Abstract
The dorsolateral prefrontal and the posterior parietal cortex have both been implicated in the guidance of visual attention. Traditionally, posterior parietal cortex has been thought to guide visual bottom-up attention, whereas prefrontal cortex to bias attention through top-down information. More recent studies suggest a parallel time course of activation of the two areas in bottom-up attention tasks, suggesting a common involvement, though these results do not necessarily imply identical roles, either. To address the specific roles of the two areas, we examined the influence of neuronal activity recorded from the prefrontal and parietal cortex of monkeys as they performed attention tasks based on choice probability and correlation between reaction time and neuronal activity. The results revealed that posterior parietal but not dorsolateral prefrontal activity correlated with behavioral choice during the fixation period, prior to the appearance of the stimulus, resembling a bias factor. This preferential influence of posterior parietal activity on behavior was transient, so that dorsolateral prefrontal activity predicted choice after the appearance of the stimulus. Additionally, reaction time was better predicted by posterior parietal activity. These findings confirm an involvement of both dorsolateral prefrontal and posterior parietal cortex in the bottom-up guidance of visual attention but indicate different roles of the two areas in the guidance of attention and a dynamic time course of their effects, influencing behavior at different stages of the task.
Keywords: monkey, neurophysiology, principal sulcus, intraparietal sulcus
INTRODUCTION
The guidance of visual attention in humans and non-human primates is thought to be controlled by a fronto-parietal network of brain areas including the dorsolateral prefrontal (dlPFC) and posterior parietal (PPC) cortex (Corbetta & Shulman, 2002; Schall, 2002; Bisley & Goldberg, 2010). PPC and dlPFC neurons share many properties, including large receptive fields, and greatly enhanced responses to attended over unattended stimuli (Schall & Hanes, 1993; Constantinidis & Steinmetz, 2001; Katsuki & Constantinidis, 2012b). Traditionally, PPC has been thought to be relatively more important in the processing of bottom-up information for the determination of visual saliency, whereas PFC has been thought of as the source of top-down information (Buschman & Miller, 2007; Ibos et al., 2013). This dichotomy has been challenged by some studies suggesting similar courses of activation in posterior parietal areas, such as the lateral intraparietal area (LIP) and area 7a, and prefrontal areas, such as area 46 and the frontal eye field (FEF) of dlPFC, in behavioral tasks requiring bottom-up attention (Thompson et al., 1996; Thomas & Pare, 2007; Katsuki & Constantinidis, 2012a; Purcell et al., 2013). A recent study revealed that dlPFC represents a stimulus that attracts attention by bottom-up factors alone no later than PPC even though the initial visual response latency of neurons was shorter in PPC than dlPFC (Katsuki & Constantinidis, 2012a).
These results suggest an early involvement of dlPFC in the representation of bottom-up saliency, raising the possibility that behavioral choices are shaped jointly by the activity in the two areas. Evidence in support of this view includes that activity of both PFC and PPC neurons can bias behavioral choice and performance in a motion discrimination task and visual search tasks (Thompson et al., 2005; Hanks et al., 2006; Heitz et al., 2010). However, parallel time courses of stimulus representation do not necessarily imply identical roles for the two areas in the guidance of visual attention. Distinct neurophysiological patterns of responses between dlPFC and PPC have been described with respect to the representation of distracting stimuli, with dlPFC being better able to filter distractors (Qi et al., 2010; Suzuki & Gottlieb, 2013). Different behavioral effects have also been demonstrated after reversible inactivation of each area, where inactivation of PFC affected both easy and difficult search performance while inactivation of PPC affected only difficult search performance (Wardak et al., 2004; Wardak et al., 2006). Activity in the two areas may still be specialized on different respects of guidance of attention.
We therefore tested whether behavior correlated with neuronal activity, equally for PPC and dlPFC. We analyzed neuronal activity from experiments that required the guidance of attention to salient stimuli defined by bottom-up factors, where dlPFC and PPC exhibit very similar time courses of activation. We then compared the influence of activity in areas 8 and 46 of dlPFC, and area LIP of PPC on behavioral choice and behavioral reaction time. Our results revealed that neuronal activity in each area influenced reaction time and behavioral choice to a different extent, in different task epochs.
METHODS
Two male, rhesus monkeys (Macaca mulatta) weighing 5–8 kg were used in this study. All surgical and animal-use procedures in this study followed guidelines by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Research Council’s Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee.
Surgery and neurophysiology
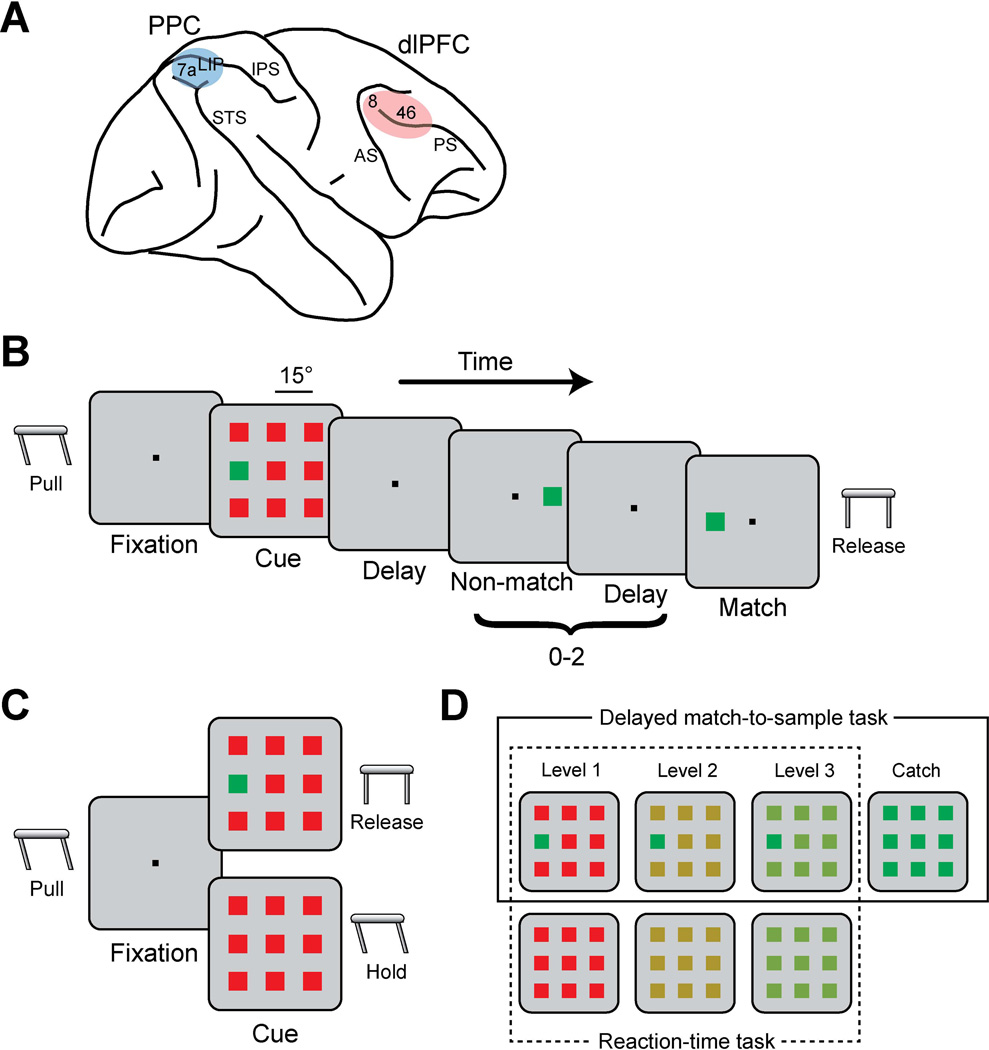
Two 20 mm diameter recording cylinders were implanted over dlPFC and PPC of the same hemisphere in each monkey (Fig. 1A). Extracellular activity of single units were recorded using arrays of 2–8 microelectrodes in each cylinder, either with glass-coated, tungsten electrodes (250 µm diameter, impedance of 1 MΩ at 1 kHz, Alpha-Omega Engineering, Nazareth, Israel) or epoxylite-coated tungsten electrodes (125 µm diameter, impedance of 4 MΩ at 1 KHz, FHC Bowdoin, ME). Electrodes were advanced individually into the cortex with a microdrive system (EPS drive, Alpha-Omega Engineering, Nazareth, Israel). The electrical signal from each electrode was amplified, band-pass filtered between 500 Hz and 8 kHz, and recorded with a modular data acquisition system at 25 µs resolution (APM system, FHC, Bowdoin, ME). The anatomical location of electrode penetration was confirmed with MR imaging of the brain obtained after implantation of the recording cylinders. In the prefrontal cortex, neuronal data were collected from areas 46 and 8a of the dlPFC including both banks of the principal sulcus, and the surface cortex dorsal to the principal sulcus and posterior but excluding the arcuate sulcus. In the posterior parietal cortex, recordings were obtained from the lateral bank of the intraparietal sulcus at depths >3 mm from the surface of the cortex excluding area 7a, which is located superficially.
