Abstract
Previous studies have found that valproic acid (VPA), a histone deacetylases (HDAC) inhibitor, improves outcomes in a rat model of spinal cord injury (SCI). The study here aimed to further illuminate the neuroprotective effects of VPA against SCI, both in vivo and in vitro. First, spinal cord injury was performed in rats using NYU impactor. Delayed VPA injection (8 h following SCI) significantly accelerated locomotor recovery. VPA therapy also suppressed SCI-induced hypoacetylation of histone and promoted expressions of BDNF and GDNF. Next, the influence of VPA on axonal growth inhibited by a myelin protein was tested. Neurons from embryonic spinal cord or hippocampus were cultured on plates coated with Nogo-A peptide, and escalating concentrations of VPA were added into the cultures. VPA treatment, in a concentration dependent manner, allowed neurons to overcome Nogo-A inhibition of neurite outgrowth. Meanwhile, VPA exposure increased the level of histone acetylation and expression of BDNF in spinal neurons. Cumulatively, these findings indicate that VPA is possibly a promising medication and deserves translational trials for spinal cord injury.
Keywords: Spinal cord injury, HDAC inhibitor, Valproic acid, Neurons
Introduction
Previous studies have shown that treatment of valproic acid (VPA), when given up to 3 h after surgery, improves locomotion in a rat model of spinal cord injury (SCI) (Lv et al., 2011a; Penas et al., 2011). Furthermore, transplantation of neural stem cells together with administration of VPA dramatically enhanced the restoration of hind limb function of SCI mice (Abematsu et al., 2010). Besides spinal cord injury, VPA exerts protective effects for various neurological diseases, including SCI, stroke, traumatic brain injury, motor neuron diseases, Parkinson’s disease, Alzheimer’s disease and Huntington’s disease (Brichta et al., 2006; Dash et al., 2010; Kidd and Schneider, 2011; Qing et al., 2008; Ren et al., 2004; Rouaux et al., 2007; Sinn et al., 2007; Zadori et al., 2009). There is now accumulating evidence that VPA may have potential in the treatment of central nervous system (CNS) disorders and the neuroprotective functions are linking with its inhibition on histone deacetylases (HDACs) (Nalivaeva et al., 2009).
HDACs play a key role in the homeostasis of histone acetylation and regulating fundamental cellular activities such as transcription (Abel and Zukin, 2008; Chuang et al., 2009). A wide range of neurological disorders have been linked to imbalances in protein acetylation levels and associated transcriptional dysfunction (Abel and Zukin, 2008; Chuang et al., 2009; Lv et al., 2011b). Because HDAC inhibitors increase histone acetylation, adjust gene transcription and upregulate neurotrophic genes, they have become a promising intervention for CNS diseases. The neural growth factors upregulated by VPA contain brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) (Bredy et al., 2007; Castro et al., 2005; Chen et al., 2006; Einat et al., 2003; Fukumoto et al., 2001; Wu et al., 2008). They play an essential role for neuron survival and neurite growth in SCI condition (Thuret et al., 2006).
It has been found that VPA promotes neurite outgrowth in some neurological conditions. For instance, valproate stimulates neurite growth in cells including primary cultured hippocampal neurons (Natori et al., 2008), SH-SY5Y cells (Yuan et al., 2001), N1E-115 neuroblastoma cells (Yamauchi et al., 2007) and PC12 cells (van Bergeijk et al., 2006). More importantly, valproic acid spreads axonal regeneration in animal models of optic nerve crush (Biermann et al., 2010), sciatic nerve axotomy (Cui et al., 2003), as well as Charcot-Marie-Tooth disease (Yamauchi et al., 2010). In addition, it inhibits the collapse of sensory neuron growth cones and increases growth cone area (Williams et al., 2002).
In this study, we further observed the efficiency of delayed VPA intervention (given 8 h after surgery) for SCI rats; and hypothesized that VPA could reduce myelin protein (Nogo-A peptide used) inhibition on axonal growth of neurons. We found that delayed VPA injection accelerated the recovery of SCI, allowed neurons to overcome Nogo-A inhibition and enhanced neurite outgrowth, associated with upregulating histone acetylation and inducing neutrophic gene.
Materials and methods
Experiment design
The study was divided into two parts. (1) Spinal cord injury was performed in Wistar rats via using NYU impactor, and then delayed injection of VPA was given 8 h after surgery for 1 week. The dosage of VPA was 300 mg/kg (dissolved in saline), i.p. twice a day (Brill et al., 2006; Lv et al., 2011a; Penas et al., 2011); for the controls, same volume of saline was injected. Neurobehavioral tests, apoptosis, lesion size, the level of Ac-H3, as well as the expressions of GDNF and BDNF were measured. (2) Neurons obtained from spinal cord and hippocampus of embryonic rat (E14) were cultured on plates coated with Nogo-A peptide; the medium contained escalating doses of VPA, 0.3, 0.6 and 1.2 mM. Neurite outgrowth was recorded to examine whether VPA could overcome Nogo-A inhibition and enhance axon extension. Then the level of histone acetylation and expressions of GDNF and BDNF mRNA were observed in neurons of spinal cord.
