Figure 2A). In contrast, mean serum leptin levels in the AdBetagal and PBS treated groups remained at 1.0–1.2 ng/ml. Both male and female rats rendered hyperleptinemic with AdLeptin exhibited significantly lower body weights relative to other treatment groups (Figure 2 B,C). Although we did not measure the dietary intake of AdLeptin pretreated rats, the lower weight gain exhibited by these rats may be attributable to the reported anorexigenic effects of leptin [27]. We conclude from this prevention trial that hyperleptinemia induced by AdLeptin prevents autoimmune infiltration of pancreatic islets and protects BBDR rats from pIC+KRV induced autoimmune diabetes. However, the adenovirus vector conferred some degree of protection from autoimmune diabetes, as only 50% of the AdBetagal pretreated rats developed the disease compared with 90% in the PBS treated group.
Figure 2.
Serum leptin levels and body weight gain in BBDR rats. BBDR rats were treated with AdLeptin, AdBetagal or PBS prior to pIC+KRV treatment, or treated with PBS alone as described in Methods. A. BBDR rats were bled on days 0, 14, and 45 relative to infection with KRV on day 0, and serum leptin levels were determined by radioimmunoassay. B. Body weights of male BBDR rats. C. Body weights of female BBDR rats. Data in A-C show the mean ± SD; n = number of rats in each group. Data include all diabetics and non-diabetics in each group. All diabetics were maintained on insulin pellets to the end of the observation period.
rrLeptin Protects BBDR Rats from Type 1 Diabetes as Effectively as AdLeptin
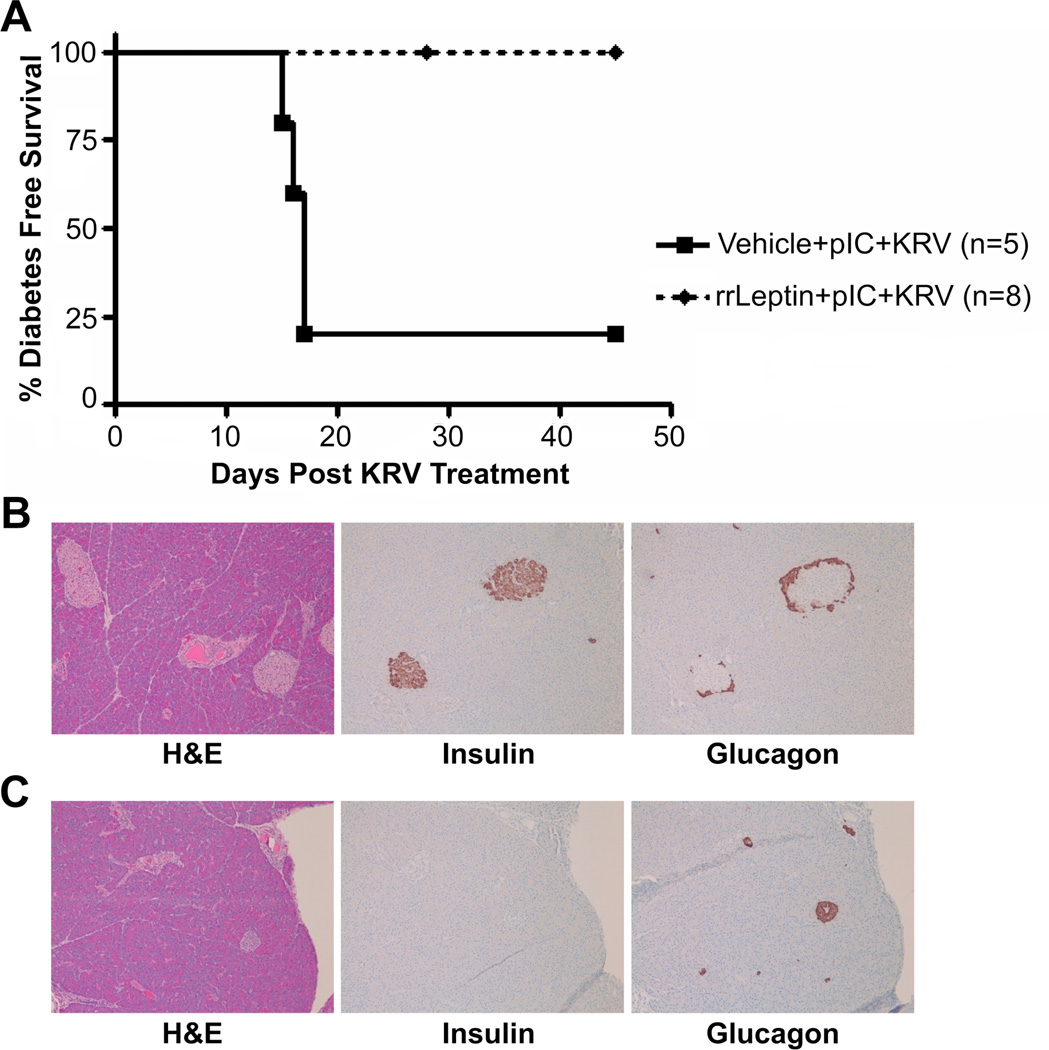
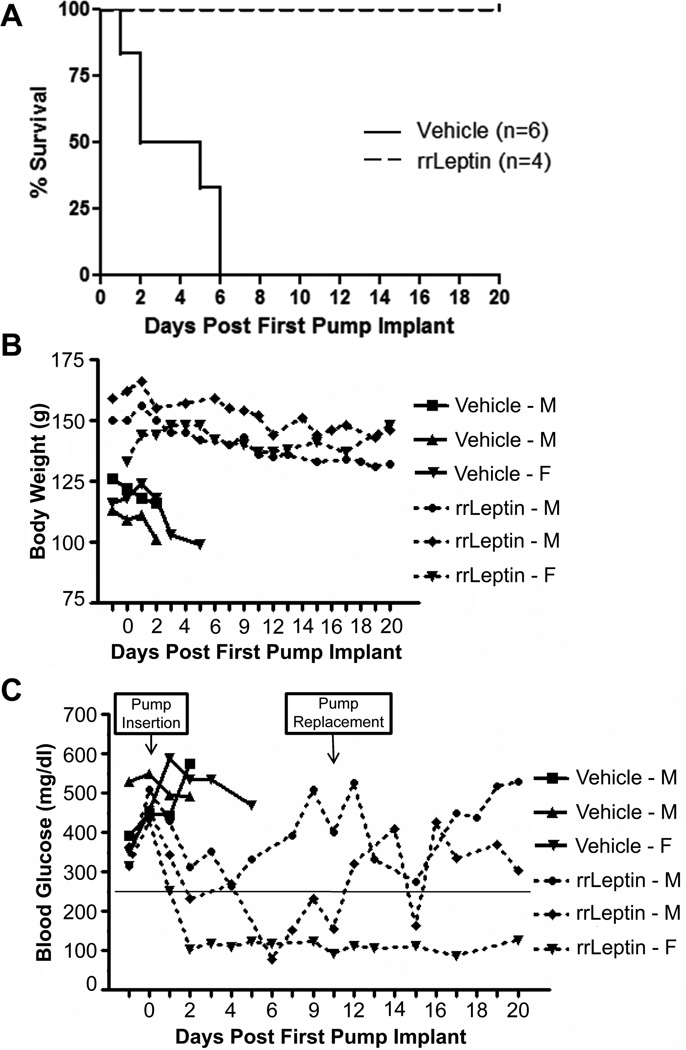
To address the confounding effects of the adenoviral vector, we repeated the protection study using rat recombinant leptin (rrLeptin). Based on pharmacokinetic studies, we determined that weanling rats treated with two alzet pumps, each delivering rrLeptin at a dose of 1.2 µg/g body weight/day for