Figure 1. Recording areas and behavioral tasks.
A. Schematic diagram of the monkey brain. Colored areas indicate the locations where recordings were performed. Abbreviations: AS, arcuate sulcus; IPS, intraparietal sulcus; PS, principal sulcus; STS, superior temporal sulcus. B. Delayed match-to-sample task. A salient stimulus was presented in the cue frame followed by 0–2 non-match frames and a match frame. Monkeys were required to release a lever after the match frame. C. Reaction-time task. The cue frame was presented with or without a target. The monkey was required to release the lever within 0.8 s of the cue onset if there was a target in the cue frame (Go trials) and continue to hold if there was no target (NoGo trials). D. Color variations of distractor stimuli for the difficult tasks when salient stimulus color was green. There were also color variations with red salient stimulus. Color variations in a box with solid line are for the delayed match-to-sample task, and the variations in a box with dotted line are for the reaction-time task (Top:Go trials, Bottom: NoGo trials).
Behavioral tasks
The monkeys faced a computer monitor 60 cm away in a dark room with their head fixed. Eye position was sampled at 240 Hz, digitized, and recorded with an infrared eye position tracking system (model RK-716; ISCAN, Burlington, MA). The visual stimulus presentation and behavior monitoring were controlled by in-house software (Meyer & Constantinidis, 2005) using the Psychophysics Toolbox (Brainard, 1997). The system was implemented in the MATLAB computational environment (Mathworks, Natick, MA).
Two different tasks were used in the present study: the delayed match-to-sample task (Fig. 1B) and the reaction-time task (Fig. 1C). In the delayed match-to-sample task, the monkeys were trained to locate a salient stimulus surrounded by distractors and to release a lever when a following stimulus appeared at the location of the salient stimulus (Fig. 1B). After the animals pulled a behavioral lever and fixated at a white fixation target (0.2° in size) located at the center of the monitor, the cue was displayed at the middle left or middle right position of a 3×3 grid. Distractor stimuli took up the other 8 locations of the grid, with a 15° separation between neighboring stimuli (diagonal stimuli appeared at an eccentricity of 21°). Stimuli were squares of 1.5° in size, and the cue stimulus (green/red) was rendered salient due to its difference in color from distractors. Four levels of difficulty were used by varying color similarity between the cue and distractors (Fig. 1D, solid line box): one level involved a green cue among red distractor stimuli or vice versa, two levels involved cue and distractors of intermediate levels of chromatic difference, and a fourth level involved distractor stimuli identical to the target, which constituted a “catch trial” that was rewarded randomly. The location of the stimulus and the colors of cue and distractors were randomly interleaved from trial to trial with equal probability so as to make it impossible for the monkeys to predict either the location or the identity of the salient stimulus. A trial consisted of a 0.5 s fixation period, a 0.5 s cue period, a 1.0 s delay period, a pseudorandom sequence of 0–2 non-match period each lasting 0.5 s and separated by delay periods of 0.5 s, and a 0.5 s match period in which the stimulus appeared at the same location as the cue. When the monkeys successfully held the lever until the match period and released the lever within 0.5 s after the match stimulus disappeared, they were rewarded with fruit juice. Release of the lever at any other time during the trial or breaking fixation exceeding a 2° window led to the immediate termination of the trial without reward.
In the reaction-time task (Fig. 1C), the monkeys were trained to release the lever as quickly as possible if a salient stimulus was present in the stimulus array (Go trial) and keep holding the lever if there was no salient stimulus (NoGo trial). The monkeys were rewarded if they successfully released the lever within 0.8 s after the stimuli presentation in the Go trials, or kept holding the lever longer than 0.8 s in the NoGo trials. The duration of the fixation period in this task varied randomly (0.5 s to 1.0 s) so that the monkeys were not able to time the lever release. For the standard version of the reaction-time task, a red target was presented among the green distractor stimuli (1.5° in size), and vice versa. For the difficult version of the reaction-time task, the color of the distractors varied in the same fashion as described for the delayed match-to-sample task (Fig.1D, dotted line box). This task did not involve catch trials – displays without a salient stimulus, by definition consisted NoGo trials. The size of each stimulus in an array was 4° in the difficult version of the reaction-time task.
Neuron selection
Recorded spike waveforms were sorted into separate units using an automated cluster analysis method referred to as the KlustaKwik algorithm (Harris et al., 2000), which applied principal component analysis of the waveforms. Neurons with significant elevation of firing rate during the presentation of visual stimuli were identified by comparing the firing rate in the 0.5 s (0.3 s in the reaction-time task) interval of a stimulus presentation with the 0.5 s interval of fixation (paired t-test; p<0.05). The spatial tuning of visually responsive neurons was determined by comparing the firing rates during the presentation of cue stimulus of either color (used level-1 difficulty) at the different locations. Neurons with spatial selectivity for the location of the single stimulus, evidenced by a significant main effect of stimulus location (2-way ANOVA; p<0.05), were included in analysis.
Time of target discrimination
Neuronal time of target discrimination was computed by comparing population firing rates of salient stimulus in receptive fields and distractor in receptive fields. Significance of firing rate difference was determined for 10 ms bin windows stepped by 1 ms (paired t-test, p<0.05). Target discrimination time was identified as the time point of the first of 10 consecutive bins with significantly greater responses to a salient stimulus than to distractors (Katsuki & Constantinidis, 2012a).
Choice probability analysis
In order to quantify the trial-to-trial association between perceptual choice and neuronal activity, we analyzed trials that resulted in correct choices and incorrect choices in the delayed match-to-sample task and the reaction-time task using the choice probability analysis based on signal detection theory (Britten et al., 1996). We first identified the stimulus location with the highest firing rate for each neuron. Firing rates of correct and error trials when the identical stimulus appeared at this location were pooled separately. A receiver operating characteristic (ROC) curve was computed from these two distributions of firing rates. The choice probability, a measurement of correlation between the behavioral choice and neuronal activity, was defined as the area under the ROC curve. A choice probability value of 1 indicates that there is a perfect correlation between the behavioral choices and the neuronal discharge rates; a value of 0.5 indicates a random correlation between the two. Time-resolved choice probabilities were computed from the spikes in 250 ms time windows, stepped by 50 ms intervals. The choice of bin size was dictated by the discharge rate of the population of neurons and number of trials available in each condition, particularly error trials. To obtain sufficient number of error trials and spikes to analyze, we only used the trials with most difficult stimulus level (Level-3 in Fig. 1D) and relied on neurons with at least 3 error trials for this condition. Only trials with lever errors during the match/non-match period in the delayed match-to-sample task and the trials with lever errors after the cue presentation in the reaction-time task were used as error trials. Error trials due to breaks in fixation, blinks, and releases of the lever before the offset of the stimulus (in the delayed match-to-sample task) were excluded. There were two types of error trials in the reaction-time task: miss trials in which the target was present (and should have been Go trials) but the monkeys did not release the lever, and false alarms in which the target was absent (and should have been NoGo trials) but the monkeys released the lever. We computed the choice probabilities for these error types separately: 1) correct detection of target in Go trials vs. miss trials, 2) false detection of target (false alarm) vs. correct rejection in NoGo trials. The choice probabilities were computed in the same fashion based on 0.3 s of the fixation period or 0.3 s of the cue period, in the reaction-time task. Choice probabilities were computed for each neuron and distributions of values across neurons were then compared for neurons recorded from PPC and dlPFC.
Fano factor
The variability of neuron’s firing rate across trials was estimated by the Fano factor, defined as the variance of spike counts divided by the mean. The Fano factor was computed based on the algorithm developed by Churchland and colleagues (Churchland et al., 2010). First, the variance and mean of the spike count were computed in each trial type, and then a regression of the variance to the mean was performed. The Fano factor reported here was the slope of this regression. Spike counts were computed in a 150 ms sliding window moving in 10 ms steps. The Fano factor was computed in three separate task periods in the delayed match-to-sample task, the fixation period (0.5 s), the cue period (0.5 s), and the delay period (1.0 s). We computed the Fano factor for correct and error trials separately for target in receptive field and target out receptive field conditions. Neurons with at least 5 trials per condition were used for this analysis.
Firing rate vs. reaction time
To evaluate the relationship between the trial-to-trial neuronal activity and behavioral reaction time, we computed a correlation coefficient between firing rate and reaction time using data from the standard version of the reaction-time task (Fig. 1C). Firing rate when the stimulus appeared at the best location for each neuron was calculated for each 100 ms window, sliding in 20 ms intervals for each trial. A correlation coefficient was computed for each bin between the firing rates and corresponding reaction times. A correlation coefficient was also calculated for the fixation period (0.3 s) or the cue period (0.3 s). A correlation value was determined thus for each neuron. The distributions of correlation values were then compared across areas.
