Abstract
BACKGROUND
Adverse childhood experiences (ACEs) are associated with poor physical and mental health outcomes in adulthood. Adverse childhood experiences are also associated with shortened leukocyte telomere length (LTL) in adults, suggesting accelerated cell aging. No studies have yet assessed the relationship of ACEs to LTL in individuals with major depressive disorder (MDD), despite the high incidence of antecedent ACEs in individuals with MDD. Further, no studies in any population have assessed the relationship of ACEs to the activity of telomerase, the major enzyme responsible for maintaining LTL, or the relationship between telomerase and LTL in individuals with ACEs.
METHODS
Twenty healthy, unmedicated adults with MDD and 20 healthy age-, sex- and ethnicity-matched controls had ACEs assessed and had blood drawn for LTL and peripheral blood mononuclear cell (PBMC) resting telomerase activity.
RESULTS
In healthy controls, greater ACE exposure was associated with shorter LTL (p< 0.05) but was unassociated with telomerase activity. In MDD, however, the opposite pattern was seen: Greater ACE exposure was unrelated to LTL but was associated with increased telomerase activity (p< 0.05) and with a higher telomerase: LTL ratio (p= 0.022).
LIMITATIONS
Study limitations include the small sample size, a single timepoint assessment of telomerase activity, and the use of retrospective self-report to assess ACEs.
CONCLUSIONS
These results replicate prior findings of shortened LTL in healthy adults with histories of multiple ACEs. However, in MDD, this relationship was substantially altered, raising the possibility that activation of telomerase in ACE-exposed individuals with MDD could represent a compensatory response to endangered telomeres.
Keywords: Depression, Childhood Adversity, Telomere Length, Telomerase Activity
Introduction
Serious adverse childhood experiences (ACEs) are remarkably prevalent, with between 52–64% of individuals in the United States experiencing at least one serious ACE before the age of 18 and between 6.2–12.5% of individuals experiencing four or more serious ACEs before that age (Anda et al., 2006; Felitti et al., 1998). ACEs are associated with increased risk of adult physical and mental disease and with shortened life expectancy (Anda, Butchart, Felitti, & Brown, 2010; Brown et al., 2009; Chapman, et al., 2004). The mechanisms underlying this increased risk are unknown, but one possibility is that ACEs are associated with premature biological aging. An emerging measure of biological age at the cellular level is the length of telomeres in circulating leukocytes. Shorter leukocyte telomere length (LTL) is associated with earlier onset or elevated risk of several common diseases of aging (Andrews, Fujii, Goronzy, & Weyand, 2010; Epel et al., 2006).
Telomeres are deoxyribonucleic acid (DNA)-protein complexes found at the ends of linear chromosomes that cap and protect the genome from damage. Telomere shortening can occur with repeated cell division as well as with chronic exposure to cytotoxic stressors such as oxidative stress and inflammation (O’Donovan, Pantell, et al., 2011; von Zglinicki, 2002), and telomere length may provide a biomarker for assessing an individual’s cumulative exposure to, or ability to cope with, stressful conditions (Kotrschal, Ilmonen, & Penn, 2007). Telomere shortening can be counteracted or reversed by telomerase, an enzyme that elongates telomeres (Blackburn & Colins, 2011). However, the amount of telomerase in most somatic cells is insufficient to maintain telomere length indefinitely (Beyne-Rauzy, 2005; Kotrschal et al., 2007), and when telomeres reach a critically short length, cells become susceptible to senescence and apoptosis (Price, Kao, Burgers, Carpenter, & Tyrka, 2013; Epel et al., 2006).