up to 30 days, would achieve equivalent serum leptin levels as the adenovirus vector delivery system. Twenty-one to 24 day old female BBDR rats were treated with rrLeptin or vehicle prior to pIC+KRV treatment. Serum leptin levels in rrLeptin treated rats peaked at 51.5 ng/ml on day 4 following the first pump insertions, and remained elevated for the duration of the treatment period, confirming the efficiency of the alzet pump delivery system (data not shown). In this trial, 100% of rats treated with rrLeptin were protected from pIC+KRV induced diabetes compared with 20% in the vehicle control group (Figure 3A, p=0.002). Similar to AdLeptin treatment, rrLeptin treated female rats gained less weight relative to vehicle treated controls (data not shown). Islets of rrLeptin treated rats were free of insulitis and positive for insulin and glucagon by immunohistochemistry (Figure 3B), while islets from diabetic vehicle treated rats maintained on insulin to the end of the experiment appeared end-stage, with central glucagon staining, no insulin staining, and no remnant insulitis (Figure 3C).
Figure 3.
rrLeptin protects BBDR rats from pIC+KRV induced diabetes. Female BBDR rats were treated with rrLeptin or vehicle prior to pIC+KRV treatment as described in Methods. Alzet pumps were replaced every five days for up to 29 days. A. Kaplan Meier survival curve depicts the percent of rats exhibiting diabetes free survival; n = number of rats in each group. Data are representative of two independent experiments; rrLeptin + pIC+KRV vs. vehicle + pIC+KRV (p=0.002). B. Representative pancreas sections from day 45 of a rrLeptin + pIC+KRV treated rat protected from diabetes. C. Representative pancreas sections from day 45 of a diabetic rat treated with vehicle + pIC+KRV and maintained on insulin pellets to the end of the observation period. Sections in B and C show H&E, insulin, and glucagon staining as indicated; all images are at 10x magnification.