Surgical procedures
The animal study was approved by the Committee on the Use and Care of Animals, Fudan University and all the principles of Laboratory Animal Care (NIH publication No. 86-23, revised 1985) were followed. Female Wistar rats (weighing 200–230 g) were anesthetized with inhalational 1.5–2% isoflurane, and body temperature was maintained using a homoeothermic blanket. A midline incision was performed to expose the spinal column of T8–11, and laminectomy was operated at T9. Then the New York University (NYU) impactor was used to induce a moderate contusion (Basso et al., 1996). The NYU impactor is a weight-drop device that releases a 10 g rod from various heights onto the exposed spinal cord. It is a thoroughly tested and recognized apparatus for spinal cord injury. In our study, the rod was dropped from a height of 12.5 mm. After surgery, the rats received manual bladder expression twice daily, with appropriate veterinary care as needed.
Behavioral assessment
The rats were exposed to the testing environment twice a day for 5 consecutive days prior to surgery. Two researchers blinded to experimental groups observed the neurobehavioral tests, weekly for 6 weeks after spinal cord injury. Gross hindlimb performance was evaluated using the open field locomotor test (Basso, Beattie, Bresnahan Locomotor Rating Scale, BBB) (Basso et al., 1996). For BBB test, rats were videotaped for 4 min in a plastic wading pool with a smooth floor (90 cm diameter, 7 cm wall height). Then the scores were recorded according to the 21-point locomotor rating scale. A score of 0 is given if there is no spontaneous movement, and a score of 21 indicates normal locomotion. When an animal shows plantar stepping with full weight support and complete forelimb–hindlimb coordination, it reaches a score of 14 points. Footprint analysis was performed to define the deficits of fine motor control (Pearse et al., 2004). The animal’s hind paws were inked and footprints were made on paper covering a narrow runway of 1 m length and 7 cm width. A series of at least eight sequential steps was used to determine the mean values of limb rotation and of base of support.
Analysis of apoptosis and lenition size
One week after surgery, the rats were sacrificed for apoptosis analysis (Choi et al., 2010). Briefly, formalinfixed and OCT-embedded spinal cords were processed to sections. Serial transverse sections (20 μm thick) were collected every 100 μm from 2 mm rostral to 2 mm caudal to the lesion epicenter (total 40 sections). Then, apoptosis was detected by a TUNEL kit (Roche). Using a fluorescence microscope, 3 randomized fields (250×250 μm) in each section were selected. The observer being unaware of the group design recorded and calculated the average apoptosis ratio of each group.
At 6 weeks post-injury after behavioral tests, the animals were killed for analyzing lesion size (Penas et al., 2011). A T8–T10 spinal cord segment around the lesion epicenter was removed and serially cut (20 μm thickness) in the transverse plane. A complete series of 12 sections spanning the injury site was collected at 500 μm intervals and attached onto glass slides. After that, the sections were stained with haematoxylin and eosin. Digital photography of the serial slices was taken to outline areas using Pro software (Media Cybernetics). The total volume was calculated by summing the lesion area in each section and multiplying by the distance between sections.
Primary culture of spinal cord neurons
The spinal neurons were isolated from spinal cord segments of embryonic day 14 (E14) rats (Boato et al., 2010). The meninges were removed under sterile conditions, and the tissues were dissociated into single cells by incubation with 0.25% trypsin–EDTA for 10 min at 37 °C. After filtration and centrifugation, the cells were resuspended in Neurobasal medium (Invitrogen), supplemented with B27 (Gibco) and glutamine (500 μM). Thereafter the neurons were cultured on 24 well plates coated with 100 μg/ml poly-D-lysine (Sigma) and 8 μg/ml laminin (Sigma), and then kept in cell culture incubator, at 37 °C with a humidified atmosphere of 5% CO2 and 95% air. The medium was changed every three days.
Primary culture of hippocampus neurons
Hippocampal neurons were prepared from fetal rat at embryonic day 14 (E14) (Boato et al., 2010). Dissected pieces of hippocampi were dissociated mechanically. Following centrifugation, cells were resuspended in Neurobasal medium, supplemented with B27 and glutamine. After that, the cells were cultured on 24 well plates coated with poly-D-lysine and laminin, and kept in cell culture incubator. The culture medium was replaced every three days.
Neurite outgrowth assay
For assay of axon growth (Boato et al., 2010; Zhou et al., 2009), 200 μl of 25 nM Nogo-A peptide (Abcam) dissolved in medium was incubated in each well for 2 h (GrandPre et al., 2000). Then they were aspirated and immediately replaced with laminin. After another 2 h, laminin was discarded and the neuron was cultured, with escalating doses of VPA (0.3, 0.6 and 1.2 mM). Cells were cultured for 6 days, after which they were stained with antibodies to β-III tubulin (Tuj-1, 1:2000, Sigma). Digital images were taken and the length of axon was determined using Meta imaging software (Molecular Devices). In each well, 50 neurons were measured and average outgrowth per well was calculated. The experiment was repeated 3 times.
Western blot
The level of histone 3 acetylation was measured by western blot (Ren et al., 2004; Sinn et al., 2007). For western blot, spinal tissues or neurons were collected, sonicated and centrifuged at 12,000 rpm for 20 min after which the protein concentrations in the supernatant were measured through a bicinchoninic acid kit (BCA Protein Assay Reagent, Thermo). Total protein amounts (120 μg/lane) were separated on a 12% SDS-PAGE, transferred onto a PVDF membrane (Millipore), and then incubated with primary antibodies overnight at 4 °C: rabbit acetylated histone H3 on lysine 9 antibody (1:1000, Upstate). After incubation with secondary antibodies conjugated to horseradish peroxidase, protein bands were probed by an enhanced chemo-luminescence kit (ECL kit, Thermo). The western blots were captured and the intensities quantified with Quantity One version 16.0.