Shortened LTL has been associated with psychiatric illness, such as anxiety, and depressive disorders (Hartmann, Boehner, Goenen, & Kalb, 2010; O’Donovan et al., 2011; Simon et al., 2006), and accelerated LTL shortening has been demonstrated in adults with ACEs (Kiecolt-Glaser et al., 2011; Tyrka et al., 2010; Price, Kao, Burgers, Carpenter, & Tyrka, 2013). Despite the high prevalence of both ACEs and poor health outcomes in MDD, no studies have assessed the relationship of ACEs to LTL in individuals with major depressive disorder (MDD). Further, the role of peripheral blood mononuclear cell (PBMC) telomerase activity (TA) has not been well characterized in stressed and psychiatrically ill individuals, nor in individuals with histories of ACEs.
This study examined LTL and TA in healthy unmedicated adults with MDD and in well-matched healthy controls. We hypothesized that graded exposure to ACEs would be associated with diminished LTL in both groups. We did not hypothesize specific TA changes or telomerase: LTL ratios that would be associated with graded exposure to ACEs due to the lack of prior data.
Methods
Participants
This study was approved by the University of California San Francisco Committee on Human Research. Participants gave informed consent to participate and were reimbursed for their participation.
Twenty subjects with MDD and 20 healthy controls (individually matched on age ± 3 years, gender and ethnicity) particpated and completed all procedures. The individuals with MDD and 18 of the controls have been described in other publications using different measures and testing different hypotheses (Wolkowitz et al., 2012). MDD diagnoses were made using the Structured Clinical Interview for DSM-IV-TR (First, Spitzer, Gibbon, & Williams, 2002) and verified through clinical interview with a Board-certified psychiatrist. Depressed subjects were required to have a minimum rating of 17 on the 17-item Hamilton Depression Rating Scale (Hamilton, 1960). Healthy controls were required to have no present or lifetime history of any DSM-IV Axis I diagnosis. All subjects were medically healthy, as assessed by physical examination, vital signs and standard laboratory screening tests. All subjects were free of acute illnesses at the time of testing. For at least 6 weeks prior to participation, no subjects had received vaccinations, immunizations, psychotropic medications, or other medications thought to affect LTL, TA, oxidative stress or inflammation (except prn short-acting sleep medication, up to 3 times per week, but none within one week of the study visit). Subjects with lifetime diagnoses of bipolar or psychotic illness or with diagnoses of alcohol or substance abuse within the preceding six months were excluded, as were subjects with symptoms of PTSD in the past month. Other comorbid anxiety diagnoses were permitted within the MDD group if MDD was considered the primary diagnosis.
Procedures
Subjects were admitted as outpatients to the UCSF Clinical Translational Science Institute at 8:00 am following a 12-hour overnight fast (except for water). After subjects rested quietly, an indwelling intravenous catheter was placed for blood drawing.
Assays
LTL assay procedures were adapted from the published original method (Cawthon, 2002). Whole blood was drawn into lavender top EDTA Vacutainer tubes, and buffy coat was saved for LTL assay. High molecular weight DNA was extracted from frozen whole blood using commercially available reagents (Puregene, Gentra Systems, Qiagen, Valencia, CA). DNA quality and quantity were assessed with a nanodrop spectrophotometer and random samples were also assessed by agarose gel electrophoresis. The T (telomeric) and S (single copy gene) values of each sample were determined by quantitative polymerase chain reaction (PCR) using the following primers: tel1b [59-CGGTTT( GTTTGG)5GTT-39] and tel2b [59 GGCTTG(CCTTAC)-5CCT-39] for T and hbg1 [59 GCTTCTGACACAACTGTGTTCACTAGC-39] and hbg2 [59 CACCAACTTCATCCACGTTCACC-39] for S (human beta-globin). Genomic DNA from HeLa cells was used as the reference to quantify the T and S values relative to the reference DNA sample by the standard curve method. All PCRs were carried out on a Roche Lightcycler 480 real-time PCR machine with 384-tube capacity (Roche Diagnostics Corporation, Indianapolis, IN). The telomere thermal cycling profile consisted of: cycling for T (telomeric) PCR: denature at 96uC for 1 second, anneal/extend at 54uC for 60 seconds, with fluorescence data collection, 30 cycles; cycling for S (single copy gene) PCR: denature at 95uC for 15 seconds, anneal at 58uC for 1 second, extend at 72uC for 20 seconds, 8 cycles; followed by denature at 96uC for 1 second, anneal at 58uC for 1 second, extend at 72uC for 20 seconds, hold at 83uC for 5 seconds with data collection, 35 cycles. Blood samples from MDD participants and their matched controls were assayed in the same batch. The inter-assay coefficient of variation (CV) for telomere length measurement was 4%.