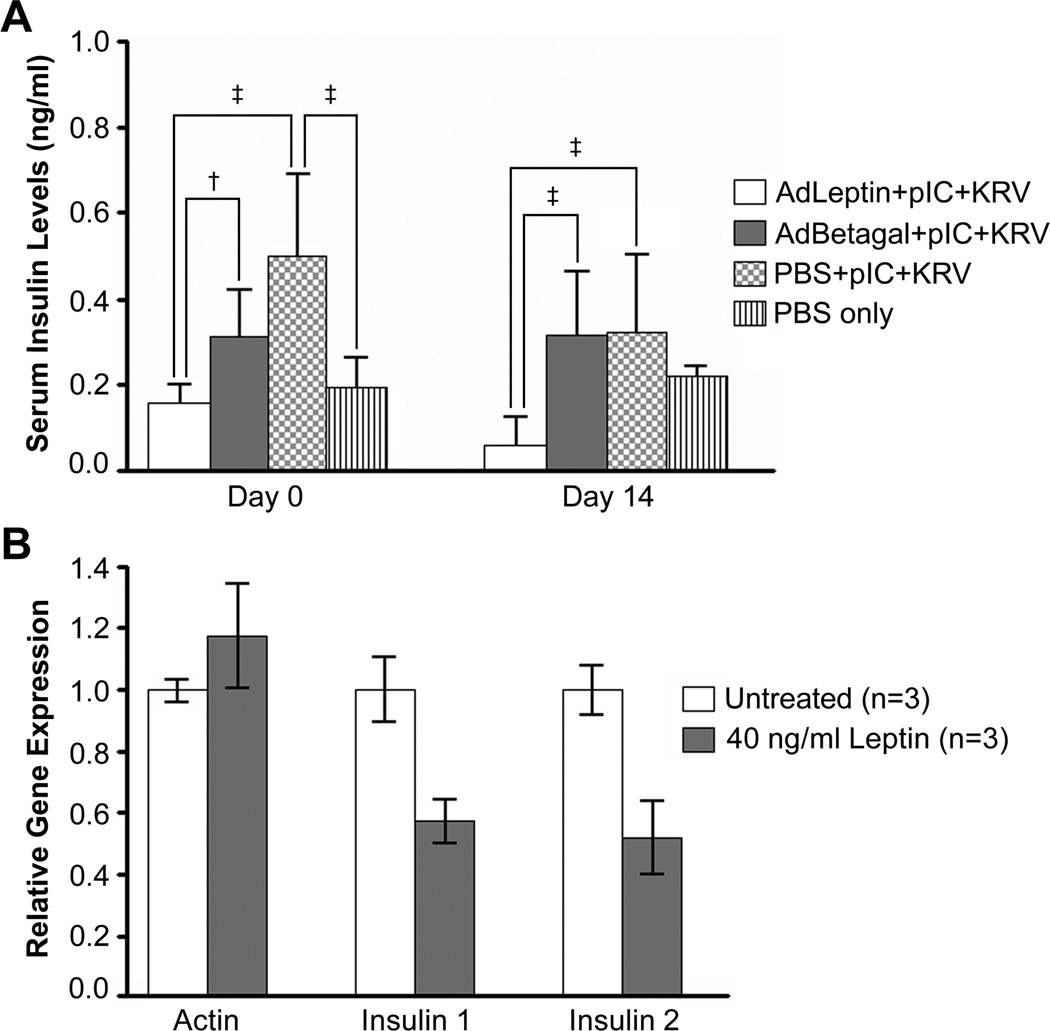
Leptin Treatment Lowers Non-fasting Serum Insulin Levels and Decreases Insulin Gene Expression
To investigate the effects of hyperleptinemia on serum insulin levels, we assayed sera from rats in our diabetes prevention trial. Among pIC+KRV treated rats, we found that nonfasting serum insulin levels were significantly lower in the AdLeptin treated rats on days 0 and 14 when compared with AdBetagal or PBS pretreated rats (Figure 4A). These data suggest that the rise in serum insulin levels elicited by pIC+KRV treatment could be suppressed by the hyperleptinemia induced by AdLeptin, a finding consistent with reports that leptin inhibits insulin secretion [14, 28, 29]. We also determined that leptin treatment of rat insulinoma cells in culture caused a significant decrease in both insulin 1 and insulin 2 gene expression as measured by real time PCR (Figure 4B). These data suggest that leptin may protect beta cells from autoimmune attack by inducing a state of “quiescence” which in turn may reduce their production and/or presentation of autoantigens.
Figure 4.
Serum insulin levels and gene expression are decreased with leptin treatment. A. Sera from BBDR rats in the AdLeptin + pIC+KRV, AdBetagal + pIC+KRV, PBS + pIC+KRV or PBS only groups were analyzed for insulin levels by radioimmunoassay on day 0 (day of KRV, rats bled prior to KRV infection) or day 14 following KRV infection. All samples were tested in duplicate, and mean serum values calculated for each sample; †p < 0.05, ‡p < 0.001. Data from day 14 represent only nondiabetic rats in each group (on day 0, n=10 for each group; on day 14, n=10 for AdLeptin + pIC+KRV, n=7 for AdBetagal + pIC+KRV, and n=6 for PBS + pIC+KRV). B. Leptin treatment of cultured cells in vitro causes a decrease in insulin gene expression. Rat insulinoma cells (INS-1832/13) were cultured with or without 40 ng/mL rat recombinant leptin for 7 consecutive days. Actin, insulin 1, and insulin 2 mRNA expression levels were quantified by real-time PCR. Error bars are ± 1 SD of triplicate determinations.
rrLeptin Prevents Rapid Weight Loss and DKA in New Onset BBDR Diabetic Rats
At the time of diabetes onset in BBDR rats most islets are compromised with significant insulitis, but some beta cell mass (~10%) is still preserved (unpublished results) and may retain function if protected from continued autoimmune attack. To investigate whether rrLeptin treatment at the time of diabetes onset could remit disease, we induced diabetes in BBDR rats by injection of pIC+KRV. On the second day of high blood glucose (> 250 mg/dl), diabetic rats were implanted with a single 2ML2 alzet pump containing rrLeptin (1.5µg/g/day) or vehicle control; neither group received exogenous insulin. Rats were monitored daily and euthanized when one or more of these criteria were met: (1) loss of ≥20% of body weight, (2) urine ketone positivity for two consecutive days, or (3) distressed appearance. Surviving rats were followed for 20 days post pump implant, with a single pump replacement on day 10. All vehicle treated rats had significantly shorter survival (p=0.0046) and lost weight more rapidly than rats treated with rrLeptin (Figure 5A,B). The vehicle treated rats became urine ketone positive within 1–6 days following the first vehicle pump insertion, whereas none of the rrLeptin treated rats became urine ketone positive during the 20 day period following diabetes onset.
