Abstract
Postoperative abdominal/pelvic peritoneal adhesions are a major source of morbidity (bowel obstruction, infertility, ectopic gestation as well as chronic pelvic pain) in women. In this study, we screened various transduction and transcription modifications of adenovirus (Ad) to identify those that support maximal Ad-mediated gene delivery to human adhesion fibroblasts, which in turn would enhance the efficacy of this novel treatment/preventative strategy for postoperative adhesion. We transduced primary cultures of human peritoneal adhesion fibroblasts with fiber-modified Ad vectors Ad5-RGD-luc, Ad5-Sigma-luc, Ad5/3-luc and Ad5-CAV2-luc as well as transcriptional targeting of viruses Ad5-survivin-luc, Ad5-heparanase-luc, Ad5-mesothelin (MSLN)-CRAd-luc and Ad5-secretory leukoprotease inhibitor (SLPI)-luc, and compared their activity to wild-type Ad5-luc. At 48 h, luciferase activity was measured and normalized to the total protein content in the cells. Among the fiber-modified Ad vectors Ad5-Sigma-luc and among the the transcription targeting modified Ad Ad5-MSLN-CRAd-luc showed significantly increased expression levels of luciferase activity at 5, 10 and 50 plaque forming units/cell in adhesion fibroblast cells compared with wild-type Ad5-luc (p < 0.05). Specific modifications of Ad improve their gene delivery efficiency towards human peritoneal adhesion fibroblasts. Developing a safe localized method to prevent/treat postoperative adhesion formation would have a major impact on women health.
Keywords: Adhesion fibroblasts, Gene therapy, Luciferase activity, Modified adenovirus vectors, Postoperative adhesion
Introduction
Postoperative adhesions are a common cause of morbidity with a high incidence following abdominal surgery (60-90%) [1] and open gynecological pelvic surgeries (90%) [2]. Common consequences of postoperative adhesions are pain, intestinal obstruction, female infertility, and ectopic gestation [3]. The management of postoperative adhesions causes a large surgical load on patients and an expenditure burden on the health care system. Current approaches of reducing surgical trauma by microsurgical techniques or adhesion-reducing agents are not efficacious in preventing adhesion formation [4].
Adhesion develops via trauma-induced local changes, including inhibition of fibrinolysis, deposition of collagen, formation of intrinsic vasculature and a reduction in the activity of tissue plasminogen activator (tPA). Human and animal studies have confirmed that reduced tPA activity and increased release of plasminogen activator inhibitors PAI-1 and PAI-2 are marked in severe adhesions [5-8]. Several pharmacologic agents have shown moderate success in reducing adhesion development in experimental models. Intraperitoneal use of recombinant human tPA (rhtPA) has shown consistent success in reducing adhesion formation [9-12]. Since the action of tPA is localized to fibrin deposits, fibrinolytic activity is limited to this site, thus preventing indiscriminate fibrinolysis [13]. However, the short half-life (few minutes) of rhtPA limits its fibrinolytic effect for a sufficient duration of time (3-5 days) until complete healing of peritoneal surfaces [11-14]. Because of the inherent limitation of local molecular therapy, an alternative strategy using gene therapy has recently been employed to correct molecular aberration induced by surgical trauma in a regulated well-controlled manner. Inherent biologic features of postoperative peritoneal adhesions such as the localized site of occurrence and short period of development after injury make it a perfect target for gene therapy using noninte-grating vectors. Adenovirus (Ad) vectors have many positive attributes, such as their ability to provide efficient in vivo gene transfer to both dividing and nondividing cells, their high in vivo stability, and the nonintegrating nature into the host genome. These merits make Ad vectors suitable for proof-of-principle experimental studies. The broad tropism of Ad allows the virus to infect many cell types and is responsible for virus dissemination to distant organs. In our previous work, we have shown that a first-generation replication-incompetent Ad vector encoding htPA can be successfully used for regulating levels of tPA/PAI without increasing the risk of postoperative bleeding, mortality, or postoperative complications, and decreasing de novo and recurrent peritoneal adhesion formation in a rat model [15]. Since then, various modifications of the Ad vector have been developed to improve its selective cell targeting and gene delivery ability in various cell types.