Quantitative RT-PCR analysis
Quantitative PCR (q-PCR) analysis was performed to access the influences of VPA on the expressions of GDNF and BDNF (Bredy et al., 2007; Chen et al., 2006). Total RNA was extracted by TRIzol reagent (Sigma) and purified with RNeasy kit (Qiagen). cDNA prepared from mRNA was amplified using the following primer sets: GAPDH-forward (5′-TTC ACC ACC ATG GAG AAG GC-3′) and GAPDH-reverse (5′-GGC ATG GAC TGT GGT CAT GA-3′), GDNF-forward (5′-ACG AAA CCA AGG AGG AAC TGA T-3′) and GDNF-reverse (5′-CCG TTT AGC GGA ATG CTT T-3′), BDNF-forward (5′ TGA GCG TGT GTG ACA GTA TTA GC-3′) and BDNF-reverse (5′-GCA GCC TTC CTT CGT GTA ACC-3′). Quantitative real-time PCR was performed in an iCycler (Bio-Rad) with the use of SYBR green DNA PCR kit (Applied Biosystems). The threshold cycle for each sample was determined from the linear range and converted to a starting quantity by interpolation from a standard curve run on the same plate for each set of primers. The levels of BDNF and GDNF mRNA were normalized to the GAPDH mRNA and each PCR reaction was repeated three independent times.
Statistical analysis
Data were presented as mean±SEM values. Repeated measures ANOVA followed by the Tukey–Kramer test was used for comparison of weekly BBB scores. The difference between two groups was compared by Student’s t test. Multiple comparisons among different groups were performed through one-way ANOVA followed by the Bonferroni post hoc test. All statistical analyses were performed by using SPSS 16.0. Differences were accepted to be statistically significant at p<0.05 and p<0.01.
Results
Delayed VPA injection improved neurobehavioral function
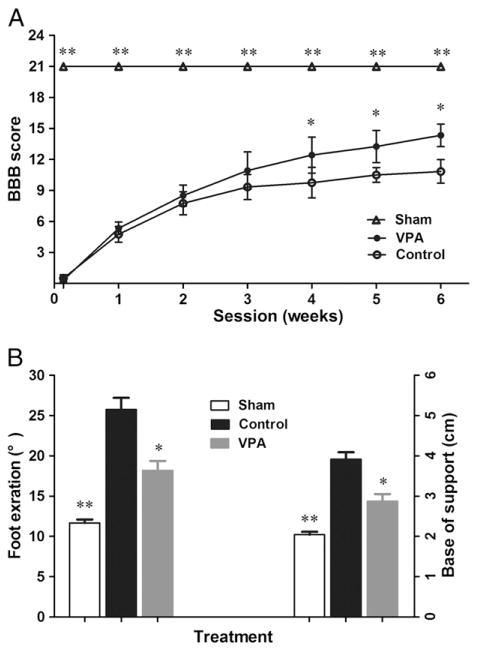
Two different neurobehavioral tests were evaluated once a week for 6 weeks (n=12). There were no obvious differences of BBB score between the treatment groups during the first 3 weeks. By week 4, however, the animals receiving VPA attained significantly higher scores in BBB (p<0.05, Fig. 1A and supplemental videos). The accelerated recovery of locomotion in the delayed VPA-treatment group was maintained until week 6 which was the end time point of the study (p<0.05, Fig. 1A). Meanwhile, VPA also improved the performance of minor movement. Compared to controls, rats from VPA group showed smaller rotation angles and decreased base of support (p<0.05 respectively, Fig. 1B).
Fig. 1.
Delayed administration of VPA improved functional recovery. SCI rats were treated by VPA or saline, staring 8 h after surgery. Compared to the controls, VPA significantly accelerated locomotion recovery after SCI. (A) The rats treated by VPA had higher scores in BBB test, starting from week 4 and lasting to endpoint. (B) At week 6, SCI rats injected by VPA showed better performance in footprint analysis. Reduced foot exorotation and support were found in animals with VPA-injection. Data were presented as mean±SEM (n=12), and p<0.05*, p<0.01**.
Delayed VPA treatment reduced apoptotic cells and lesion size
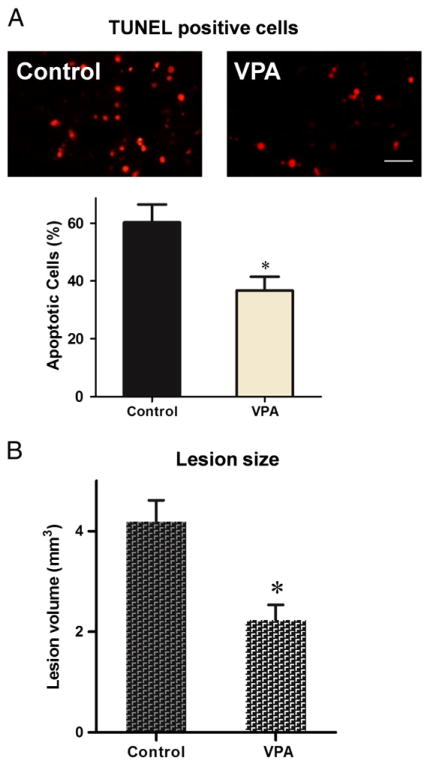
The effects of VPA on cell dearth and tissue sparing were measured respectively at different time points after spinal cord injury. Analysis of apoptosis was performed by TUNEL kit at week 1 post operative. Average rate of apoptotic cells was compared between different treatment groups. Delayed VPA treatment significantly reduced apoptotic cells induced by spinal cord injury (p<0.05, Fig. 2A). Lesion size was analyzed at 6 weeks after surgery. The mean lesion volume of rats treated by VPA was much smaller than that of controls (p<0.05, Fig. 2B).