Figure 5.
rrLeptin treated diabetics survive in the absence of exogenous insulin. BBDR rats induced to become diabetic following pIC+KRV treatment were given rrLeptin or vehicle as described in Methods. A. Kaplan Meier curve of the survival of recent onset diabetic BBDR rats treated with rrLeptin or vehicle control (p=0.0046). Rats were monitored for up to 20 days, with pump replacement 10 days following the first pump insertion. Any rat that met one of three criteria was euthanized: (1) 20% weight loss, (2) urine ketone positivity, or (3) distressed status. Median DKA free survival time was 3.7 days in the vehicle control and > 20 days in the rrLeptin group. B. rrLeptin ameliorates the rapid weight loss in new onset diabetics. Body weight changes in rats treated with rrLeptin or vehicle control. C. rrLeptin temporarily restores normoglycemia in new onset diabetics. Non-fasting blood glucose levels from the same rats shown in B. In B and C, three representative animals are shown from each group; M = male and F = female rat.
In the absence of exogenous insulin, rrLeptin treated rats experienced an immediate fall in their blood glucose levels (Figure 5C) and a temporary restoration of euglycemia (blood glucose < 250 mg/dl) in all of the rrLeptin treated rats. In contrast to the diabetes prevention studies where rrLeptin treated rats failed to gain as much weight as vehicle treated controls, new onset diabetics treated with rrLeptin failed to lose weight as rapidly as did vehicle treated controls. This apparent dichotomy of action may be explained by the anorexigenic property of leptin to prevent weight gain in normal rats, whereas in new onset diabetics, leptin may help lower hyperglycemia and thereby prevent the rapid weight loss commensurate with hyperglycemic osmotic diuresis.
AdLeptin Protects Transplanted Islet Grafts from Diabetes Recurrence
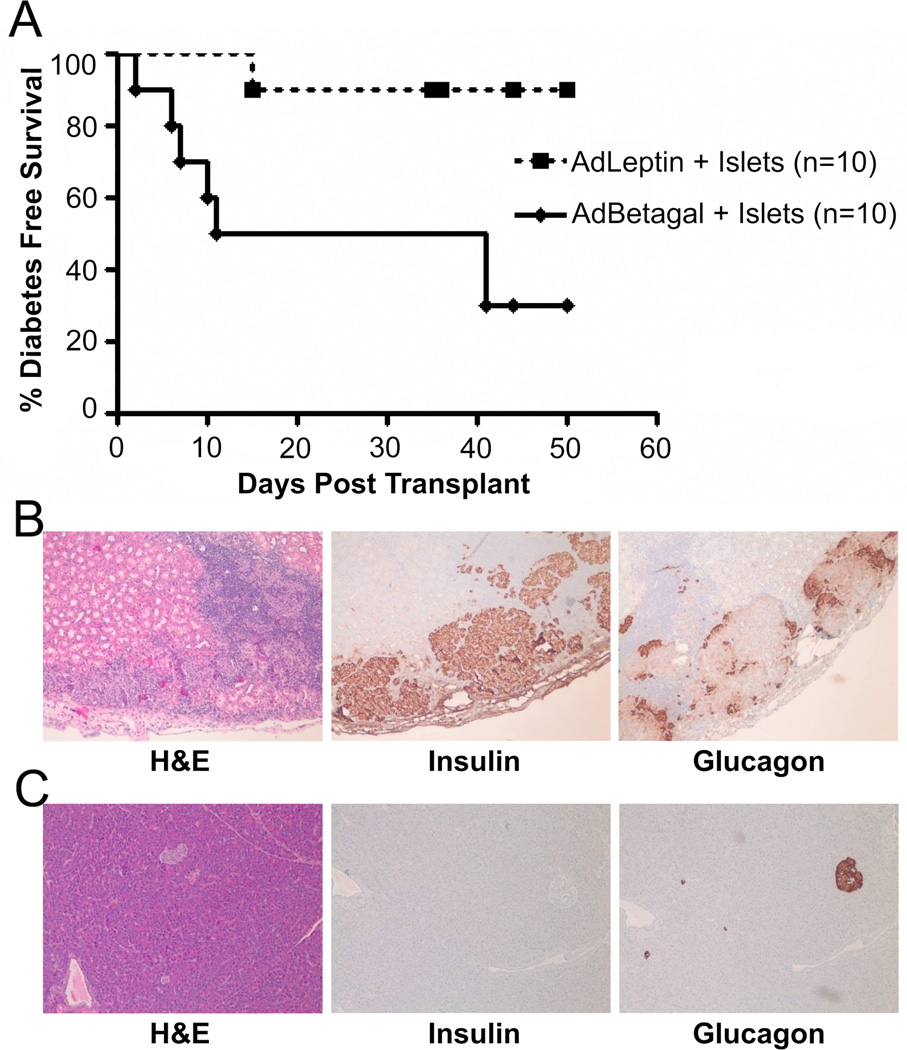
We next tested the efficacy of leptin in preventing autoimmune diabetes recurrence in a syngeneic islet transplant model. To avoid multiple surgical manipulations necessitated by the alzet pump delivery system, we used the AdLeptin delivery system in the islet transplantation studies. BBDR rats were rendered diabetic with pIC+KRV, and implanted with an insulin pellet on the second day of high blood glucose (> 250 mg/dl). After an additional 3–4 days, rats were treated with 1x1010 PFU AdLeptin or AdBetagal/100g body weight and maintained for 4–6 days to allow for adenovirus mediated gene expression. Insulin pellets were then removed and 10 BBDR islets/g bodyweight were transplanted into each rat. Animals in both groups were followed for up to 50 days post transplant and taken down at diabetes recurrence (two consecutive days of blood glucose >250 mg/dl). At day 50, any non-diabetic rats remaining had their graft-bearing kidney removed and were monitored for reversion to hyperglycemia.