Increasing Ad transduction and transcription within human adhesion fibroblasts would enhance the efficacy of this novel treatment/preventative strategy for postoperative adhesions. Testing various Ad vectors with modifications at either the fiber or promoter region to determine the best modification for a safe and effective treatment of postoperative adhesion is essentially required. To optimize the future use of the rhtPA gene delivered via an Ad vector and to enhance the safety of this novel strategy, we compared modified Ad vectors in the human adhesion cell line with a goal to identify the most robust modified Ad vector to support the highest level of rtPA expression in human adhesion cells with minimal scarring or cell damage.
Materials and Methods
Recombinant Ad
Ad vectors with a typical batch yield of 2 × 1010 plaque forming units (PFU)/ml have been used. These vectors were prepared on a large scale in the laboratory as we have described previously [16] and listed in table 1.
Table 1. Description of the Ad vectors used in this study.
| Virus | Virus description | Modification site | Promoter | Reference |
|---|---|---|---|---|
| Ad5-luc | E1/E3 deleted, a luc gene under the CMV promoter in place of E1 | wild | CMV | Krasnykh et al. (1996) |
| Ad5-RGD-luc | E1/E3 deleted, a luc gene under the CMV promoter in place of E1, RGD-4c modification in the H1 loop of the knob domain | fiber | CMV | Dmitriev et al. (1998) |
| Ad5-Sigma-luc | E1/E3 deleted, a luc gene under the CMV promoter in place of E1, chimeric fiber with the tail and shaft from Ad5 and the knob domain of reovirus | fiber | CMV | Mercier et al. (2004) |
| Ad5/3-luc | E1/E3 deleted, a luc gene under the CMV promoter in place of E1, chimeric fiber with the tail and shaft from Ad5 and the knob domain of Ad3 | fiber | CMV | Kanerva et al. (2002) |
| Ad5-CAV2-luc | E1/E3 deleted, a luc gene under the CMV promoter in place of E1, Ad5 fiber knob switching to that of canine Ad serotype 2 | fiber | CMV | Schagen et al. (2006); Bruner-Tran et al. (2006) |
| Ad5-survivin-luc | E1/E3 deleted, a luc gene under the survivin promoter in place of E1 | promoter | survivin | Van Houdt et al. (2006) |
| Ad5-heparanase-luc | E1/E3 deleted, a luc gene under the heparanase promoter in place of E1 | promoter | heparanase | Breidenbach et al. (2006) |
| Ad5-SLPI-luc | E1/E3 deleted, a luc gene under the SLPI promoter in place of E1 | promoter | SLPI | Barker et al. [21] (2003) |
| Ad5-MSLN-CRAd-luc | a luc and E1 gene under the MSLN promoter | promoter based CRAd | MSLN | Tsuruta et al. [20] (2005) |
Source and Culture of Fibroblasts
Primary cultures of fibroblasts were obtained from adhesion tissue from human subjects at the time of surgery under the approval of the Institutional Review Board of Wayne State University. A 38-year-old nonpregnant female was admitted for laparoscopic adhesiolysis, who gave informed consent for collection of adhesion tissue from the right and left pelvic side wall. The woman had no endometriosis. Pathological evaluation of the tissue indicated that it was adhesion tissue. Cells were obtained from the collected tissue and cultured as described in our earlier study [17].
Transfection of Human Adhesion Fibroblasts with Ad Vectors and Luciferase Reporter Assay
Transfection with various Ad vectors was performed as we have described previously [18, 19]. Briefly, peritoneal adhesion fibroblasts were cultured in DMEM with high glucose and 10% FBS with 1% antibiotic and antimycotic mix. To avoid potential variability in primary cells at different passages, all experiments were conducted on cells less than passage 4. Cells were cultured in 12-well plates at 105/well and transduced with vectors to be screened at 5, 10 and 50 PFU/cell. This was done in medium with continuous gentle shaking for 4 h, which was then replaced with fresh regular medium, and incubation was continued for an additional 48 h. Luciferase activation was measured using luciferase enzyme assay systems, according to the manufacturer's instructions (Promega, Madison, Wisc., USA). Total protein content was determined using a BCA kit (Pierce Biotech, Rockford, Ill., US) and values were used to normalize luciferase activity measured in the transfected cells. All samples were run in duplicate and experiments repeated three times to ensure reproducibility of results.
Statistical Analysis
The results of the luciferase transactivation were expressed as means ± SEM of three different experiments. Statistical analysis was determined using two-tailed Student's t test to compare groups (p < 0.05 was considered significant).