RESULTS
Neurophysiological data were collected from areas 8 and 46 of the dlPFC and LIP of the PPC in two monkeys (Fig. 1A), while the animals were performing tasks requiring them to detect a salient stimulus in a visual display (Fig. 1B–C). We were particularly interested in the role of bottom up information in the guidance of attention, therefore the saliency of the target stimulus was achieved by virtue of color difference from surrounding (distractor) stimuli. The monkeys had no prior knowledge of the stimulus color or location in each trial, making the detection of the stimulus entirely defined by bottom-up factors. Additionally, since planning of eye movements is intricately connected with visual attention circuits (Kustov & Robinson, 1996; Moore & Fallah, 2001), we required monkeys to maintain fixation throughout the trial and signal the location or presence of the salient stimulus with the release of a lever, instead. Neural activity recorded during the task allowed us to test the correlation between neuronal activity in the two areas and salient stimulus detection, rather than execution of eye movements. A first set of experiments relied on spatial version of a delayed match-to-sample task, which required localization of the salient stimulus. A second set of experiments used a reaction-time variant of the task, requiring an immediate behavioral response after detection of the stimulus. The tasks allowed us to probe different aspects of the guidance of attention.
Choice probability in the delayed match-to-sample task
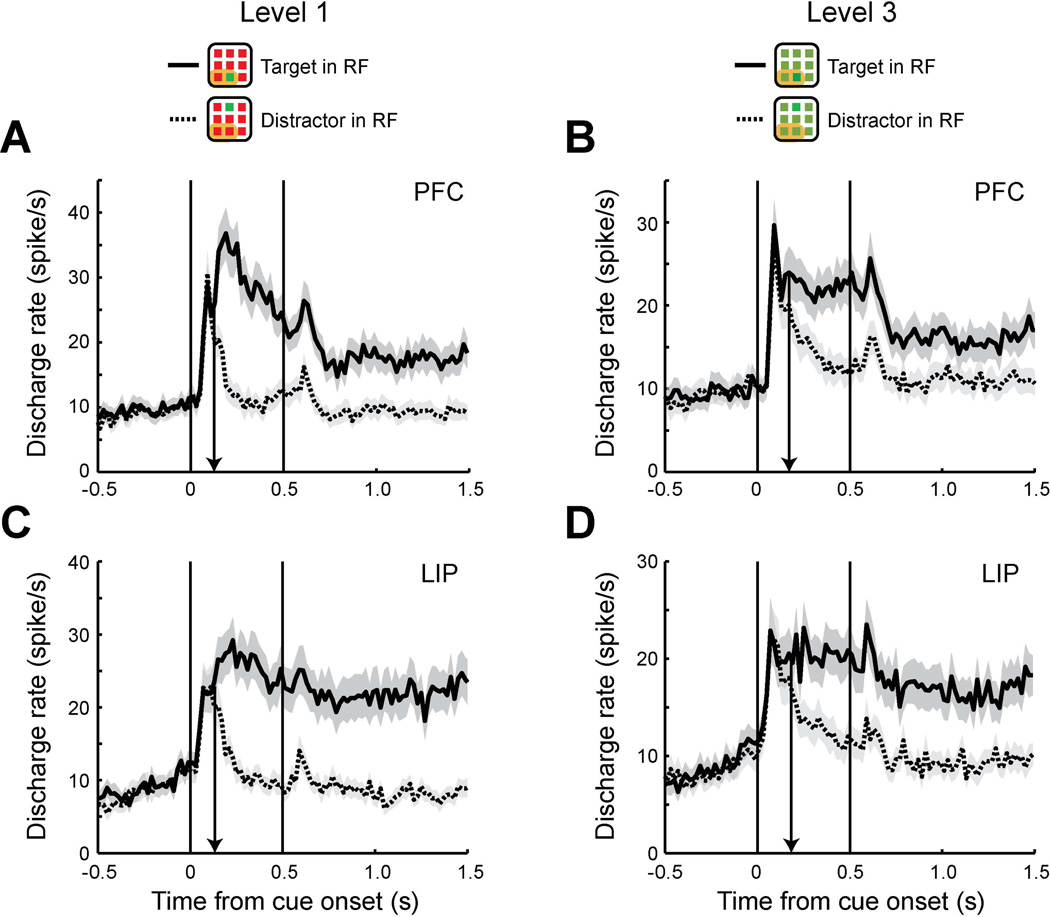
The first question we wished to address with respect to the influence of dlPFC and PPC on behavior was whether neuronal activity correlated with behavioral choices equally strongly in the two areas. We therefore analyzed data from a behavioral task which required monkeys to identify the location of a salient color stimulus in an array of stimuli and decide whether a subsequent single stimulus matched it in spatial location or not, by releasing a lever (delayed match-to-sample task, Fig. 1B). The task involved trials of four levels of increasing difficulty by adjusting the similarity of the distractor colors relative to the cue (Fig. 1D, solid box): One level of difficulty involved trials with a red distractor stimulus when the cue was green or vice versa; two levels of difficulty involved trials with intermediate levels of chromatic difference between cue and distractors; and a fourth level of difficulty involved trials with distractor stimuli identical to the target (catch trials), which were rewarded randomly. In order to have sufficient numbers of error trials, we only used trials of the third level of difficulty for this analysis. During the course of the experiments, we repeatedly alternated recording in dlPFC and LIP, and also obtained simultaneous recordings from the two areas (25% and 33% of sessions used in each area involved simultaneous recordings). As a consequence, an equivalent level of behavioral performance was obtained in the recording sessions from the two areas. There was no significant difference in behavioral performance between the sessions of the dlPFC and LIP recordings (57% and 55% for the level-3 trials, respectively, t-test, t32=0.63, p>0.5). We relied on neurons that had spatial selectivity for the location of the stimuli, whose discharge rate was therefore informative about the location of the salient stimuli, and with at least 3 error trials in the level-3 difficulty condition (Fig. 1D). A total of 63 neurons from dlPFC and 62 neurons from LIP satisfied these criteria and were used in this analysis. The time of target discrimination was computed for each area by comparing the responses to the salient stimulus in receptive field with distractors in receptive fields, using correct trials from stimulus presentations of difficulty level-1 (Fig. 2A, C) and 3 (Fig. 2B, D). Consistent with a previous study from our laboratory that reported an early involvement of the dlPFC in bottom-up attention (Katsuki & Constantinidis, 2012a), the times of target discrimination were similar in this sample of neurons too, and in fact slightly earlier in dlPFC than LIP, for both level-1 stimulus (126 ms after stimulus onset, in dlPFC, 133 ms in LIP) and level-3 stimulus (171 ms in dlPFC and 183 ms in LIP).
Figure 2. Time of target discrimination in delayed match-to-sample task.
A. Population peri-stimulus time histograms (PSTH) of dlPFC neurons (N=63). Average discharge rates are plotted for target in receptive field (RF) trials (solid line) and distractors in receptive field trials (dotted line) using correct trials of difficulty level-1. Shaded area along each plot represents standard error of the mean computed across neurons. A black arrow indicates the time of target discrimination. Receptive filed location is schematically depicted as an orange area in insets (receptive field location differed across neurons). Although green targets are used in the insets for illustration purpose, trials with either red or green color scheme are included in the plots. Plotting bin size is 20 ms. B. Population PSTH of LIP neurons (N=62) using correct trials of difficulty level-1. C. Population PSTH of dlPFC using the same neurons as A, but using correct trials of difficulty level-3. D. Population PSTH of LIP using the same neurons as B with correct trials of difficulty level-3.
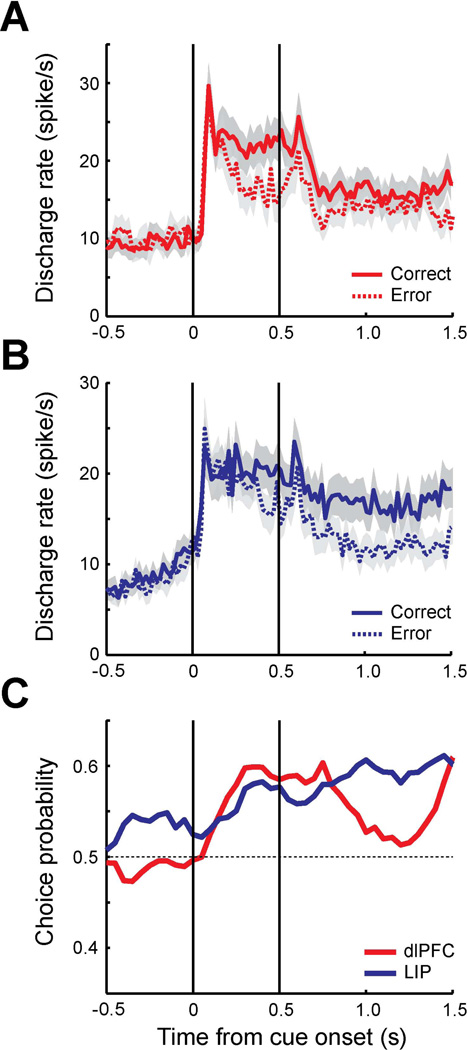
Behavioral outcomes were categorized into two groups, corresponding to correct and error trials. Only trials with lever errors following the match or non-match periods were identified as error trials for this analysis; errors due to breaks in fixation at any point, or releases of the lever before the offset of the stimulus were excluded from analysis. Average firing rates of correct trials (dlPFC: 1140 trials, LIP: 1208 trials) and error trials (dlPFC: 525 trials, LIP: 832 trials) were plotted separately for each area (Fig. 3A–B). On average, the firing rates of error trials were lower than the correct trials in both dlPFC and LIP. To quantify the relationship between behavioral choices and neuronal responses, we performed a receiver operating characteristic (ROC) analysis to compute the probability of distinguishing between the distributions of error and correct trials, involving identical stimulus conditions, a quantity also known as choice probability (Britten et al., 1996), based on signal detection theory (see methods). The area under the ROC curve using the firing rate of correct trials and error trials represents the choice probability for each neuron. The choice probability was computed in a time-resolved fashion, in 250 ms windows, sliding in 50 ms intervals (Fig. 3C).