Islet transplanted BBDR rats (90%) treated with AdLeptin remained diabetes free to the end of the observation period (50 days) as compared to 30% diabetes-free survival in rats treated with AdBetagal (p=0.01) (Figure 6A). Histological analysis of the grafts of non-diabetic, AdLeptin treated rats revealed intact islets with positive insulin and glucagon staining (Figure 6B). H&E staining did reveal the presence of lymphocytes within and around the islet graft, but the abundance of insulin-positive cells observed in these islet grafts suggested that the lymphocytes were unable to efficiently kill beta cells. In contrast, the endogenous pancreata of non-diabetic Adleptin treated rats revealed glucagon staining with no detectable insulin staining (Figure 6C).
Figure 6.
AdLeptin protects syngeneic islet grafts in diabetic recipients. BBDR rats induced to become diabetic following pIC+KRV treatment were given AdLeptin or AdBetagal and transplanted with syngeneic islets as described in Methods. A. Diabetes free survival following transplantation of syngeneic islets. Data presented are pooled from two independent experiments; AdLeptin vs. AdBetagal (p=0.01). Median diabetes free survival time was 26 days in the islet engrafted AdBetagal group and > 50 days in the AdLeptin group. B. AdLeptin protected islet grafts are surrounded by lymphocytic infiltrates but maintained positive insulin and glucagon staining. Representative islet graft from an AdLeptin treated, non-diabetic rat was identified under the kidney capsule. C. AdLeptin treated rats that remained normoglycemic following islet transplantation had their graft-bearing kidney removed to confirm reversion to hyperglycemia. Pancreata were recovered and processed for immunohistochemistry. Serial sections in B and C were stained with H&E, insulin, and glucagon; all images are at 10x magnification.
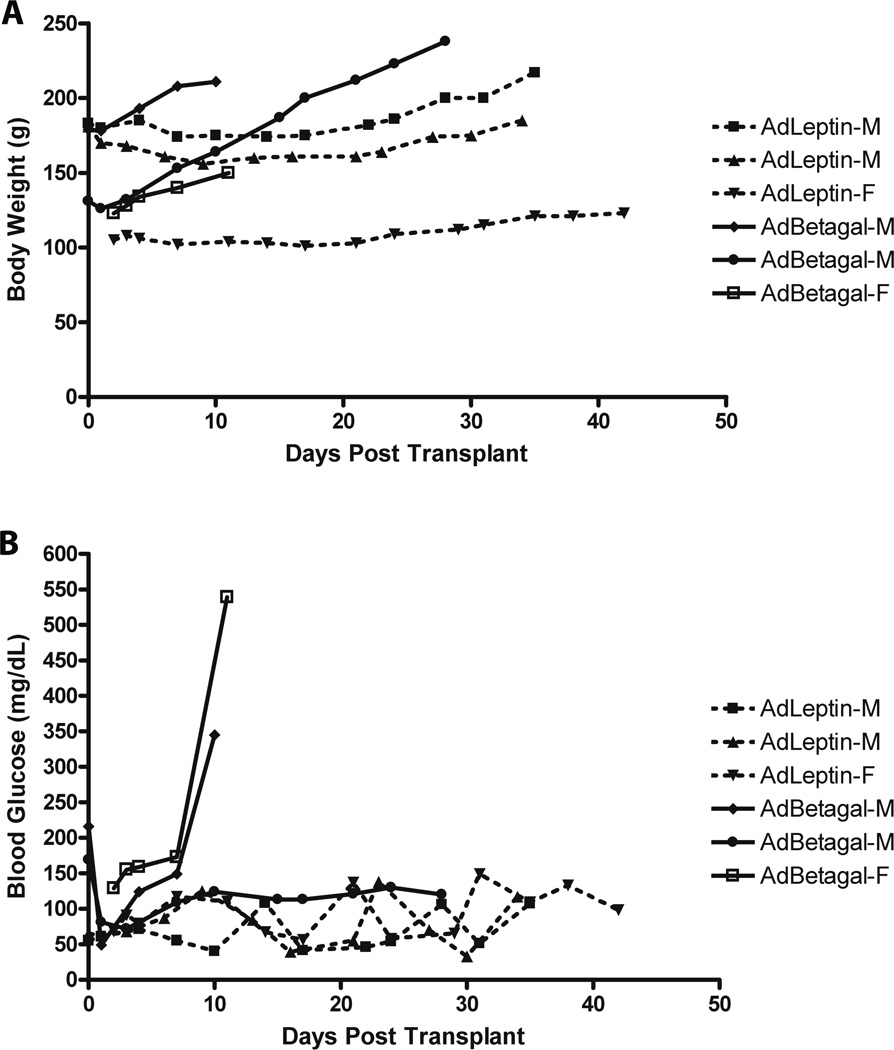
In the AdLeptin group, serum leptin levels rose from 0.8±0.4 ng/ml (n=6) prior to adenovirus treatment to 37±12.4 ng/ml (n=6) immediately prior to islet transplantation. Among representative AdBetagal treated rats, serum leptin remained at 1.4±0.5 ng/ml (n=6). AdLeptin treated rats gained less weight following islet transplantation compared to AdBetagal treated rats (Figure 7A). Analysis of non-fasting blood glucose levels demonstrated that all non-diabetic AdLeptin treated rats maintained normoglycemia throughout the observation period (Figure 7B). Following removal of the graft-bearing kidney, reversion to hyperglycemia in these animals indicated that the islet graft, rather than remnant or regenerative endogenous islets, was responsible for maintaining euglycemia.