Results
Fiber-Modified Virus Ad5-Sigma-luc Support Significantly Higher Gene Expression in Human Adhesion Fibroblasts
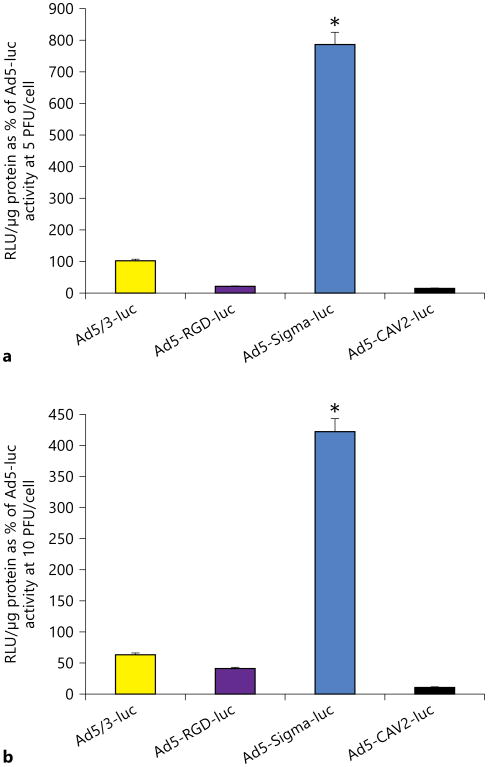
We tested the gene delivery efficiency of fiber-modified Ad vectors at three dose levels (5, 10 and 50 PFU/cell) by measuring their luciferase activity in primary human adhesion fibroblasts and compared it with the activity of wild-type (unmodified) virus (Ad5-luc). Among the four modified vectors tested, Ad5-Sigma-luc supported higher luciferase transactivation than Ad5-luc (wild type) at 5 and 10 PFU/cell dose levels (fig. 1). At the three tested doses (5, 10 and 50 PFU/cell), infectivity of Ad5-Sigma-luc was 786, 422 and 275%, respectively, of Ad5-luc activity (p < 0.05). Ad5-Sigma-luc supported the highest gene expression in comparison to other tested Ad fiber modifications (fig. 1); the latter also showed minimal luciferase activity in comparison to wild-type Ad5-luc activity.
Fig. 1.
Evaluation of fiber-modified Ad for the transduction of human adhesion fibroblasts. Ad5/3-luc, Ad5-RGD-luc, Ad5-Sig-ma-luc and Ad5-CAV2-luc were evaluated for transduction efficiency by their level of expressed luciferase activity (in relative light units, RLU). Ad constructs were used to transduce adhesion cells at 5 (a) and 10 PFU/cell (b). Ad5-Sigma-luc shows the highest luciferase expression at both 5 and 10 PFU/cell. Luciferase activity was normalized to the protein content and expressed as mean of three experiments ± SE and plotted as percentage of Ad5-luc activity (* p < 0.05).
Evaluation of the Activity of Transcriptionally Modified Ad Vectors in Human Peritoneal Adhesion Cells
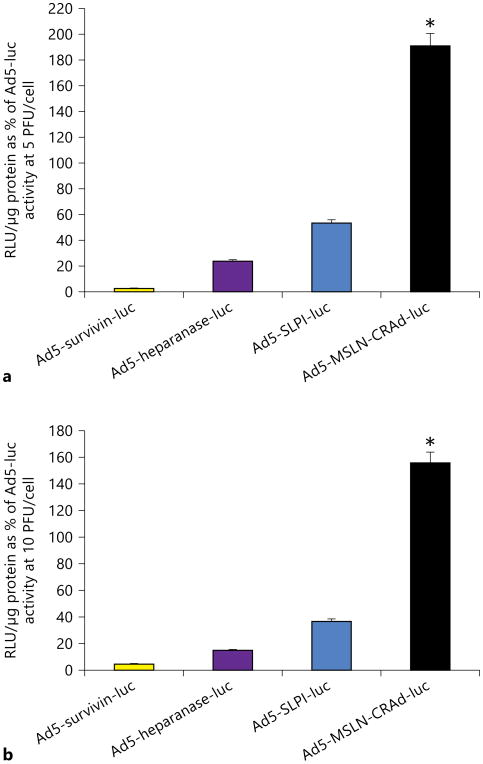
Among the four tumor-specific promoters (TSPs) tested, Ad5-mesothelin (MSLN)-CRAd-luc showed the highest luciferase activity in adhesion cells at 5, 10 and 50 PFU/cell compared to wild-type Ad5-luc activity (fig. 2; data for 50 PFU/cell not shown). At doses of 5 and 10 PFU/cell, infectivity of Ad5-MSLN-CRAd-luc was 190 and 156%, respectively, higher compared with wild-type Ad5-luc (p < 0.05). None of the other modified viruses like Ad5-survivin, Ad5-heparanase and Ad5-secretory leukoprotease inhibitor (SLPI)-luc demonstrated significant transduction compared to equivalent PFU doses of the wild-type Ad5-luc.