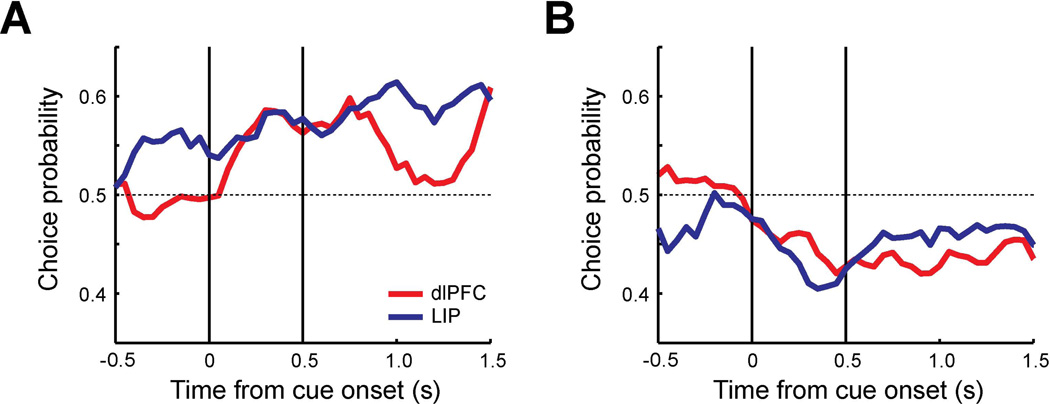
Figure 3. Choice probability of trials with target in receptive field in delayed match-to-sample task.
A. Population PSTH of dlPFC neurons (N=63). Average discharge rates are plotted for correct trials (solid line, 1140 trials) and error trials (dotted line, 525 trials) in which targets were presented in the receptive field. Shaded area along each plot represents standard error of the mean computed across neurons. The vertical lines represent the time of cue onset and offset. Bin size is 20 ms. B. Population PSTH of LIP neurons (N=62) for correct trials (1208 trials) and error trials (832 trials). C. Population choice probability of dlPFC (red) and LIP (blue). Average area under the ROC curve was computed as a choice probability using correct trials and error trials (250 ms time window stepped by every 50 ms).
The average dlPFC choice probability was significantly different from 0.5 for the cue and delay period (t-test, Cue: t62=5,15, p<10−5, Delay: t62=4.25, p<10−4), while significantly higher LIP choice probability than 0.5 was observed in all three task epochs (t-test, Fixation: t61=3.91, p<0.001, Cue: t61=5.31, p<10−5, Delay: t61=7.05, p<10−8). A significant difference was present between areas in terms of choice probability. The average LIP choice probability during the fixation period was significantly different from the dlPFC choice probability (t-test, t123=−3.96, p<0.001). This suggests that ongoing LIP activity even before the stimulus array is presented was more likely to influence the outcome of the behavioral trial. No significant difference was apparent during the stimulus presentation interval (t-test, t123=0.78, p>0.4), although we saw a trend towards higher dlPFC values after ∼150 ms, at the time interval when a significant difference between salient stimulus and distractors emerges in both areas. A higher choice probability in LIP neurons compared to dlPFC neurons was also observed in the second 0.5 s of the delay period (t-test, t123=−3.09, p<0.01). The results indicate that higher firing rate of LIP neurons during the fixation and the delay period is more likely to result in correct performance of the task involving discrimination of a salient stimulus, when it appears in the neuron’s preferred location.
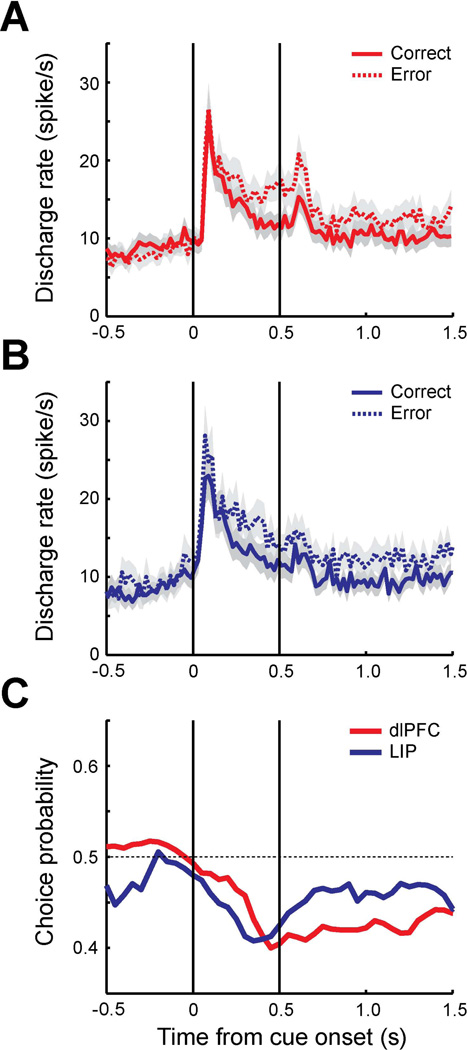
The analysis presented so far was performed with trials in which a salient stimulus appeared in neurons’ preferred location which is characterized by a greater neural response to the salient stimulus compared to the distractors. Suppression of responses to non-target stimuli could also be an important factor to detect the salient stimulus correctly. To further investigate how response to distractors affects behavioral choice, we conducted an analysis of trials in which a distractor appeared in the neuron’s receptive field instead of the salient stimulus (Fig. 4). A total of 73 neurons from dlPFC and 57 neurons from LIP were used in this analysis. In contrast to the trials with the salient stimulus in receptive filed, the firing rate of trials with the distractor in the receptive field (dlPFC: 1243 trials, LIP: 665 trials) tended to be higher in error than in correct trials (dlPFC: 1341 trials, LIP: 1108 trials); this was true for both areas (Fig. 4 A–B). Choice probability was now generally lower than 0.5; it was significantly different from 0.5 for both dlPFC and LIP during the cue (t-test, PFC: t72=−4.89, p<10−5, LIP: t56=−4.63, p<10−4) and delay period (t-test, PFC: t72=−7.38, p<10−9, LIP: t56=−2.62, p<0.05). A difference between dlPFC and LIP in the average choice probability was again present during the fixation (t-test, t128=2.04, p<0.05) and the first 0.5 s of the delay period (t-test, t128=−2.24, p<0.05). Similar to the condition of the salient stimulus in the receptive field, LIP activity during the fixation period correlated more strongly with behavioral choice than the equivalent activity in dlPFC, though in this condition (when distractors appeared in the receptive field) elevated LIP activity during the fixation period was associated with a higher probability of an erroneous report. Elevated activity in dlPFC during the delay period affected the behavioral outcome more than LIP activity, again being associated with an error, when the distractor was in the receptive field. The results suggest that lower LIP responses during the fixation period and lower dlPFC responses during the delay period tend to lead to correct performance in discrimination of a salient stimulus when the distractor was in the receptive field instead of the salient stimulus.
Figure 4. Choice probability of trials with distractor in receptive field in delayed match-to-sample task.
A. Population PSTH of dlPFC neurons (N=73). Average discharge rates are plotted for correct trials (solid line, 1341 trials) and error trials (dotted line, 1243 trials) in which distractors were presented in the receptive field. Shaded area along each plot represents standard error of the mean computed across neurons. The vertical lines represent the time of cue onset and offset. Bin size is 20 ms. B. Population PSTH of LIP neurons (N=57) for correct trials (1108 trials) and error trials (665 trials). C. Population choice probability of dlPFC (red) and LIP (blue).