Figure 7.
Body weight and non-fasting blood glucose data from pIC+KRV induced diabetic BBDR rats treated with AdLeptin or AdBetagal and given islet grafts under the kidney capsule. A. Individual body weights of rats treated with AdLeptin or AdBetagal. B. Non-fasting blood glucose levels from the same rats shown in A. In A and B, three representative animals are shown from each group; M = male and F = female rat.
DISCUSSION
The in vivo studies described here have evaluated leptin as a therapeutic agent for use at multiple stages of type 1 diabetes in the virus induced BBDR rat model. We find that leptin has a protective effect during various windows of disease vulnerability and therapeutic accessibility including prediabetes, at diabetes onset, and following islet transplantation of diabetic rats. Although leptin treatment has been tested in the prevention of type 1 diabetes [20] and in new onset diabetics in the NOD mouse [9], to our knowledge our study is the first to test the effects of leptin in an autoimmune diabetes recurrence model with islet transplantation.
Prevention of virus induced type 1 diabetes with leptin is not consistent with much of the literature which details pro-inflammatory and pro-autoimmune activities of leptin [20, 30, 31, 32, 33]. Matarese and colleagues [20] reported that treatment of newborn female NOD mice with recombinant leptin accelerates diabetes onset, promotes insulitis in the pancreatic islets, and predisposes diabetics to fatal DKA. Interestingly, this proautoimmune effect was not observed in male NODs that underwent the same treatment protocol, implying a gender bias of the effects of leptin in NOD mice. In contrast, in our study, treatment with either AdLeptin, or continuous treatment with rrLeptin using alzet pumps followed by pIC+KRV treatment protected between 90–100% of both male and female BBDR rats from diabetes. Moreover, the islets from these animals were insulitis-free. The apparently contradictory findings between the leptin studies in the NOD mouse and the BBDR rat may reflect the dose or timing of leptin treatment, or may result from species-specific effects of leptin. Alternatively, it is likely that leptin’s protective effects in this study may reflect different etiopathogenic mechanisms in the induction of diabetes between the NOD and BBDR models.
The protective effect of leptin in our virus (and pIC) induced BBDR model is consistent with the beneficial role of leptin observed in innate immune mediated arthritis models [18, 22]. We also found that leptin treatment in BBDR rats produced the reported metabolic effects attributable to leptin, such as lack of weight gain and hypoinsulinemia, observed in other studies [9, 24]. In the study by Yu et al [9], it is noteworthy that leptin (without exogenous insulin) reverses hyperglycemia of STZ-diabetic rats, whereas a restricted diet alone (pair fed with leptin treated rats) did not. Together, these data suggest that lack of weight gain, by itself, is not sufficient to reverse hyperglycemia.
AdLeptin treatment has also been shown to improve the survival of transplanted islets in chemically induced diabetic rats [34]. Our study has extended these findings by demonstrating that AdLeptin treatment protects syngeneic islet grafts in autoimmune diabetic rats. We found that treatment with AdLeptin and its associated serum hyperleptinemia was highly effective in protecting syngeneic islet grafts from autoimmune recurrence. Although islet grafts from all non-diabetic AdLeptin treated rats stained positively for both insulin and glucagon, we did observe lymphocytic infiltrates within or surrounding the grafts. In contrast, islet grafts from diabetic rats in the AdBetagal group maintained on insulin pellets to the end of the experimental period contained glucagon- but not insulin-positive cells, and had little to no remnant immune infiltrate surrounding the graft.
The effect of leptin on insulitis in the diabetes recurrence model is in contrast to that observed in our prevention studies where there was minimal or no insulitis. The lack of insulitis in the islets of leptin pretreated rats may have resulted from suppression of the expansion or homing of autoimmune effector cells to the pancreas. In support of this, preliminary findings from our lab indicate that 6 days after KRV infection leptin treated rats exhibited decreased splenic cellularity and spleen to body weight ratio compared with vehicle controls, implying that the KRV induced lymphocytosis normally observed in the spleen may be abrograted by an induced hyperleptinemic state. Further studies underway are evaluating whether differences in the splenic cell subsets, particularly effector CD4+CD8+APC, and Treg populations, may contribute to the protective effects of leptin in our model. Alternatively, the immunomodulatory effects may be secondary to leptin’s actions on the beta cells. Numerous reports show that leptin inhibits insulin secretion [14, 28, 29], and we observed that AdLeptin treated rats had decreased serum insulin levels compared to AdBetagal or PBS control rats. Although the mechanisms remain to be elucidated, taken together these data indicate that endogenous or engrafted beta cells rendered quiescent by leptin treatment may have multiple mechanisms to “escape” autoimmune attack.