Fig. 2.
Delayed VPA treatment reduced apoptotic cells and lesion size. (A) Apoptotic cells were detected by TUNEL kit week 1 post operative. Delayed VPA treatment significantly decreased apoptotic cells. (B) Lesion volume was measured 6 weeks after injury. Delayed VPA treatment also significantly reduced lesion size. Data were presented as means±SEM (n=8), Bar=20 μm, and p<0.05*, as compared to controls.
Delayed VPA therapy promoted histone acetylation
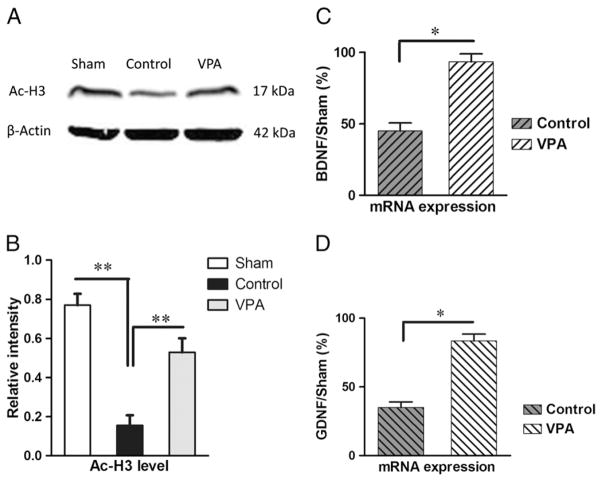
Spinal cords of animals from sham surgery, control and VPA treatment group were collected one week after spinal cord injury. Weston blot was performed to investigate the level of histone 3 acetylation and the optical densities representing Ac-H3 were normalized to β-actin. Spinal cord injury resulted in low levels of acetylated H3 (p<0.01, Fig. 3A/B). VPA, as an HDAC inhibitor, significantly suppressed hypoacetylation of Histone 3 induced by SCI (p<0.01, Fig. 3A/B).
Fig. 3.
Delayed VPA therapy unregulated H3 acetylation, and mRNA expressions of BDNF and GDNF. Rats were sacrificed to investigate acetylation of histone and expressions of neurotrophic genes. (A/B) VPA injection ameliorated SCI-induced hypoacetylation of H3. (C/D) The mRNA expressions of BDNF and GDNF were also elevated by VPA therapy. Data were presented as means±SEM (n=6), and p<0.05*, p<0.01**.
VPA increased GDNF and BDNF expression in SCI rats
Rats suffering from spinal cord injury were treated by VPA or saline separately. To explore the effect of delayed VPA injection on the expressions of neurotrophic genes, q-PCR analysis was applied to measure the levels of GDNF and BDNF mRNA. Each PCR reaction was repeated three independent times. Compared to controls, animals injected by VPA showed significant increase in BDNF (p<0.05, Fig. 3C) transcript. Meanwhile, VPA also unregulated the expression of GDNF (p<0.05, Fig. 3D).
VPA attenuated Nogo-A inhibition on axonal growth of neurons
The efficiency of delayed VPA intervention (given 8 h after surgery) for rats with spinal cord injury was supported by in vivo study. Then we hypothesized that VPA exposure could reduce myelin protein inhibition on axonal growth of neurons. For in vitro experiment, Nogo-A peptide was used to inhibit neurite growth of neurons and the impact of VPA was tested. Both neurons from spinal cord and hippocampus were primarily cultured and different concentrations of VPA were added to the culture medium.