Blood for PBMC TA determination was collected into Cell Preparation Tubes (Becton-Dickinson, Franklin Lakes, NJ, USA, Vacutainer CPT), which contain a Ficoll separation gradient. Blood processing procedures have been described in detail previously (Wolkowitz et al., 2012). Telomerase activity was assayed with the telomere repeat amplification protocol (TRAP). TA assay was optimized on the basis of the commercially available kit TRAPeze (Chemicon, Temecula, CA, USA). Telomerase activity is defined as 1 unit = the amount of product from one 293T cell/ 10 000 PBMC’s. Blood samples from MDD participants and their matched controls were assayed in the same batch. Inter-assay CV of PBMC telomerase activity was 6.8%.
Ratings
Subjects reported depressive symptoms over the preceding week using the Quick Inventory of Depressive Symptoms Scale (QIDS; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996). Subjects reported on ACEs using the self-administered 8-item Adverse Childhood Experiences scale (Felitti et al., 1998). This scale has been well-validated (Anda et al., 2010) and assesses history of personal abuse, neglect, and household dysfunction. Scores range from zero (no history of ACEs) to eight (all eight types of ACEs). Sleep quality was was assessed with the Insomnia Severity Index (Morin, Belleville, Bélanger & Ivers, 2011).. Subjective socioeconomic status was measured using a 10-rung ladder version of the MacArthur Scale of Subjective Social Status (Adler, Epel, Castellazzo, & Ickovics, 2000).
All variables were screened for normality, and non-normal distributions were natural log-transformed. Independent sample t-tests and chi-square tests were used to compare groups on demographic variables, including age, gender, ethnicity, socioeconomic variables (e.g., education), exercise activity, tobacco and alcohol use, and insomnia. Age was significantly associated with telomere length (r = −.52, p < .05) among depressed individuals but not among controls (r = −.07, p = .78). In the control group only, effects were found between gender and telomere length (telomere length was longer in males; r = .51, p < .05), and between age and telomerase activity (r = −.63, p < .01).
Results
MDD and control groups did not significantly differ in age, sex, ethnicity, educational level, socioeconomic status, or alcohol or tobacco use. Individuals with MDD reported more difficulties with insomnia (t(22.8) = −4.89, p = .000), compared to healthy controls, but sleep was not associated with LTL or TA, in either group.
As expected, individuals with MDD reported a higher severity of depressive symptoms on the QIDS than healthy controls (t(17.5) = −11.64, p=0.000) as well as a greater number of ACEs (M= 3.90, SD ±2.05 t(38)= −3.04, p=0.004). Among individuals with MDD, 65% had ACE scores of > 4 (out of a maximum score of 8), compared to 25% of the controls (χ2 (1,n=40)=14.07, p < .001). As reported previously with a subset of this sample (Wolkowitz et al., 2012), individuals with MDD had higher TA than healthy controls (t(36)= −2.53, p=0.016) and did not have significantly shorter leukocyte telomeres than controls. Finally, controlling for age and gender, individuals with MDD had increased TA: LTL ratios compared to healthy controls (F= 6.06, p= 0.02).