In new onset BBDR diabetics with an ongoing autoimmune response, we also observed a significant survival benefit with rrLeptin treatment. Even in the absence of exogenous insulin therapy, rrLeptin treatment of diabetic rats prevented DKA and partially restored euglycemia. These findings are similar to those of Yu and colleagues who demonstrated that AdLeptin induced hyperleptinemia in new onset diabetic NOD mice led to a rapid decline in blood glucose levels within 9 days post adenovirus infection in the absence of either endogenous or exogenous insulin [9]. Similar reversion to normoglycemia was not observed in diabetic mice treated with the control vector, AdBetagal; these animals experienced weight loss, hyperglycemia and ketosis. Similarly, in our study, leptin treated rats did not become urine ketone positive, nor did they experience the rapid weight loss commensurate with osmotic diuresis resulting from severe hyperglycemia.
The insulin deficient state in type 1 diabetes results in uncontrolled hyperglucagonemia which, in turn, contributes to the extreme catabolic state that leads to hepatic overproduction of glucose and ketones [35, 36, 37]. Consequently, Yu and colleagues suggested that the reversal of diabetes in NOD hyperleptinemic mice may be attributed to leptin mediated suppression of hyperglucagonemia, since AdLeptin treated mice had significantly lower serum glucagon levels compared with the AdBetagal treated mice [9]. Thus, leptin mediated suppression of glucagon may help prevent excessive ketosis in both the NOD and BBDR models. Intriguingly, Yu and colleagues also discovered that leptin treatment led to upregulation of IGF-1 expression and serum levels that was associated with functional signaling through the IGF-1 receptor in skeletal muscle, as demonstrated by IGF-1 receptor phosphorylation [9]. Speculatively, the effects of leptin to enhance the insulinomimetic action of IGF-1 on skeletal muscle and other insulin responsive tissues, combined with suppression of glucagon action on the liver, may work together to reverse the catabolic consequences of the lack of insulin.
Given the historical association of virus exposure with type 1 diabetes [38, 39, 40], as well as reports of enterovirus infection in patients with recent onset diabetes [41, 42, 43, 44], the virus induced BB rat is a biologically relevant model for investigation of human type 1 diabetes and potential therapies. Although further investigation is warranted, studies with two different species (NOD mice and now BBDR rats) and two different type 1 diabetes models (spontaneous and virus induced) provide evidence that leptin treatment can restore euglycemia in the absence of exogenous insulin therapy in new onset diabetics. In addition, our study is the first to show a role for leptin in blocking or ameliorating autoimmune diabetes recurrence following islet transplantation. These effects of leptin can perhaps be manipulated in a clinical setting to (1) improve glycemic control and reduce the insulin requirement in new onset diabetics, and/or (2) slow or halt the memory autoimmune response in those receiving islet transplants. Leptin or its analogs may prove useful as an adjunct, metabolic or immune modulatory therapy for diabetic patients at multiple stages of their disease.
ACKNOWLEDGEMENTS
We thank Linda Leehy, Elaine Norowski, Cindy Bell and Michael Bates for technical assistance. Research supported in part by a grant from the ADA, an institutional Diabetes Endocrinology Research Center (DERC) grant DK32520 from the National Institutes of Health, the Beta Cell Biology Consortium, grants from the Juvenile Diabetes Research Foundation, International, the Brehm Foundation, and the Helmsley Foundation. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
The authors have no conflict of interest to report.