To ensure that the differences in choice probability that we observed in these experiments was not the result of differential color selectivity in the two areas, we repeated the analysis after excluding neurons exhibiting significant color selectivity concurrently with spatial selectivity (p<0.05 in 2-way ANOVA test, using spatial location and color as factors). This possibility seemed unlikely from the outset, because comparable percentages of neurons exhibited significant selectivity for the color of our stimuli in LIP and dlPFC (12% and 13%, respectively) and because the choice probability analysis pools trials with the salient stimulus of either color, together. Nonetheless, when we only analyzed non color selective neurons (PFC: N=48, LIP: N=50), the choice probability was still significantly different between areas during the fixation (t-test, t96=−4.63, p<0.0001) and the second 0.5 s delay periods (t-test, t96=−2.85, p<0.01) for the target in receptive field trials (Fig. 5A). Similar trends were observed for trials involving the distractor appearing in the receptive field in the sample of non-color selective neurons (compare Fig. 5B with 4C), though differences between areas failed to reach statistical significance in this smaller sample.
Figure 5. Choice probability of neurons with different selectivity in delayed match-to-sample task.
A–B. Population choice probability of dlPFC (red, N=48) and LIP (blue, N=50) neurons excluding neurons with color selectivity. A. Targets in the receptive filed condition. B. Distractors in the receptive field condition.
Neuronal variability in the two areas
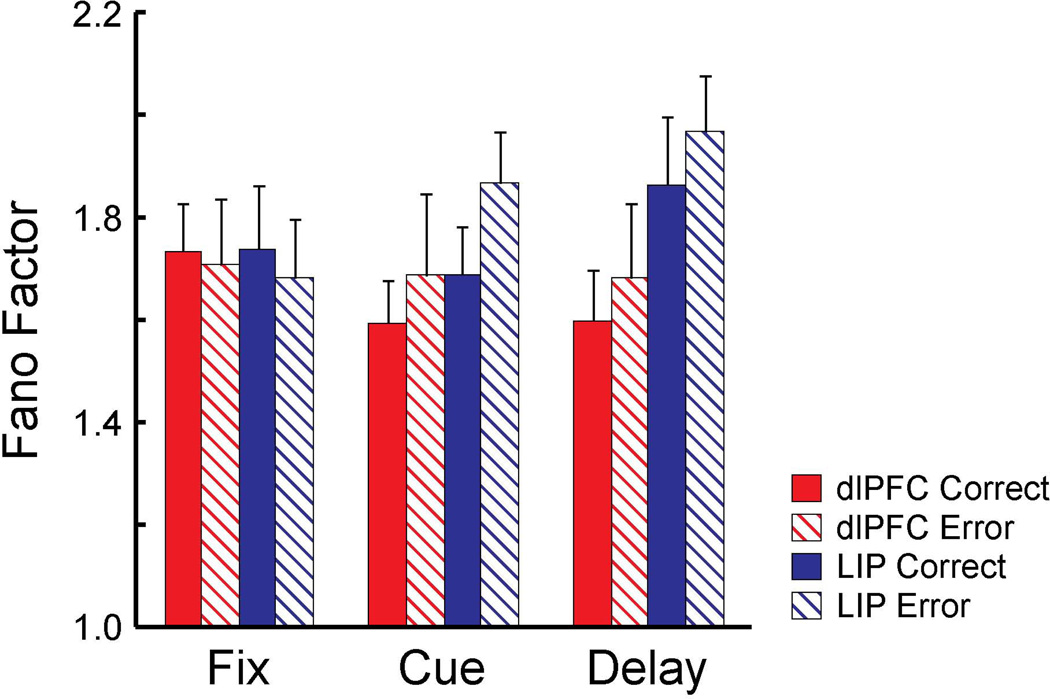
The differential contribution of two areas to the behavioral choice could be possibly attributed to a difference in neuron’s response variability between areas. To investigate this possibility, we computed the Fano factor of a neuron’s spike counts during the task, defined as the variance divided by the mean (Churchland et al., 2010). The Fano factor was estimated in separate task periods in the delayed match-to-sample task, including the fixation period (0.5 s), the cue period (0.5 s), and the delay period (1.0 s) for correct and error trials with the target in receptive field. The analysis was performed on neurons with at least 5 trials per condition in the difficulty level-3. The average Fano factor was generally lower for correct trials compared to error trials during the cue period and the delay period in both dlPFC (Fig. 6A, N=60) and LIP (Fig. 6B, N=62) although there was no significant main effects of correct vs. error or task epoch in either area (2-way ANOVA, PFC: F1, 354=0.28, p>0.5 for correct/error, F2, 354=0.28, p>0.7 for epoch, LIP: F1, 366=0.64, p>0.4 for correct/error, F2, 366=1.67, p>0.1 for epoch). We also performed 2-way ANOVA separately for correct and error conditions using area and task epoch as main factors. No significant main effects of area and task epoch were found in either correct or error condition (2-way ANOVA, Correct: F1, 360=2.04, p>0.1 for area, F2, 360=0.52, p>0.5 for epoch, Error: F1, 360=1.9, p>0.1 for area, F2, 360=0.54, p>0.5 for epoch). The results indicate that the Fano factor was equivalent in the two areas and the different contribution of the two areas on behavioral choice could not be accounted for by a difference in response variability between areas.
Figure 6. Fano factor of correct and error trials in delayed match-to-sample task.
Fano factor was estimated for separate task periods in delayed match-to-sample task for dlPFC (red, N=60) and LIP (blue, N=62) using correct (solid) and error (striped) trials. Spikes counted were computed in a 150 ms sliding window moving in 10 ms steps.
Choice probability in the reaction-time task
Analysis of choice probability in the delayed match-to-sample task revealed systematic differences between the effects of neuronal activity in each area on behavior, however the nature of errors in this task could involve multiple factors. Since the monkeys were only allowed to make behavioral responses after a delay and a subsequent match/non-match stimulus presentation, error responses could be caused by a target discrimination failure, or failure to maintain the location of the salient stimulus in memory. To test more directly whether the relationship between neuronal activity and detection of the salient stimulus differed in the parietal and prefrontal cortex, we analyzed choice probability in a reaction-time version of the task (Fig. 1C). In this task variant, the monkeys were trained to report the presence or absence of the salient stimulus as soon as the stimulus array was presented. When the salient stimulus was present (Go trials), the animals were required to release the lever as fast as possible to receive a reward. When the salient stimulus was absent (NoGo trials), the monkeys were required to keep holding the lever. A reward was delivered after 0.8 s of continuing to hold the lever, in this case. Analysis of choice probability in this task allowed us therefore to determine the influence of neuronal activity in detecting the salient target, per se.
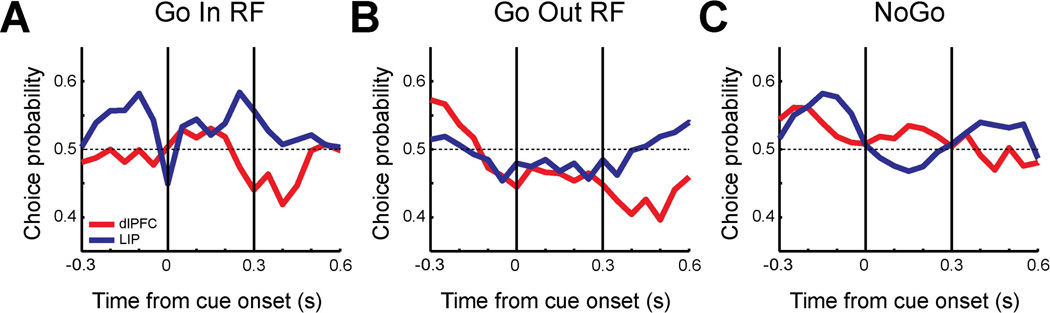
This task had three difficulty levels using the same color scheme as the delayed match-to-sample task (Fig. 1D, dotted box). Error trials were categorized into two groups: 1) miss trials in which the monkeys did not release the lever when the salient stimulus was presented (that should have been Go trials), 2) false alarm trials in which the monkeys falsely reported the presence of the salient stimulus when it was not presented (that should have been NoGo trials). We again identified neurons with at least 3 error trials per condition, resulting in a total of 17 dlPFC neurons and 14 LIP neurons that were used for this analysis. Behavioral performance in the sessions of the dlPFC and LIP recordings was not significantly different (61% and 57% for the level-3 trials, respectively, t-test, t12=1.80, p>0.09). Choice probability was computed using trials of the most difficult levels (level-3) with at least three error trials. Time-resolved choice probabilities were computed for Go trials when the salient stimulus appeared in the neuron’s preferred location (correct detections vs. miss trials). Choice probabilities were computed separately for all NoGo trials pooled together (based on false alarms vs. correct rejections).