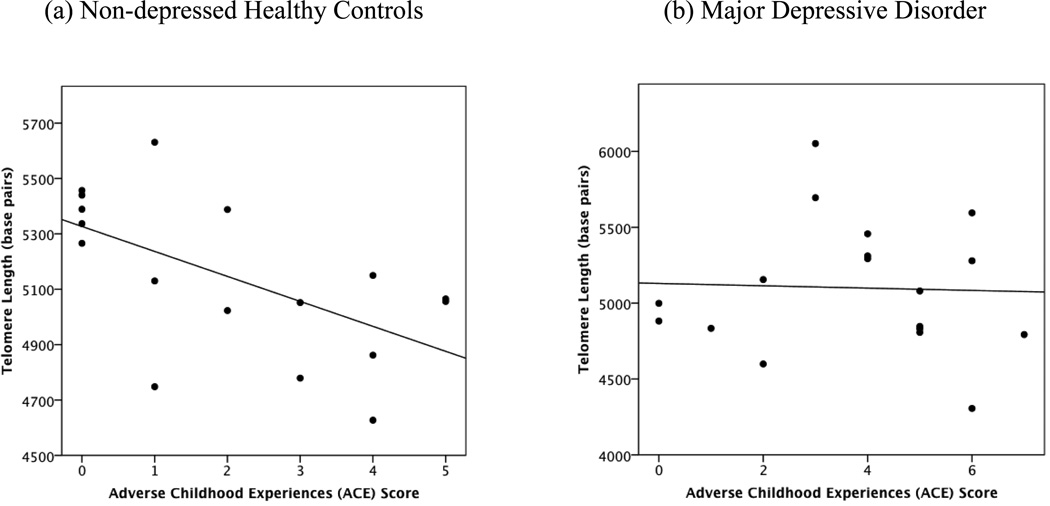
Partial correlations examined associations between ACEs and LTL controlling for participants’ age and gender. Among healthy controls, ACEs were significantly inversely correlated with LTL (r = −.61, p < .05) (Fig 1a) but were not significantly correlated with TA (r= −.22, p > .10) (Fig 2a). or with the TA: LTL ratio (r =.12, p > .10). By contrast, among individuals with MDD, ACEs were not significantly correlated with LTL (r = −.13, p > .10) (Fig. 1b), but were positively correlated with TA (r = .58, p < .05) (Fig. 2b), and the TA: LTL ratio (r= 0.60, p < 0.01).
Figures 1.
a and b. Associations between Adverse Childhood Experiences and leukocyte telomere length in non-depressed individuals (r = −.61; p < .05) (1a) and individuals with Major Depressive Disorder (r = −.13, p > .10) (1b).
Figures 2.
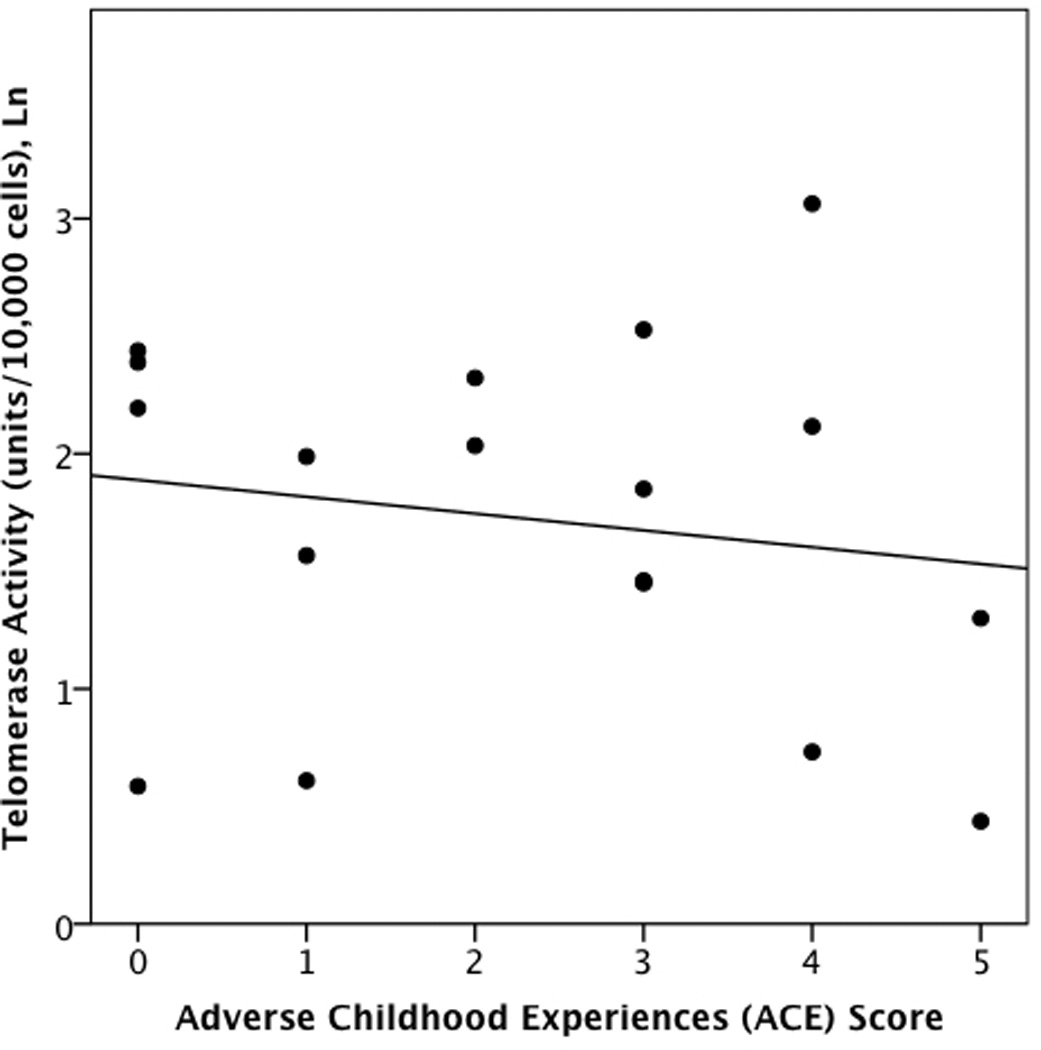
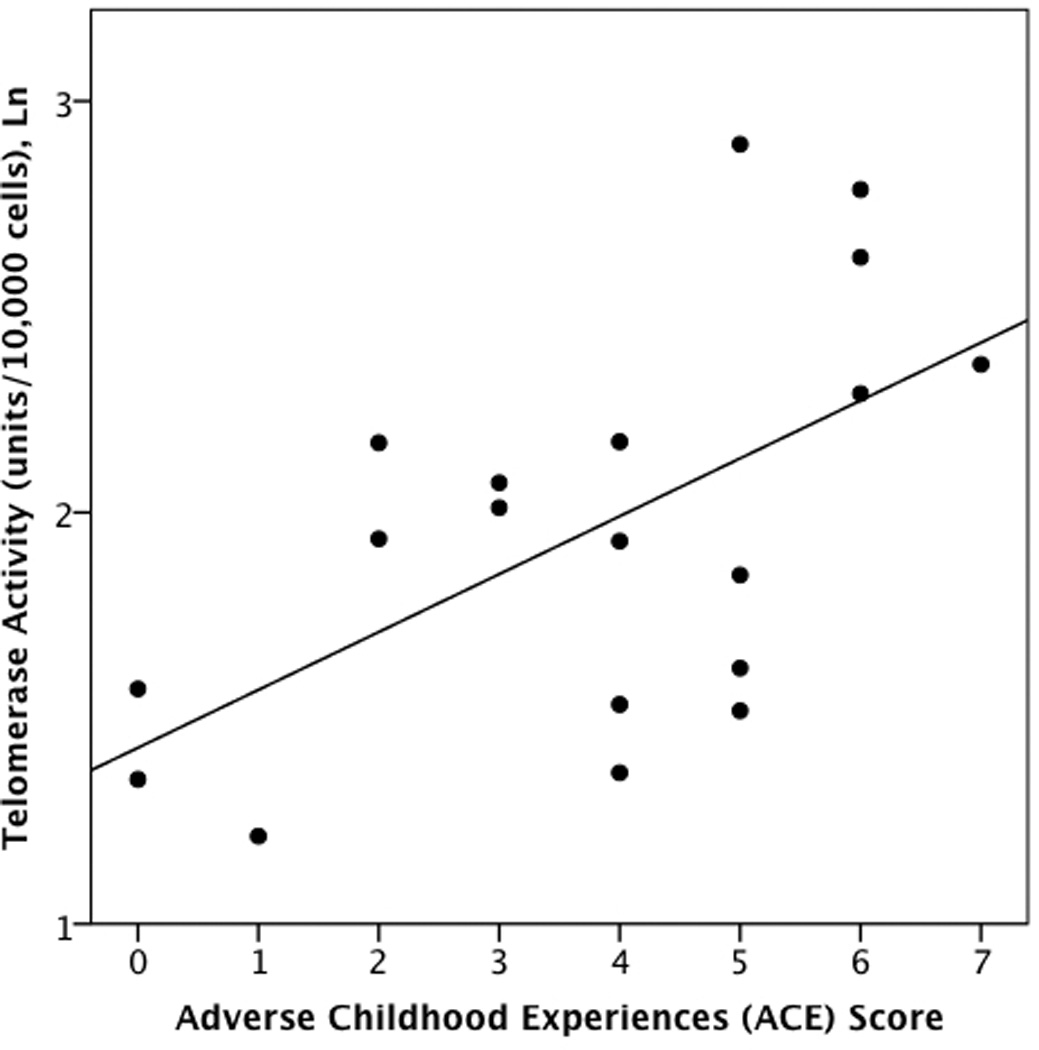