REFERENCES
- 1.Giarratana N, Penna G, Adorini L. Animal models of spontaneous autoimmune disease: type 1 diabetes in the nonobese diabetic mouse. Methods Mol Biol. 2007;380:285–311. doi: 10.1007/978-1-59745-395-0_17. [DOI] [PubMed] [Google Scholar]
- 2.Zipris D. Epidemiology of type 1 diabetes and what animal models teach us about the role of viruses in disease mechanisms. Clin Immunol. 2009;131:11–23. doi: 10.1016/j.clim.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Mordes JP, Serreze DV, Greiner DL, Rossini AA. Animal models of autoimmune diabetes mellitus. In: LeRoith D, Taylor SI, Olefski JM, editors. Diabetes Mellitus: A Fundamental and Clinical Text. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 591–604. [Google Scholar]
- 4.Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 2004;45:278–291. doi: 10.1093/ilar.45.3.278. [DOI] [PubMed] [Google Scholar]
- 5.Ramanathan S, Bihoreau M-T, Paterson AD, Marandi L, Gauguier D, Poussier P. Thymectomy and radiation-induced type 1 diabetes in nonlymphopenic BB rats. Diabetes. 2002;51:2975–2981. doi: 10.2337/diabetes.51.10.2975. [DOI] [PubMed] [Google Scholar]
- 6.Zipris D, Lien E, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J Immunol. 2005;174:131–142. doi: 10.4049/jimmunol.174.1.131. [DOI] [PubMed] [Google Scholar]
- 7.Mordes JP, Bortell R, Doukas J, Rigby M, Whalen B, Zipris D, et al. The BB/Wor rat and the balance hypothesis of autoimmunity. Diabetes Metab Rev. 1996;12:103–109. doi: 10.1002/(SICI)1099-0895(199607)12:2<103::AID-DMR161>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulation protein, and adiponectin. Curr Opin Lipidol. 2008;13:51–59. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kapica M, Puzio I, Kato I, Kuwahara A, Zabielski R. Role of feed-regulating peptides on pancreatic exocrine secretion. J Physiol Pharmacol 59 Suppl. 2008;2:145–159. [PubMed] [Google Scholar]
- 12.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 13.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 14.Okuya S, Tanabe K, Tanizawa Y, Oka Y. Leptin increases the viability of isolated rat pancreatic islets by suppressing apoptosis. Endocrinology. 2001;142:4827–4830. doi: 10.1210/endo.142.11.8494. [DOI] [PubMed] [Google Scholar]
- 15.Roduit R, Thorens B. Inhibition of glucose-induced insulin secretion by long-term preexposure of pancreatic islets to leptin. FEBS Lett. 1997;415:179–182. doi: 10.1016/s0014-5793(97)01115-0. [DOI] [PubMed] [Google Scholar]
- 16.Shimabukuro M, Wang MY, Zhou YT, Newgard CB, Unger RH. Protection against lipoapoptosis of beta cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci USA. 1998;95:9558–9561. doi: 10.1073/pnas.95.16.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger RH, Zhou YT, Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc Natl Acad Sci USA. 1999;96:2327–2332. doi: 10.1073/pnas.96.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernotiene E, Palmer G, Gabay C. The role of leptin in innate and adaptive immune responses. Arthritis Research & Therapy. 2006;8:217–226. doi: 10.1186/ar2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 20.Matarese G, Sanna V, Lechler RI, Sarvetnick N, Fontana S, Zappacosta S, La Cava A. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- 21.Keystone EC, Schorlemmer HU, Pope C, Allison AC. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977;20:1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- 22.Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptindeficient mice. Arthritis Res Ther. 2004;6:R256–R263. doi: 10.1186/ar1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hultgren OH, Tarkowski A. Leptin in septic arthritis: decreased levels during infection and amelioration of disease activity upon its administration. Arthritis Res. 2001;3:389–394. doi: 10.1186/ar332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Koyama K, Yuan X, Lee Y, Zhou YT, O’Doherty R, et al. Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proc Natl Acad Sci USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb PA, Berrios JP, Mariani G, Handler ES, Greiner D, Mordes JP, et al. Autoimmune destruction of islets transplanted into RT6-depleted diabetes-resistant BB/Wor rats. Diabetes. 1990;39:643–645. doi: 10.2337/diab.39.5.643. [DOI] [PubMed] [Google Scholar]
- 26.Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu H, Inoue K, Mori M. The leptin-dependent and -independent melanocortin signaling system: regulation of feeding and energy expenditure. J Endocrinol. 2007;193:1–9. doi: 10.1677/JOE-06-0144. [DOI] [PubMed] [Google Scholar]
- 28.Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci USA. 1999;96:674–679. doi: 10.1073/pnas.96.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 32.Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Bloom SR, et al. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol. 2001;31:1324–1332. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 33.Tuzun A, Uygun A, Yesilova Z, Ozel AM, Erdil A, Yaman H, et al. Leptin levels in the acute stage of ulcerative colitis. J Gastroenterol Hepatol. 2004;19:429–432. doi: 10.1111/j.1440-1746.2003.03300.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes. 2007;56:2295–2301. doi: 10.2337/db07-0460. [DOI] [PubMed] [Google Scholar]
- 35.Muller WA, Faloona GR, Unger RH. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971;50:1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai H, Dobbs RE, Unger RH. The role of glucagon in the pathogenesis of the endogenous hyperglycemia of diabetes mellitus. Metabolism. 1975;24:1287–1297. doi: 10.1016/0026-0495(75)90067-0. [DOI] [PubMed] [Google Scholar]
- 37.Dobbs R, Sakurai H, Sasaki H, Faloona G, Valverde I, Baetens D, et al. Glucagon: role in the hyperglycemia of diabetes mellitus. Science. 1975;187:544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 38.Filippi C, von Herrath M. How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol. 2005;233:125–132. doi: 10.1016/j.cellimm.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 39.van der Werf N, Kroese FG, Rozing J, Hillebrands JL. Viral infections as potential triggers of type 1 diabetes. Diabetes Metab Res Rev. 2007;23:169–183. doi: 10.1002/dmrr.695. [DOI] [PubMed] [Google Scholar]
- 40.von Herrath M. Can we learn from viruses how to prevent type 1 diabetes?: the role of viral infections in the pathogenesis of type 1 diabetes and the development of novel combination therapies. Diabetes. 2009;58:2–11. doi: 10.2337/db08-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreoletti L, Hober D, Hober-Vandenberghe C, Belaich S, Vantyglem MC, Lefebvre J, et al. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J Med Virol. 1997;52:121–127. doi: 10.1002/(sici)1096-9071(199706)52:2<121::aid-jmv1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Clements GB, Galbraith DN, Taylor KW. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–223. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 43.Hyoty H, Hiltunen M, Knip M, Laakkonen M, Vahasalo P, Karjalainen J, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44:652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 44.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]