Fig. 2.
Evaluation of TSP-modified Ad for transduction of human adhesion fibroblasts. Ad5-survivin-luc, Ad5-heparanase-luc, Ad5-SLPI-luc and Ad5-MSLN-CRAd-luc were evaluated for transduction efficiency by their level of luciferase activity expressed. Ad constructs were used to transduce adhesion cells at 5 (a) and 10 PFU/cell (b). Ad5-MSLN-CRAd-luc shows the highest luciferase expression and activity in adhesion cells at both 5 and 10 PFU/cell. Luciferase activity (in relative light units, RLU) was normalized to the protein content and expressed as the mean of three experiments ± SE and plotted as percentage of Ad5-luc activity (* p < 0.05).
Discussion
We report here that Ad vectors with specific modifications enhancing both transduction and transcription of the vector-supported gene delivery can be utilized in postoperative adhesions by targeting therapeutic genes like htPA to adhesion fibroblast cells which are peritoneal fibroblasts transformed at cellular and molecular level into adhesion fibroblasts. In this study, we recorded the Ad5-mediated gene delivery to primary human adhesion fibroblast cells by screening two modified Ad panels, which include the fiber-modified panel (Ad5-RGD-luc, Ad5/3-luc, Ad5-Sigma-luc and Ad5-CAV2-luc) and the TSP-containing panel (Ad5-SLPI-luc, Ad5-survivin-luc, Ad5-heparanase-luc and Ad5-MSLN-CRAd-luc). In these sets of experiments, we compared the modified Ad to wild-type Ad5 in order to identify the most robust Ad vector modification. We used separate panels of Ad in this study which covered two main strategies: Ad5-trans-ductional targeting and Ad5-transcriptional targeting. Transductional targeting aims at deletion of the broad tropism of Ad5 toward normal epithelial cells and/or enhances virus infectivity of primary CAR (Coxsackie/adeno receptor)-deficient cells. We determined the transduction levels in the context of human adhesion fibroblasts using transductionally enhanced Ad5 as in Ad5-RGD-luc, Ad5/3-luc, Ad5-Sigma-luc and Ad5-CAV2-luc. We showed that Ad5-Sigma-luc supports significantly higher reporter gene activity in human adhesion fibroblasts than unmodified Ad5-luc at all tested multiplicities of infection (5, 10 and 50 PFU/cell; fig. 1). Ad5-Sigma is an Ad vector with fibers of Ad5 and reovirus, and the receptor-binding molecule of serotype T3D (type 3Dearing) reovirus called the σ1 protein that binds sialic acid and junction adhesion molecule 1 which determine the T3D reovirus tropism in enhancing the infectivity of the virus vector and gene transfer ranging from 2.3-to 45-fold in all tested cell lines [20]. Our data demonstrate that Ad5-Sigma-luc consistently exhibited the highest gene transfer among all fiber-modified viruses in human adhesion cells. It remains to be seen if such observations will be reproduced in vivo in suitable animal adhesion models.
The Ad5-transcriptional targeting strategy also represents a potential molecular approach to achieve adhesion fibroblast-specific expression of transgenes encoded with viral vectors. We tested four TSPs for their activity in human adhesion fibroblasts: survivin, SLPI, heparanase, and MSLN promoters. Activity of these four promoters has not been tested previously in adhesion fibroblasts. Since their TSP activity has been reported to be highly expressed in many cell types [21, 22], they were considered to be potential candidates for adhesion-specific transcriptional targeting of Ad-mediated gene expression. Our results showed consistently higher reporter gene activities of Ad5-MSLN-CRAd-luc in adhesion fibroblasts than unmodified Ad5-luc at all tested multiplicities of infection. Ad5-MSLN-CRAd is a type II promoter-inducible conditionally replicative Ad (CRAd), with the capability of replacing the endogenous viral promoters with MSLN TSP, to control early translated genes (E1A) of the virus [23, 24]. Conditionally replicative Ad are designed with the purpose of targeting selective abnormal cells like tumor cells or adhesion cells for viral replication rather than normal cells [23]. In order to generate CRAd, the original promoter of the wild-type Ad is replaced by a specific promoter which can help in Ad replication including E1. In Ad5-MSLN-CRAd, replication is seen only in target tissues but not in normal tissue as the viral promoter has been replaced with MSLN TSP.