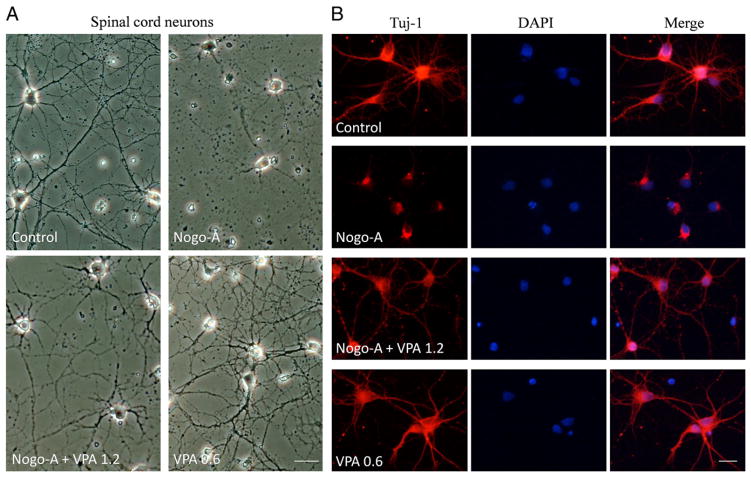
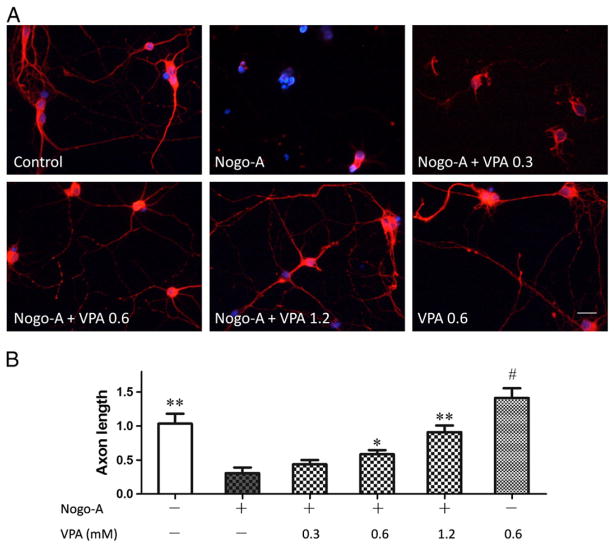
Spinal cord neurons were primarily cultured on 24-wells coated with or without Nogo-A peptide. Compared to control, Nogo-A inhibited the axonal growth of spinal cord neurons. VPA was added into the culture medium to test its efficiency on axonal regeneration. Neurons were immunostained with antibodies to β-III tubulin to trace neurite. Surprisingly, VPA exposure attenuated Nogo-A inhibition (Fig. 4) and significantly enhanced neurite growth in a dose dependent manner (Fig. 5).
Fig. 4.
VPA attenuated Nogo-A inhibition on axonal growth of spinal neurons. (A) Spinal neurons were cultured on 24-well coated with or without Nogo-A peptide. Compared to control, Nogo-A inhibited axonal growth. However, VPA exposure attenuated Nogo-A inhibition and promoted neurite outgrowth. (B) Neurons were immunostained with antibodies to β-III tubulin to trace neurite (red), and nuclei were stained by DAPI (blue). VPA obviously enhanced neurite generation. Bar A=40 μm, Bar B=100 μm.
Fig. 5.
VPA enhances axon outgrowth in a concentration dependent manner. (A) Spinal neurons were stained with antibodies to β-III tubulin (red) and digital images were taken for morphometrical analysis. (B) The length of axon was determined and average outgrowth was calculated. VPA attenuated the inhibitory effect of Nogo-A and enhanced neurite regeneration in a concentration dependent manner. Neurite outgrowth was expressed as a ratio to control and presented as mean±SEM (n=3). Bar=100 μm, and p<0.05*, p<0.01** as compared to Nogo-A group; p<0.05# as compared to control group.
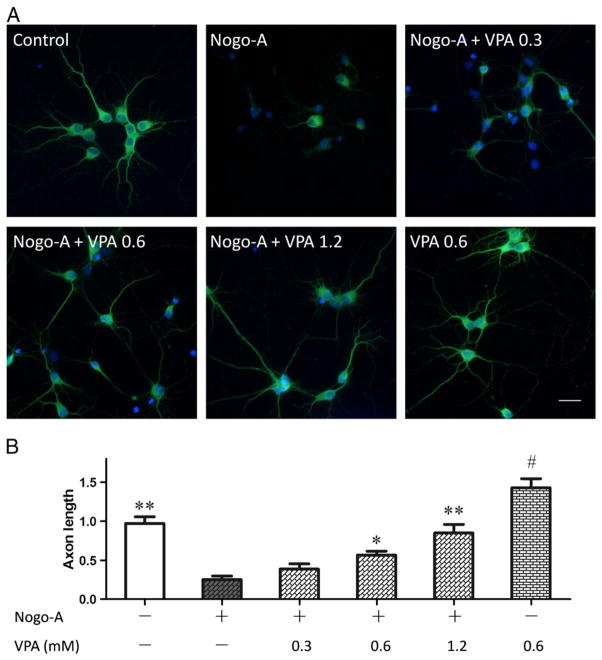
To further prove the effect of VPA on neurite outgrowth inhibited by Nogo-A peptide, hippocampus neurons were primarily cultured and stained with antibodies to β-III tubulin. Similarly to the effect on spinal cord neurons, VPA obviously relieved Nogo-A inhibition and promoted neurite outgrowth in a dose dependent manner for hippocampus neurons (Fig. 6).
Fig. 6.
VPA enhances axonal growth of hippocampus neurons. (A) Immunostaining of β-III tubulin (green) was performed in hippocampus neurons. (B) The average outgrowth of neurite was analyzed. VPA in a concentration dependent manner enhanced axonal regeneration. Neurite outgrowth was expressed as a ratio to control and presented as mean± SEM (n=3). Bar=100 μm, and p<0.05*, p<0.01** as compared to Nogo-A group; p<0.05# as compared to control group.