a and b. Associations between Adverse Childhood Experiences and peripheral blood mononuclear cell telomerase activity in non-depressed individuals (r = −.22, p > .10) (2a) and individuals with Major Depressive Disorder (r =.58, p < .05) (2b).
Discussion
We replicated previous findings of shortened LTL in healthy non-depressed individuals with extensive ACEs (Kiecolt-Glaser et al., 2011; Tyrka et al., 2010; Price et al., 2013), but found a distinctly different pattern in healthy unmedicated individuals with MDD. Specifically, greater exposure to ACEs was correlated with significantly shorter LTL among the healthy controls but not among the MDDs. By contrast, greater exposure to ACEs was significantly correlated with increased TA in the individuals with MDD but not in healthy controls. Likewise, greater exposure to ACEs was associated with greater TA: LTL ratios in the individuals with MDD but not in the healthy controls.
To our knowledge, no study has previously examined the relationship between ACEs and LTL in individuals with MDD, despite the fact that histories of ACEs are common in adults with MDD (Anda et al., 2006), and no study has yet examined the relationship between ACEs and TA in any population. The present findings suggest that ACEs may be an important factor to consider in studies of LTL and may explain some of the variability reported acrosss studies (Price et al., 2013; Shalev, Moffit, et al., 2013; Shalev, Entringer et al., 2013;).
The mechanisms by which ACEs, at least in non-MDD individuals, come to be associated with shortened LTL are not known, and it is unknown if a causal relationship exists. However, there are multiple pathways through which early adversity may become “biologically embedded” throughout the lifespan (Shalev et al., 2013; Shalev, 2012), including excessive oxidative stress and inflammation (Tyrka et al., 2013; Fagundes, Glaser, & Kiecolt-Glaser, 2013; Danese & McEwen, 2012) and dysfunction of the HPA and noradrenergic stress response systems (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008).