The current study is a proof-of-principle study to assess the feasibility of a panel of Ad vectors with altered infectivity via transductional or transcriptional modification at either the knob or the promoter region. Our study demonstrates that Ad5-Sigma-luc and Ad5-MSLN-CRAd, which come under two separate panels, have enhanced luciferase activity in the adhesion cells. Hence, a single vector with more than one modification can be used as a suitable vector with enhanced efficacy at transduction as well as TSP transcription activity making it suitable for therapeutic gene delivery. This would also be a novel approach in targeting adhesion cells offering distinct advantages over vectors with single modifications. Ad has shown a tendency to localize in the liver and low Ad5 titers have been detected in the bloodstream. The safety of these modifications has already been tested in vitro in THLE3 human hepatocyte cells in our laboratory [17]. Among current approaches to limit or overcome adhesion, physical barriers have been reported to be most effective in spite of their limitations in delivery and performance criteria like shape and presence of peritoneal fluid for example [25]. The efficacy of these barriers can potentially be enhanced by adding a biological element to their physical functionality. As a future application, we can possibly use physical barriers anchored with dual-modified Ad vectors at both transductional and transcriptional level. This will enhance the utility of Ad vectors as it will localize their delivery to areas of injury as well as enhance the efficacy of the physical barriers, by adding a biologically active component to their adhesion prevention strategy. In our laboratory, we are exploring such an approach in suitable animal models for postoperative peritoneal adhesion.
In conclusion, we have shown that Ad5-Sigma-luc and Ad5-MSLN-CRAd-luc showed higher reporter gene expression in human adhesion fibroblasts. These modifications appear to be promising in the delivery of therapeutic genes such as tPA. As such, we believe that our cell model used in this study is appropriate. In addition to preventing de novo adhesion formation, this strategy is being proposed to prevent adhesion reformation, in which the cell phenotype has already acquired the adhesion profile as we have described before [15]. In a clinical setting, a physical barrier anchored with modified Ad vectors could possibly be administered into the pelvic cavity at the end of surgical manipulations to prevent/reduce the development of postoperative adhesion. Feasibility of such a combination should be tested in stringent model systems and successful outcome could potentially lead to major clinical advancement in the area of prevention or treatment of postoperative adhesions.
Acknowledgments
The study was supported by RCMI grant G12 RR 03032 and USDA B1 58-3148-5-125 (in cooperation with the Egyptian Science and Technology Development Fund under Project 739).
References
- 1.Menzies D, Ellis H. Intestinal obstruction from adhesions - how big is the problem? Ann R Coll Surg Engl. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 2.Group OLS. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Operative Laparoscopy Study Group. Fertil Steril. 1991;55:700–704. [PubMed] [Google Scholar]
- 3.Liakakos T, Homakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–273. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 4.Alpay Z, Saed GM, Diamond MP. Postoperative adhesions: from formation to prevention. Semin Reprod Med. 2008;26:313–321. doi: 10.1055/s-0028-1082389. [DOI] [PubMed] [Google Scholar]
- 5.Holmdahl L, Eriksson E, Eriksson BI, Risberg B. Depression of peritoneal fibrinolysis during operation is a local response to trauma. Surgery. 1998;123:539–544. doi: 10.1067/msy.1998.86984. [DOI] [PubMed] [Google Scholar]
- 6.Ivarsson ML, Bergstrom M, Eriksson E, Risberg B, Holmdahl L. Tissue markers as predictors of postoperative adhesions. Br J Surg. 1998;85:1549–1554. doi: 10.1046/j.1365-2168.1998.00859.x. [DOI] [PubMed] [Google Scholar]