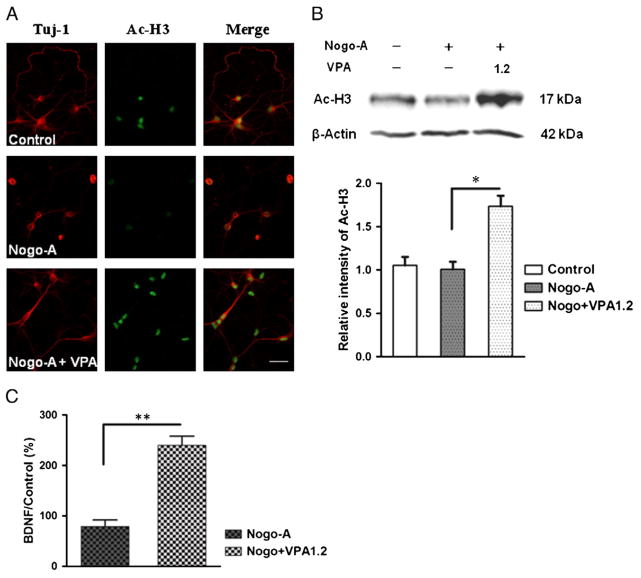
VPA enhanced Ac-H3 in spinal cord neurons
The in vivo experiment suggested VPA increased histone acetylation in rat model of SCI. But for in vitro study, the possible impact of VPA is still unknown. Therefore, in neurons from spinal cord, the levels of histone acetylation were investigated. Compared to controls, higher-dosage VPA (1.2 mM) obviously upregulated Ac-H3 (p<0.01, Fig. 7), however, lower concentrations of VPA (0.3 and 0.6 mM) failed to enhance acetylation of histone 3 (data not shown).
Fig. 7.
VPA enhanced H3 acetylating and BDNF transcript in spinal neurons. (A) Neurons were immunostained with antibodies to β-III tubulin (red) and acetylated histone 3 (green). Compared to controls, VPA (1.2 mM) increased Ac-H3. (B) Weston blot was performed to quantify Ac-H3. VPA significantly enhanced H3 acetylating in neurons attained from spinal cord. (C) The level of BDNF mRNA in spinal cord neurons was measured by q-PCR. Compared to controls, VPA significantly upregulated BDNF transcript. Data were presented as means±SEM (n=3), Bar=100 μm, p<0.05* and p<0.01**.
VPA increased BDNF expression in spinal neurons
The further evaluate the impact of VPA on gene expression, quantitative RT-PCR was used to evaluate the transcripts of GDNF and BDNF in spinal neurons. BDNF mRNA was significantly increased by higher concentration of VPA treatment (p<0.01, 1.2 mM, Fig. 7C), not lower dosages (0.3 and 0.6 mM, data not shown). But VPA did not change the levels of GDNF mRNA (data now shown).
Discussion
We report here delayed injection of VPA promoted the recovery of SCI rats, with a marked increased levels of GDNF and BDNF transcripts, which further explains the protective effects of VPA against SCI (Lv et al., 2011a; Penas et al., 2011). However, it is still unknown whether VPA could reduce the inhibitory effects of myelin proteins on axon outgrowth. Thus, the spinal cord and hippocampus neurons were cultured to elucidate the influence of VPA on axonal growth inhibited by Nogo-A peptide. Surprisingly, VPA enhanced axon regeneration, which is associated with elevated levels of histone acetylation and BDNF mRNA.
A large number of experiments, both in vitro and in vivo, have found that VPA has a potent ability to promote neurite outgrowth. VPA stimulate neurite growth primary cultured hippocampal neurons (Natori et al., 2008) and other cell lines (van Bergeijk et al., 2006; Yuan et al., 2001). It promotes axonal regeneration in animal models of neurological disorders (Biermann et al., 2010; Cui et al., 2003; Yamauchi et al., 2010). Our results demonstrated that VPA significantly attenuated Nogo-A inhibition of axonal growth in neurons derived from spinal cord and. But the current research in not sufficient to clarify whether VPA could reverse the inhibitory effects of myelin which contains at least three inhibitors, Nogo-A, MAG and OMgp, as MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma (Cafferty et al., 2010). Future studies should focus on whether exposure of neurons to VPA could prevent myelin inhibition of neurite growth.
The neuroprotective properties of VPA involve modulation of neurotrophic factors, such as BDNF and GDNF. VPA, as an HDAC inhibitor, up-regulate GDNF and BDNF gene transcription in astrocyte (Wu et al., 2008) and C6 glioma cells (Castro et al., 2005), as well as BDNF gene expression in prefrontal cortex (Bredy et al., 2007). Valproate also increased the expression of BDNF protein in the frontal cortex and hippocampus (Einat et al., 2003; Fukumoto et al., 2001). In this study, both in vivo and in vitro results showed that VPA increased BDNF mRNA, with elevated Ac-H3. VPA also increased the expressions of Bcl-2 and HSP70 based on another study (Lv et al., 2011a). VPA possibly triggers the expressions of BDNF, Bcl-2 and HSP70 through increasing histone acetylation and chromatin relaxation which facilitates transcription factor interaction with specific gene promoters and gene expression. VPA may also affect other genes, such as NT-3 (Walz et al., 2008), neuropeptide Y (Brill et al., 2006), and melatonin receptor MT1 (Castro et al., 2005) whose ligand melatonin possesses antiapoptotic activity in various neurodegenerative diseases (Wang, 2009). In the future study, microarray and chromatin immunoprecipitation (ChIP) should be performed to study the expressions of genes influenced by VPA and the association of modified histone and gene promoter.