Our overall findings may be best explained by the balance between TA and LTL. Specifically, the increased TA observed among individuals with MDD may reflect a compensatory response that maintains LTL or mitigates telomere shortening, as previously hypothesized (Wolkowitz, Reus & Mellon, 2011; Damjanovic et al., 2007). This mechanistic explanation might be consistent with prior preclinical and human studies that indicate upregulation of TA in response to cell damage (Baek, Bu, Kim, & Kim, 2004; Mattson, Fu, & Zhang, 2001), and a preferential elongation by telomerase of shorter telomeres (Britt-Compton, Capper, Rowson, & Baird, 2009). Finally, the associations between ACEs and increased TA: LTL ratios in the MDD subjects are consistent with several recent studies that also indicate higher ratios in other unhealthy or high-risk conditions (Damjanovic et al., 2007; Kroenke, Pletcher et al., 2012). Higher TA relative to LTL could indicate greater telomere endangerment, requiring greater telomerase activation in an attempt to maintain telomere homeostasis.
This putative compensatory telomerase response is absent in the controls, who exhibit LTL shortening but no TA in proportion to their history of ACEs. It is unknown why telomerase activation did not accompany shorter leukocyte telomeres in the healthy controls. It is possible that additional biochemical alterations seen in MDD (e.g., NFkB activation, oxidative stress, inflammation) contribute to telomerase activation (Schiavone, Jaquet, Trabace, & Krause, 2013; Yamagiwa, Meng, & Patel, 2006). Larger studies with prospective designs will be needed to further assess issues of mediation and causality.
Strengths of the present study include the use of well-screened and characterized subjects who were medically healthy and who had been off of psychoactive and other interfering medications for a minimum of 6-weeks before participation. Another strength was the assessment of both telomere length and telomerase activity at the same time in the same subjects. Limitations include the small sample size, and the use of only single timepoint TA assessment. Whereas LTL is a relatively stable marker, TA can change more quickly (Epel et al., 2010). Another limitation is the significant mean difference in ACE scores in the control and MDD groups, calling into question whether the different findings in the two groups are related to diagnosis vs. level of antecedent ACE. Future stuides utilizing flow cytometry with cell separation will be needed to assess whether changes in average LTL are due to changes on a per-cell basis or due to a redistribution of leukocyte subpopulations (Lin et al., 2010). Finally, ACEs were assessed through retrospective self-reports, which may be subject to bias (Hardt & Rutter, 2004).
Conclusions
The present data are the first to relate ACEs to TA in any population and the first to relate ACEs to LTL in individuals with MDD. These data highlight biological sequellae of early life psychological and physical trauma and suggest that these sequellae may differ in depressed vs. non-depressed individuals. ACE-related alterations in cell aging in certain populations might also contribute to, and help explain, the excess medical morbidity and early mortality seen in adults with histories of multiple ACEs. In addition, given recent findings suggesting that parent-child relationships may play a key role in the associations between childhood adversity and telomere length (Asok, Bernard, Roth, Rosen, & Dozier, 2013; Brody, Yu, Beach, & Philibert, 2014), interventions promoting secure attachment relationships among children exposed to adversity (Ghosh Ippen, Harris, Van Horn, & Lieberman, 2011; Lieberman, Van Horn, & Ghosh Ippen, 2005) can also be examined for long-term biological benefits.
Acknowledgement
This work was supported by the following grants: NIMH 1 R01 MH083784 (Co-PIs: OMW, ESE, SHM) and a grant from the Barbro and Barney Fund to EHB. This work was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors also gratefully acknowledge the generous financial support of the O’Shaughnessy Foundation and the Tinberg family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
EHB, JL and ESE were co-founders of a company providing telomere length measures.
Contributors
Stephen H. Chen, Elissa S. Epel, Synthia H. Mellon, Jue Lin, Victor I. Reus, Rebecca Rosser, Eve Kupferman, Heather Burke, Laura Mahan, Elizabeth H. Blackburn, Owen M. Wolkowitz
None of the funding sources played a role in the conduct of the study, the interpretation of the data or the publication of the results.