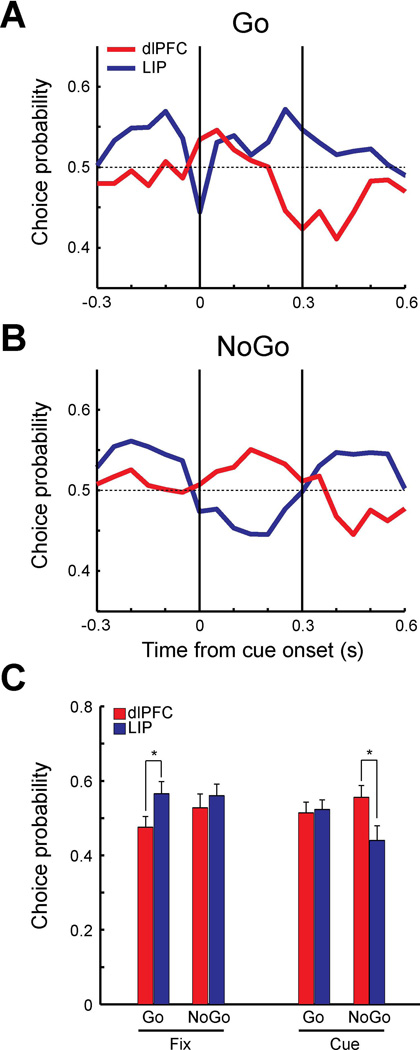
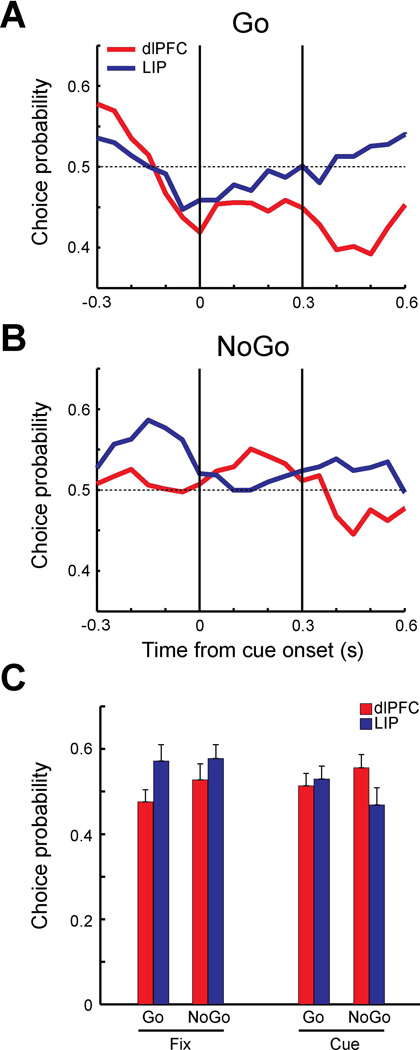
For both types of Go and NoGo trials, the choice probabilities of LIP neurons during the fixation period (−0.3 to 0 s) were higher than the dlPFC values (Fig. 7A–B), as was the case in the delayed match-to-sample task. The choice probability of LIP and dlPFC fluctuated somewhat in NoGo trials (Fig. 7B), however no period had a value significantly different from 0.5 (t-test, p>0.05 for all comparisons). Statistical significance was reached between areas during the fixation period in the Go condition (Fig. 7A,C; t-test, t29=−2.07, p<0.05). During the cue presentation period, choice probabilities of dlPFC neurons increased in both Go and NoGo trials. The difference between dlPFC and LIP during the cue presentation (0 to 0.3 s) in NoGo trials was significant (Fig. 7C, t-test, t29=0.14, p<0.05). The results indicate that when the firing rate of LIP neurons during the fixation period was higher, monkeys were more likely to report detecting the salient stimulus, either correctly or falsely. On the other hand, when the firing rate of dlPFC neurons to the stimulus in the receptive field was higher during the cue presentation, monkeys were more likely to falsely detect the stimulus as the salient stimulus. We repeated this analysis on trials in which the salient stimulus appeared out of the receptive field and distractors appeared in the neuron’s preferred location (Fig. 8). A total of 17 neurons from dlPFC and 14 neurons from LIP were used. The pattern of responses during the Go trials (Fig. 8A) was reminiscent of the effect we observed in the delayed match-to-sample task (Fig. 4C), with choice probabilities dipping below 0.5 for both areas, though no difference between areas reached statistical significance in this sample.
Figure 7. Choice probability in trials with target in receptive field in the reaction-time task.
A. Population choice probability of Go trials for dlPFC (red, N=17) and LIP (blue, N=14) when targets were presented in the receptive field. Choice probability was computed for each 250 ms time window stepped by every 50 ms using correct go trials (dlPFC: 160 trials, LIP: 140 trials) vs. miss trials (dlPFC: 175 trials, LIP:219 trials). Vertical lines indicate cue onset time and approximate duration of cue presentation. B. Population choice probability of NoGo trials using false alarm trials (dlPFC 106 trials, LIP: 74 trials) vs. correct NoGo trials (dlPFC: 320 trials, LIP: 280 trials). C. Population choice probabilities during 300 ms of fixation period and 300 ms of cue period (dlPFC: red, LIP: blue). Average choice probabilities were plotted separately for Go trials and NoGo trials for each period. Asterisks represent statistically significant differences (t-test, p<0.05).
Figure 8. Choice probability of trials with distractor in receptive field in reaction-time task.
A. Population choice probability of Go trials for dlPFC (red, N=17) and LIP (blue, N=14) in which distractors were presented in the receptive field. Choice probability was computed using correct go trials (dlPFC: 160 trials, LIP: 140 trials) vs. miss trials (dlPFC: 134 trials, LIP:188 trials). Vertical lines indicate cue onset time and approximate duration of cue presentation. B. Population choice probability of NoGo trials using false alarm trials (dlPFC 106 trials, LIP: 86 trials) vs. correct NoGo trials (dlPFC: 320 trials, LIP: 280 trials). C. Population choice probabilities during 300 ms of fixation period and 300 ms of cue period (dlPFC: red, LIP: blue). Average choice probabilities were plotted separately for Go trials and NoGo trials for each period.
To ensure again that the effect of neuronal responses to behavior was not associated with selectivity for color, we repeated our analysis on the sample of neurons without significant (2-way ANOVA, p<0.05) color selectivity (Fig. 9A–C). Analysis of this sample (dlPFC: N=15, LIP: N=12) produced very similar results as those shown in Figure 6 and Figure 7. For the Go trials with target in receptive field, there was a significant difference between areas during the fixation period (Fig. 9A, t-test, t25=−2.13, p<0.05). No significant difference between areas was observed in the Go trials with distractor in receptive field (Fig. 9B) or Nogo trials (Fig. 9C).
Figure 9. Choice probability of neurons with different selectivity in reaction-time task.
A–C. Population choice probability of dlPFC (red, N=15) and LIP (blue, N=12) neurons excluding neurons with color selectivity. Choice probabilities are plotted separately for A. Go trials with targets in the receptive filed, B. Go trials with distractors in the receptive field, and C. NoGo trials (no target).
Firing rate and behavioral reaction time
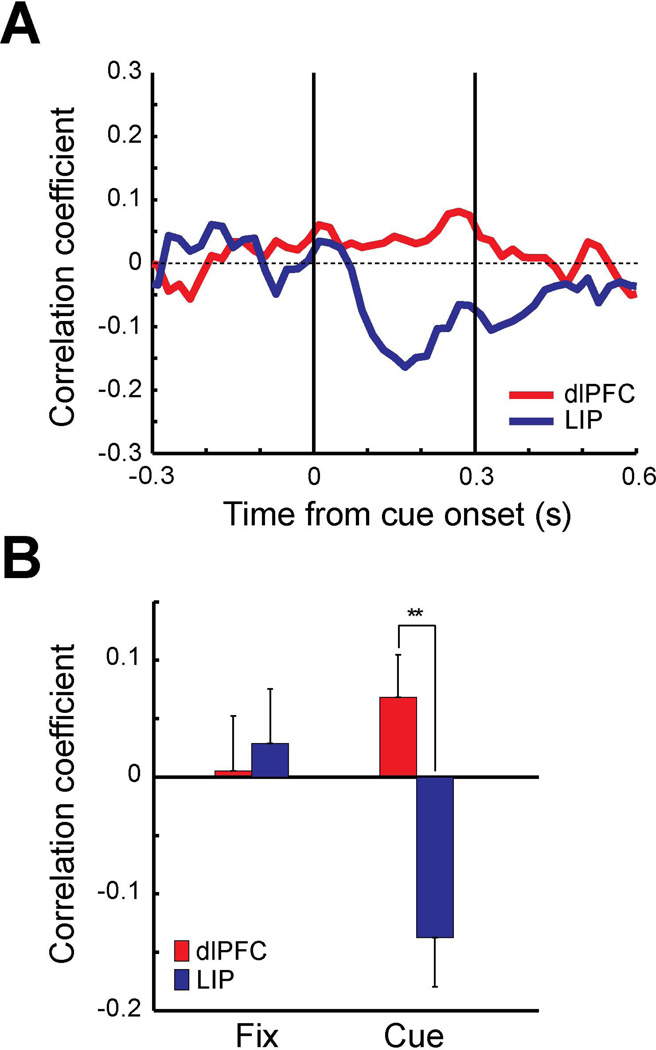
The influence of neuronal firing on behavioral outcomes is not limited only to choice probability; cortical firing rate is also known to determine the speed of responses (Hanes & Schall, 1996). The reaction-time version of our task provided information of how fast the monkey released the lever in response to detecting a salient stimulus. We were therefore able to compare the relationship between firing rate in dlPFC and PPC, and behavioral reaction time. Neuronal activity and behavioral reaction time (lever releasing time) were recorded while the monkey was performing the standard reaction-time task (Fig. 1C). For each neuron, firing rate was calculated in successive 100 ms windows, sliding by 20 ms, for trials in which the salient stimulus appeared at the neuron’s preferred location. The correlation coefficient was calculated using the firing rates and the corresponding behavioral reaction times for each neuron. A total of 42 neurons from dlPFC and 36 neurons from LIP were used for this analysis. Similar neuronal times of target discrimination were observed in the two areas areas (dlPFC: 107 ms, LIP: 105 ms).