The neuroprotective potential of isoflurane is controversial. For instance, 2% isoflurane worsened physiological and neurological outcomes in a rat model of ischemia hyperglycemia-induced hemorrhagic transformation (Hu et al., 2011). Low isoflurane concentration (1%) caused spatial learning impairment and more neurodegeneration in mice (Valentim et al., 2010). However, for spinal cord ischemia, pre-ischemic 2.8% isoflurane exposure exerted protective effects (Sung et al., 2010). Isoflurane (2%) application at the onset of reperfusion reduced brain ischemic injury in rats (Li and Zuo, 2011). In our study, anesthesia was induced by 2% isoflurane and maintained by 1.5% for 25 min during the surgery. It is unlikely that isoflurane with such low concentration and short time could influence the outcomes, even though further study is needed to determine the effect of isoflurane exposure on spinal cord injury.
Timing is of great importance when it comes to the early intervention of spinal cord injury, because the quality and timeliness of treatment will influence the long-term prognosis of the survivor. VPA was administrated as late as 3 h after SCI in a rat model based on recent reports (Lv et al., 2011a; Penas et al., 2011). However, it is hardly possible to give medications to the patients within 3 h after injury. It is worthy to test the efficiency of delayed treatment of VPA against injury. Therefore, the first dose of VPA was given 8 h following surgery and the efficiency was tested in our in vivo study. Even though VPA was not given immediately, it exerted robust neuroprotective effects. Delayed VPA treatment improved locomotion of rats suffering from SCI, suggesting the possible clinical application of valproate for patients.
There is now accumulating evidence that valproate, as an HDAC inhibitor, may have potential in the treatment of neurodegenerative diseases (Nalivaeva et al., 2009). VPA has been clinically used to treat epilepsy for many years. The dosage of VPA in our study is similar with the dose used for rat model of epilepsy (Nissinen and Pitkanen, 2007). The dose administered has been shown to result in serum VPA levels similar to those seen in patients receiving VPA treatment (Loscher and Honack, 1995). It is noteworthy that VPA, at a dose used in epilepsy, did not slow disease progression in ALS patients (Piepers et al., 2009). However, it is still unknown the effect of VPA for the patients with spinal cord injury. Our result combined with previous studies (Abematsu et al., 2010; Lv et al., 2011a; Penas et al., 2011) suggests that VPA in a clinical relevant concentration induces protective functions for models of spinal cord trauma. VPA has much potential as a brain penetrant, clinically available and well tested drug (Nalivaeva et al., 2009). Therefore, it is rational to further evaluate its translational potential for patients.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81070935 to Q. D.), Science and Technology Commission of Shanghai Municipality (11ZR1405400 to X. H.), Muscular Dystrophy Association, USA (157511 to X. W.), and National Institutes of Health, USA (NS055072 to X. W.).
Footnotes
Statement of interest
None.
Supplementary materials related to this article can be found online at doi:10.1016/j.expneurol.2011.11.042.
References
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Investig. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagreze WA. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Investig Ophthalmol Vis Sci. 2010;51:526–534. doi: 10.1167/iovs.09-3903. [DOI] [PubMed] [Google Scholar]
- Boato F, Hendrix S, Huelsenbeck SC, Hofmann F, Grosse G, Djalali S, Klimaschewski L, Auer M, Just I, Ahnert-Hilger G, Holtje M. C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J Cell Sci. 2010;123:1652–1662. doi: 10.1242/jcs.066050. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta L, Holker I, Haug K, Klockgether T, Wirth B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann Neurol. 2006;59:970–975. doi: 10.1002/ana.20836. [DOI] [PubMed] [Google Scholar]
- Brill J, Lee M, Zhao S, Fernald RD, Huguenard JR. Chronic valproic acid treatment triggers increased neuropeptide y expression and signaling in rat nucleus reticularis thalami. J Neurosci. 2006;26:6813–6822. doi: 10.1523/JNEUROSCI.5320-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro LM, Gallant M, Niles LP. Novel targets for valproic acid: up-regulation of melatonin receptors and neurotrophic factors in C6 glioma cells. J Neurochem. 2005;95:1227–1236. doi: 10.1111/j.1471-4159.2005.03457.x. [DOI] [PubMed] [Google Scholar]
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- Choi DC, Lee JY, Moon YJ, Kim SW, Oh TH, Yune TY. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol Dis. 2010;39:272–282. doi: 10.1016/j.nbd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui SS, Yang CP, Bowen RC, Bai O, Li XM, Jiang W, Zhang X. Valproic acid enhances axonal regeneration and recovery of motor function after sciatic nerve axotomy in adult rats. Brain Res. 2003;975:229–236. doi: 10.1016/s0006-8993(03)02699-4. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, Moore AN. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One. 2010;5:e11383. doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology. 2001;158:100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, Zhang J. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–1756. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd SK, Schneider JS. Protective effects of valproic acid on the nigrostriatal dopamine system in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2011;194:189–194. doi: 10.1016/j.neuroscience.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Honack D. Comparison of anticonvulsant efficacy of valproate during prolonged treatment with one and three daily doses or continuous (“controlled release”) administration in a model of generalized seizures in rats. Epilepsia. 1995;36:929–937. doi: 10.1111/j.1528-1157.1995.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q. Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res. 2011a;1396:60–68. doi: 10.1016/j.brainres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Lv L, Tang YP, Han X, Wang X, Dong Q. Therapeutic Application of Histone Deacetylase Inhibitors for Stroke. Cent Nerv Syst Agents Med Chem. 2011b;11:138–149. doi: 10.2174/187152411796011330. [DOI] [PubMed] [Google Scholar]