- 7.Holmdahl L, Falkenberg M, Ivarsson ML, Risberg B. Plasminogen activators and inhibitors in peritoneal tissue. APMIS. 1997;105:25–30. doi: 10.1111/j.1699-0463.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 8.Molinas CR, Elkelani O, Campo R, Luttun A, Carmeliet P, Koninckx PR. Role of the plasminogen system in basal adhesion formation and carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80:184–192. doi: 10.1016/s0015-0282(03)00496-5. [DOI] [PubMed] [Google Scholar]
- 9.Buckenmaier CC, III, Summers MA, Hetz SP. Effect of the antiadhesive treatments, car-boxymethylcellulose combined with recombinant tissue plasminogen activator and Se-prafilm, on bowel anastomosis in the rat. Am Surg. 2000;66:1041–1045. [PubMed] [Google Scholar]
- 10.Doody KJ, Dunn RC, Buttram VC., Jr Recombinant tissue plasminogen activator reduces adhesion formation in a rabbit uterine horn model. Fertil Steril. 1989;51:509–512. doi: 10.1016/s0015-0282(16)60563-0. [DOI] [PubMed] [Google Scholar]
- 11.Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, Emeis JJ, Kooistra T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000;74:203–212. doi: 10.1016/s0015-0282(00)00656-7. [DOI] [PubMed] [Google Scholar]
- 12.Menzies D, Ellis H. The role of plasminogen activator in adhesion prevention. Surg Gynecol Obstet. 1991;172:362–366. [PubMed] [Google Scholar]
- 13.Vipond MN, Whawell SA, Scott-Coombes DM, Thompson JN, Dudley HA. Experimental adhesion prophylaxis with recombinant tissue plasminogen activator. Ann R Coll Surg Engl. 1994;76:412–415. [PMC free article] [PubMed] [Google Scholar]
- 14.Hellebrekers BW, Trimbos-Kemper TC, Boesten L, Jansen FW, Kolkman W, Trimbos JB, Press RR, van Poelgeest MI, Emeis SJ, Kooistra T. Preoperative predictors of postsurgical adhesion formation and the Prevention of Adhesions with Plasminogen Activator (PAPA-study): results of a clinical pilot study. Fertil Steril. 2009;91:1204–1214. doi: 10.1016/j.fertnstert.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 15.Atta HM, Al-Hendy A, El-Rehany MA, Dewerchin M. Adenovirus-mediated overexpression of human tissue plasminogen activator prevents peritoneal adhesion formation/reformation in rats. Surgery. 2009;146:12–17. doi: 10.1016/j.surg.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Hassan MH, Khatoon N, Curiel DT, Hamada FM, Arafa HM, Al-Hendy A. Toward gene therapy of uterine fibroids: targeting modified adenovirus to human leiomyoma cells. Hum Reprod. 2008;23:514–524. doi: 10.1093/humrep/dem410. [DOI] [PubMed] [Google Scholar]
- 17.Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763–768. doi: 10.1016/s0015-0282(00)01799-4. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–1631. doi: 10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Salama SA, Kamel M, Christman G, Wang HQ, Fouad HM, Al-Hendy A. Gene therapy of uterine leiomyoma: adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir treatment inhibits growth of human and rat leiomyoma cells in vitro and in a nude mouse model. Gynecol Obstet Invest. 2007;63:61–70. doi: 10.1159/000095627. [DOI] [PubMed] [Google Scholar]
- 20.Tsuruta Y, Pereboeva L, Glasgow JN, Luongo CL, Komarova S, Kawakami Y, Curiel DT. Reovirus σ1 fiber incorporated into adenovirus serotype 5 enhances infectivity via a CAR-independent pathway. Biochem Biophys Res Commun. 2005;335:205–214. doi: 10.1016/j.bbrc.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 21.Barker SD, Coolidge CJ, Kanerva A, et al. The secretory leukoprotease inhibitor (SLPI) promoter for ovarian cancer gene therapy. J Gene Med. 2003;5:300–310. doi: 10.1002/jgm.341. [DOI] [PubMed] [Google Scholar]
- 22.Zhu ZB, Makhija SK, Lu B, Wang M, Kaliberova L, Liu B, Rivera AA, Nettelbeck DM, Mahasreshti PJ, Leath CA, III, et al. Transcriptional targeting of tumors with a novel tumor-specific survivin promoter. Cancer Gene Ther. 2004b;11:256–262. doi: 10.1038/sj.cgt.7700679. [DOI] [PubMed] [Google Scholar]
- 23.Haviv YS, Curiel DT. Engineering regulatory elements for conditionally-replicative adenoviruses. Curr Gene Ther. 2003;3:357–385. doi: 10.2174/1566523034578311. [DOI] [PubMed] [Google Scholar]
- 24.Paupoo AA, Zhu ZB, Wang M, Rein DT, Starzinski-Powitz A, Curiel DT. A conditionally replicative adenovirus,CRAd-S-pK7, can target endometriosis with a cell-killing effect. Hum Reprod. 2010;25:2068–2083. doi: 10.1093/humrep/deq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]