Average correlation coefficient values were lower (more negative) for LIP neurons than dlPFC neurons throughout the cue presentation period (Fig. 10A), indicating that higher firing rate in LIP was more predictive of faster reaction times in the task. Correlation coefficients were also computed for the 300 ms of the fixation period (−300 to 0 ms from the cue onset) and the 300 ms of the cue period. LIP correlation coefficient of the cue period was significantly different from zero (Fig. 10B, t-test, t35=−3.24, p<0.01). No significant correlation was found in the fixation period of either area and the cue period of dlPFC. The difference between dlPFC and LIP was found to be significant in the cue period (Fig. 10B, t-test, t76=3.71, p<0.001). The results indicate that correlation between the neuronal activity and the behavioral reaction time is stronger in PPC than dlPFC. We computed Fano factors for the neurons used for this analysis and found that neuronal response variability was again not significantly different between areas and task epochs (2-way ANOVA, F1, 152=2.25, p>0.05 for area, F1, 152=0.01, p>0.9 for task epoch).
Figure 10. Firing rate vs. behavioral reaction time.
A. Average correlation coefficient of firing rates vs. behavioral reaction time for dlPFC (red, N=42) and LIP (blue, N=36). Firing rate was computed for each 100 ms window sliding 20 ms of each trial. Correlation coefficient was calculated for each neuron for each bin using the computed firing rates and corresponding behavioral reaction time of the trials. B. Average correlation coefficient computed for 300 ms of fixation period and 300 ms of cue period. Double asterisks represent statistically significant differences (t-test, p<0.01). Error bars are standard error of the mean computed across neurons.
DISCUSSION
Our study investigated the relationship between firing rate and behavioral choice in two cortical areas implicated in the guidance of visual attention. We analyzed data from two different tasks requiring localization of a visual stimulus based on bottom-up factors. Neurons in both dlPFC and LIP are activated by these tasks and demonstrate similar time courses of activation (Katsuki & Constantinidis, 2012a). Firing rate differences between target and distractors become smaller, and the time of target discrimination occurs later, in both areas, as the distance of target and distractors increases across the dimension we varied (color), similar to the effects reported by experiments comparing responses to target and distractors from neurons at different distances between the stimuli (Lennert & Martinez-Trujillo, 2011). Despite these similarities in response characteristics in LIP and dlPFC, our results reveal three main differences in the role of the two areas. First, LIP activity was critical prior to the appearance of the stimulus, correlating significantly with the monkey’s decision regarding the presence of a salient stimulus. Second, this preferential influence of LIP activity on behavior was transient; dlPFC activity predicted behavioral later in the trial, after the stimulus appearance. Third, only neuronal activity in area LIP predicted reaction time in the task. These differences were not due to variability of responses in the two areas; comparable Fano factor values were observed in the two areas and similar modulation by task epochs and errors.
Activation in bottom-up attention tasks
Visual attention can be oriented to stimuli based either on their physical distinctiveness (bottom-up selection, based on salience) or their behavioral relevance (top-down selection) based on prior information, expectations, and goals. Selective neural representation of visual stimuli based on their bottom-up saliency, in the form of enhanced responses to stimuli that pop-out and reduction of responses to background elements, is observed among multiple visual cortical areas including early stages of cortical hierarchy such as V1 and the later stages such as LIP and FEF (Knierim & van Essen, 1992; Schall & Hanes, 1993; Gottlieb et al., 1998). In order to identify the most salient stimulus in the visual field and guide bottom-up attention efficiently, it is critical to be able to integrate all types of information in the visual field as fast as possible into a map of global saliency (Koch & Ullman, 1985; Niebur & Koch, 1996). Combining both bottom-up and top-down factors, a global priority map in the brain is thought to play a role in integrating separate streams of visual information and orienting attention (Serences & Yantis, 2006; Bisley & Goldberg, 2010). So far, several different brain areas such as LIP and 7a of the posterior parietal cortex (Gottlieb et al., 1998; Constantinidis & Steinmetz, 2001), FEF, 8, and 46 of the prefrontal cortex (Schall & Hanes, 1993; Katsuki & Constantinidis, 2012a), and the superior colliculus (McPeek & Keller, 2002) are hypothesized to represent saliency/priority maps. Anatomically, these areas are interconnected with each other (Segraves & Goldberg, 1987; Cavada & Goldman-Rakic, 1989b; Felleman & Van Essen, 1991; Schall et al., 1995; Stanton et al., 1995; Pare & Wurtz, 1997) and receive projections from many visual cortical areas (Cavada & Goldman-Rakic, 1989a; Morel & Bullier, 1990; Schall et al., 1995; Lock et al., 2003). Comparisons of neuronal responses between areas indicate that a pop-out visual stimulus in the receptive field is discriminated from the background stimuli in the neuronal activity of the frontal areas (FEF, area 46) and posterior parietal areas (LIP) at comparable timing (Thompson et al., 1996; Thomas & Pare, 2007; Katsuki & Constantinidis, 2012a). Thus, representation of visual salience in these areas could be processed in parallel and may contribute to attention deployment and following behavioral responses differently.
Specialization of function
A number of studies have suggested that activity of neurons in PFC, PPC, and the superior colliculus influences behavioral choice, through accumulation of sensory evidence over time (Burman & Bruce, 1997; Schall & Thompson, 1999; Carello & Krauzlis, 2004; Hanks et al., 2006; Purcell et al., 2010). Microstimulation of LIP neurons indeed biases monkeys’ behavioral choices and their reaction times in a motion discrimination task (Hanks et al., 2006). Stronger responses to a distractor instead of a target in FEF neurons also correlate with behavioral response errors in visual search tasks (Thompson et al., 2005; Heitz et al., 2010). Although multiple brain areas might represent the selection of targets that could affect behavioral choice, the contribution of each area to the generation of movement may not be the same. Potential functional differences between the two areas can be distinguished into three (non-mutually exclusive) categories that have inspired corresponding views about the nature of functional differentiation between the two areas (reviewed by Katsuki and Constantinidis, 2012b). First, PFC can be thought of an output area that translates the outcome of cognitive operations performed largely in the parietal lobe into motor plans and shifts of attention. Neural activity related to movement preparation appears earlier in the PPC compared to PFC (Snyder et al., 1997; Cui & Andersen, 2007); microstimulation of prefrontal areas is more potent in generating eye movements than in LIP where saccades also appear with longer latency (Shibutani et al., 1984; Bruce et al., 1985). Second, the two brain areas may be uniquely specialized for different types of cognitive operations, such as categorization (Goodwin et al., 2012; Swaminathan & Freedman, 2012; Crowe et al., 2013), and filtering of distractors when information is held in working memory (Qi et al., 2010; Suzuki & Gottlieb, 2013), so that there is a division of labor in terms of cognitive operations between them. Third, the fundamental difference between the two areas may be that PFC has a supreme ability for plasticity which is essential for flexible behavior depending on context, a critical role illustrated by the effects of prefrontal lesions (Rossi et al., 2007; Buckley et al., 2009).
In the context of attention, differences we report here are consistent with the second view, revealing distinct roles of the two areas. The firing rate of both LIP and dlPFC was lower in error than correct trials when a salient stimulus was in the receptive field and was higher in error than correct trials when a distractor was in the receptive field (Fig. 3–Fig. 4). Furthermore, the activity of individual neurons in the two areas co-varied significantly with the behavioral report of the animal regarding the presence or absence of a distractor. However, the average choice probability, which was used as a measure of the ability of neurons in each area to influence the monkey’s decisions, varied systematically between the two areas, providing insights on their discrete roles. We identified three main effects in the relationship between neuronal activity and behavior.