References
- 1.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, White women. Health Psychol. 2000;19(6):586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 2.Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med. 2010;39(1):93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield CH, Perry BD, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Clin Neurosci. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2009;56(4):390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev Psychopathol. 2013;25(03):577–585. doi: 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baek S, Bu Y, Kim H, Kim H. Telomerase induction in astrocytes of Sprague–Dawley rat after ischemic brain injury. Neurosci Lett. 2004;363(1):94–96. doi: 10.1016/j.neulet.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 7.Beyne-Rauzy O, Prade-Houdellier N, Demur C, Recher C, Ayel J, Laurent G, Mansat-De Mas V. Tumor necrosis factor-α inhibits hTERT gene expression in human myeloid normal and leukemic cells. Blood. 2005;106(9):3200–3205. doi: 10.1182/blood-2005-04-1386. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn EH, Collins K. Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harb Perspect Biol. 2011;3(5):a003558. doi: 10.1101/cshperspect.a003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britt-Compton B, Capper R, Rowson J, Baird DM. Short telomeres are preferentially elongated by telomerase in human cells. FEBS Lett. 2009;583(18):3076–3080. doi: 10.1016/j.febslet.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Brody GH, Yu T, Beach SR, Philibert RA. Prevention effects ameliorate the prospective association between nonsupportive parenting and diminished telomere length. Prv Sci. 2014 Mar 6; doi: 10.1007/s11121-014-0474-2. 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37(5):389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol B. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24(4):531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felitti MD, Vincent J, Anda MD, Robert F, Nordenberg MD, Williamson MS, James S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TRAxis I Disorders, Research Version, Non-Patient Edition. SCID-I/P [Google Scholar]
- 22.Ghosh Ippen C, Harris WW, Van Horn P, Lieberman AF. Traumatic and stressful events in early childhood: Can treatment help those at highest risk? Child Abuse Negl. 2011;35(7):504–513. doi: 10.1016/j.chiabu.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton M. A rating scale for depression. J Neurology, Neurosurgery, and Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27(12):1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- 26.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotrschal A, Ilmonen P, Penn DJ. Stress impacts telomere dynamics. Biol Lett. 2007;3(2):128–130. doi: 10.1098/rsbl.2006.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke CH, Pletcher MJ, Lin J, Blackburn E, Adler N, Matthews K, Epel E. Telomerase, telomere length, and coronary artery calcium in black and white men in the CARDIA study. Atherosclerosis. 2012;220(2):506–512. doi: 10.1016/j.atherosclerosis.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman AF, Van Horn P, Ippen CG. Toward evidence-based treatment: Child-parent psychotherapy with preschoolers exposed to marital violence. J Am Acad Child Adolesc Psychiatry. 2005;44(12):1241–1248. doi: 10.1097/01.chi.0000181047.59702.58. [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal Immunol Methods. 2010;352(1):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattson MP, Fu W, Zhang P. Emerging roles for telomerase in regulating cell differentiation and survival: a neuroscientist's perspective. Mech Ageing Development. 2001;122(7):659–671. doi: 10.1016/s0047-6374(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 33.Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Epel ES. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS One. 2011;6(5):e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Medicine. 1996;26(03):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 38.Schiavone S, Jaquet V, Trabace L, Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal. 2013;18(12):1475–1490. doi: 10.1089/ars.2012.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2012;18(5):576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shalev I. Early life stress and telomere length: investigating the connection and possible mechanisms. Bioessays. 2012;34(11):943–952. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatr Scand. 2013;128(6):434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 46.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, Blackburn EH. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17(2):164–172. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011;13(1):25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamagiwa Y, Meng F, Patel T. Interleukin-6 decreases senescence and increases telomerase activity in malignant human cholangiocytes. Life Sci. 2006;78(21):2494–2502. doi: 10.1016/j.lfs.2005.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]