- Nalivaeva NN, Belyaev ND, Turner AJ. Sodium valproate: an old drug with new roles. Trends Pharmacol Sci. 2009;30:509–514. doi: 10.1016/j.tips.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Natori T, Kodaira F, Hirasawa T, Gao YY, Nagai K. Augmentation of polysialic acid by valproic acid in early postnatal mouse hippocampus and primary cultured hippocampal neurons. J Biosci Bioeng. 2008;105:164–167. doi: 10.1263/jbb.105.164. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Pitkanen A. Effect of antiepileptic drugs on spontaneous seizures in epileptic rats. Epilepsy Res. 2007;73:181–191. doi: 10.1016/j.eplepsyres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Penas C, Verdu E, Asensio-Pinilla E, Guzman-Lenis MS, Herrando-Grabulosa M, Navarro X, Casas C. Valproate reduces CHOP levels and preserves oligoden-drocytes and axons after spinal cord injury. Neuroscience. 2011;178:33–44. doi: 10.1016/j.neuroscience.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Piepers S, Veldink JH, de Jong SW, van der Tweel I, van der Pol WL, Uijtendaal EV, Schelhaas HJ, Scheffer H, de Visser M, de Jong J, Wokke JHJ, Groeneveld GJ, van den Berg LH. Randomized sequential trial of valproic acid in amyotrophic lateral sclerosis. Ann Neurol. 2009;66:227–234. doi: 10.1002/ana.21620. [DOI] [PubMed] [Google Scholar]
- Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, Zhang Z, Wei S, Sun X, Chen CH, Zhou W, Wang K, Song W. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med. 2008;205:2781–2789. doi: 10.1084/jem.20081588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Panteleeva I, Rene F, de Aguilar JLG, Echaniz-Laguna A, Dupuis L, Menger Y, Boutillier AL, Loeffler JP. Sodium valproate exerts neuroprotective effects in vivo through CREB-binding protein-dependent mechanisms but does not improve survival in an amyotrophic lateral sclerosis mouse model. J Neurosci. 2007;27:5535–5545. doi: 10.1523/JNEUROSCI.1139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, Kim JM, Park DK, Lee SK, Kim M, Roh JK. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007;26:464–472. doi: 10.1016/j.nbd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Sung YH, Lee SH, Sung JK, Han JH, Kim H, Kim CJ, Kang JM. Preconditioning of isoflurane on spinal cord ischemia can increase the number of inducible nitric oxide synthase-expressing motor neurons in rat. Korean J Anesthesiol. 2010;58:70–75. doi: 10.4097/kjae.2010.58.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IA, Antunes LM. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology. 2010;113:1099–1108. doi: 10.1097/ALN.0b013e3181f79c7c. [DOI] [PubMed] [Google Scholar]
- van Bergeijk J, Haastert K, Grothe C, Claus P. Valproic acid promotes neurite outgrowth in PC12 cells independent from regulation of the survival of motoneuron protein. Chem Biol Drug Des. 2006;67:244–247. doi: 10.1111/j.1747-0285.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- Walz JC, Frey BN, Andreazza AC, Cereser KM, Cacilhas AA, Valvassori SS, Quevedo J, Kapczinski F. Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania. J Psychiatr Res. 2008;42:416–421. doi: 10.1016/j.jpsychires.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Wang X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther. 2009;15:345–357. doi: 10.1111/j.1755-5949.2009.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- Wu XF, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, Chuang DM, Zhang WQ, Lu RB, Hong JS. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11:1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Murabe M, Fujiwara Y, Sanbe A, Fujita Y, Murase S, Tanoue A. Gadd45a, the gene induced by the mood stabilizer valproic acid, regulates neurite outgrowth through JNK and the substrate paxillin in N1E-115 neuroblastoma cells. Exp Cell Res. 2007;313:1886–1896. doi: 10.1016/j.yexcr.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Torii T, Kusakawa S, Sanbe A, Nakamura K, Takashima S, Hamasaki H, Kawaguchi S, Miyamoto Y, Tanoue A. The mood stabilizer valproic acid improves defective neurite formation caused by Charcot-Marie-Tooth disease-associated mutant Rab7 through the JNK signaling pathway. J Neurosci Res. 2010;88:3189–3197. doi: 10.1002/jnr.22460. [DOI] [PubMed] [Google Scholar]
- Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- Zadori D, Geisz A, Vamos E, Vecsei L, Klivenyi P. Valproate ameliorates the survival and the motor performance in a transgenic mouse model of Huntington’s disease. Pharmacol Biochem Behav. 2009;94:148–153. doi: 10.1016/j.pbb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Zhou ZG, Peng XM, Fink DJ, Mata M. HSV-mediated transfer of artemin overcomes myelin inhibition to improve outcome after spinal cord injury. Mol Ther. 2009;17:1173–1179. doi: 10.1038/mt.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]