First, we found that the monkey’s detection of a stimulus that was difficult to discriminate correlated significantly with LIP but not dlPFC neuronal activity during the fixation period. The difference in choice probability between areas was robust in that respect and was observed in multiple experimental conditions: A higher discharge rate of LIP neurons with receptive fields where the eventual target stimulus would appear led to a higher probability of correct detection (Fig. 3C), whereas a higher discharge rate of neurons with receptive fields away from the target, was associated with a higher probability of an error (Fig. 4C). The effect was present both in the delayed match-to-sample (Fig. 3–Fig. 4) and reaction time version of the task (Fig. 7A). This influence of firing rate prior to the appearance of a stimulus on the eventual behavioral choice is presumably the result of random fluctuation in firing rate from trial to trial, prior to any stimulus information, similar to a bias factor. This neural correlate of a decision bias has been described in area LIP before, in the context of other tasks (Shadlen & Newsome, 2001). Our present results suggest that the effect is specific for LIP and not present in dlPFC, even though the latter area is strongly responding to the task and represents the target stimuli.
Secondly, we found that this preferential correlation of area LIP activity with behavior was not present throughout the trial, but that dlPFC activity began to exert significant influence on behavioral choice during the cue presentation (as did activity in area LIP). When the stimulus appeared in the receptive field, higher rates of PFC neurons were more likely to be associated with correct detection of the salient stimulus (Fig. 3C). No significant choice probability was found, for either dlPFC or LIP, in the condition involving presentation of the distractor in the receptive field. This result is similar to the choice probability of middle temporal (MT) neurons, which is greater than chance for the neurons’ preferred direction of motion, while it remains around chance level for a non-preferred direction (Bosking & Maunsell, 2011). A significantly higher correlation of dlPFC compared to LIP activity on behavioral choice during the stimulus presentation was also detected in the NoGo condition of the reaction-time task, (Fig. 7C).
Finally, we observed that reaction time was determined primarily by neuronal activity in area LIP; a significant negative correlation between firing rate and reaction time was present only for LIP neurons (Fig. 10). Previous studies have revealed a similar relationship between neuronal firing rate and reaction time for the FEF (Hanes & Schall, 1996). Our results suggest that this is not present for dlPFC, even though robust neuronal responses were elicited in this area, in the reaction time version of our task.
In an attempt to gain further insight on the differential effects of neuronal activity on behavior, we compared the variability of neuronal responses in the two areas. In principle, lower variability of neuronal responses (e.g. in area LIP during the fixation period) may be associated with higher influence on behavioral choice. Our analysis revealed levels of Fano factor in line with prior reports (Qi & Constantinidis, 2012; Wimmer et al., 2014), but no difference between areas. Taken together, these results show that the representation of salient stimulus information in the posterior parietal and prefrontal cortex should not be viewed as redundant, with the two areas performing identical functions, and producing the same outputs. Instead, our results suggest that the output of neuronal activity in the parietal and frontal lobe can be dynamically routed to downstream targets and motor effectors during the task, and that the two areas are specialized in terms of their influence on behavior.
ACKNOWLEDGEMENTS
Research reported in this paper was supported by the National Eye Institute of the National Institutes of Health under award numbers R01 EY16773 and T32 NS073553, by the Tab Williams Family Endowment Fund, and by the Harry O’Parker Neurosciences Fund. We wish to thank Kathini Palaninathan for technical help.
Footnotes
AUTHOR CONTRIBUTIONS
F.K. and C.C. designed experiments; F.K. and M.S. performed experiments; F.K. and C.C. analyzed the data and wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosking WH, Maunsell JH. Effects of stimulus direction on the correlation between behavior and single units in area MT during a motion detection task. J Neurosci. 2011;31:8230–8238. doi: 10.1523/JNEUROSCI.0126-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis. Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- Burman DD, Bruce CJ. Suppression of task-related saccades by electrical stimulation in the primate’s frontal eye field. J. Neurophysiol. 1997;77:2252–2267. doi: 10.1152/jn.1997.77.5.2252. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 1989a;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J. Comp. Neurol. 1989b;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, Bradley DC, Smith MA, Kohn A, Movshon JA, Armstrong KM, Moore T, Chang SW, Snyder LH, Lisberger SG, Priebe NJ, Finn IM, Ferster D, Ryu SI, Santhanam G, Sahani M, Shenoy KV. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci. 2010;13:369–378. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple stimulus displays: I. Neurons encode the location of the salient stimulus. Cereb. Cortex. 2001;11:581–591. doi: 10.1093/cercor/11.7.581. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crowe DA, Goodwin SJ, Blackman RK, Sakellaridi S, Sponheim SR, Macdonald AW, 3rd, Chafee MV. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci. 2013;16:1484–1491. doi: 10.1038/nn.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Andersen RA. Posterior parietal cortex encodes autonomously selected motor plans. Neuron. 2007;56:552–559. doi: 10.1016/j.neuron.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Goodwin SJ, Blackman RK, Sakellaridi S, Chafee MV. Executive control over cognition: stronger and earlier rule-based modulation of spatial category signals in prefrontal cortex relative to parietal cortex. J Neurosci. 2012;32:3499–3515. doi: 10.1523/JNEUROSCI.3585-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci. 2006;9:682–689. doi: 10.1038/nn1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Cohen JY, Woodman GF, Schall JD. Neural correlates of correct and errant attentional selection revealed through N2pc and frontal eye field activity. J Neurophysiol. 2010;104:2433–2441. doi: 10.1152/jn.00604.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibos G, Duhamel JR, Ben Hamed S. A functional hierarchy within the parietofrontal network in stimulus selection and attention control. J Neurosci. 2013;33:8359–8369. doi: 10.1523/JNEUROSCI.4058-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C. Early involvement of prefrontal cortex in visual bottom-up attention. Nat Neurosci. 2012a;15:1160–1166. doi: 10.1038/nn.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C. Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Front Int Neurosci. 2012b;6:17. doi: 10.3389/fnint.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, van Essen DC. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J. Neurophysiol. 1992;67:961–980. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Human Neurobiology. 1985;4:219–227. [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lennert T, Martinez-Trujillo J. Strength of response suppression to distracter stimuli determines attentional-filtering performance in primate prefrontal neurons. Neuron. 2011;70:141–152. doi: 10.1016/j.neuron.2011.02.041. [DOI] [PubMed] [Google Scholar]
- Lock TM, Baizer JS, Bender DB. Distribution of corticotectal cells in macaque. Exp Brain Res. 2003;151:455–470. doi: 10.1007/s00221-003-1500-y. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- Meyer T, Constantinidis C. A software solution for the control of visual behavioral experimentation. J Neurosci Methods. 2005;142:27–34. doi: 10.1016/j.jneumeth.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci U S A. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, Bullier J. Anatomical segregation of two cortical visual pathways in the macaque monkey. Vis Neurosci. 1990;4:555–578. doi: 10.1017/s0952523800005769. [DOI] [PubMed] [Google Scholar]
- Niebur E, Koch C. Control of Selective Visual Attention: Modeling the “Where’’ Pathway. In: Touretzky DS, Mozer MC, Hasselmo ME, editors. Neural Information Processing Systems. Cambridge, MA: MIT Press; 1996. pp. 802–808. [Google Scholar]
- Pare M, Wurtz RH. Monkey posterior parietal cortex neurons antidromically activated from superior colliculus. J Neurophysiol. 1997;78:3493–3497. doi: 10.1152/jn.1997.78.6.3493. [DOI] [PubMed] [Google Scholar]
- Purcell BA, Heitz RP, Cohen JY, Schall JD, Logan GD, Palmeri TJ. Neurally constrained modeling of perceptual decision making. Psychol Rev. 2010;117:1113–1143. doi: 10.1037/a0020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BA, Schall JD, Woodman GF. On the origin of event-related potentials indexing covert attentional selection during visual search: timing of selection by macaque frontal eye field and event-related potentials during pop-out search. J Neurophysiol. 2013;109:557–569. doi: 10.1152/jn.00549.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C. Variability of prefrontal neuronal discharges before and after training in a working memory task. PLoS ONE. 2012;7:e41053. doi: 10.1371/journal.pone.0041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Katsuki F, Meyer T, Rawley JB, Zhou X, Douglas KL, Constantinidis C. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci. 2010;4:12. doi: 10.3389/fnsys.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci. 2007;27:11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc Lond B Biol Sci. 2002;357:1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J. Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu. Rev. Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Functional properties of corticotectal neurons in the monkey’s frontal eye field. J. Neurophysiol. 1987;58:1387–1419. doi: 10.1152/jn.1987.58.6.1387. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Shibutani H, Sakata H, Hyvarinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp. Brain. Res. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J. Comp. Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci. 2013;16:98–104. doi: 10.1038/nn.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan SK, Freedman DJ. Preferential encoding of visual categories in parietal cortex compared with prefrontal cortex. Nat Neurosci. 2012;15:315–320. doi: 10.1038/nn.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NW, Pare M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol. 2007;97:942–947. doi: 10.1152/jn.00413.2006. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol. 2005;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J. Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- Wimmer K, Nykamp DQ, Constantinidis C, Compte A. Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat Neurosci. 2014;17:431–439. doi: 10.1038/nn.3645. [DOI] [PubMed] [Google Scholar]