Abstract
Background
Cirrhosis is diagnosed in patients of all ages, and is the end result of many different diseases. The aim of this study was to characterize clinical and ethnic features of adult patients who were admitted to the hospital at different (young/old) ages and examine associations between age and ethnicity within these groups.
Methods
In this retrospective analysis of a diverse cohort of 2,017 patients with a clinical diagnosis of cirrhosis between January 2001 and December 2011; we focused on age, ethnicity, and outcome of patients with cirrhosis.
Results
We identified 219 patients under the age of 40, including 87/802 White (11%), 31/550 African American (6%) and 89/550 Hispanic patients (16%) (p<0.001). Ethnicity and causes of cirrhosis were found to have a significant correlation with age. Overall, Hispanic and White patients together were more than twice as likely to be diagnosed with cirrhosis at an age under 40 compared to African American patients (p<0.001). Autoimmune hepatitis caused cirrhosis at a younger age regardless of ethnicity (p<0.001), while cryptogenic/NAFLD/NASH was more likely identified at an older age (p=0.008). African American patients with cirrhosis due to either alcohol or HCV were older than Hispanic (p<0.001 and p=0.003, respectively) and White patients (p<0.001 and p<0.001, respectively) at presentation. Finally, younger patients admitted with cirrhosis had a higher in-hospital mortality rate (p<0.001).
Conclusions
The data suggest an association between ethnicity and age of cirrhosis diagnosis, both overall and in patients with certain cirrhosis etiologies. This work raises the possibility of an ethnic, and/or genetic basis for cirrhosis.
Keywords: non-alcoholic steatohepatitis, hepatitis, alcohol, Hispanic, African American, race
Introduction
Cirrhosis is the result of persistent hepatic injury from chronic liver disease. It is a wound healing process during which fibrosis and scar tissue is deposited in the space of Disse, and in other portions of the liver1,2. This has a number of important functional consequences, including alteration of normal portal blood flow. The end result of the fibrosing process is cirrhosis, which results in a number of systemic complications, including ascites and portal hypertension, which are associated with poor quality of life and approximately 30,000 deaths per year in the United States3.
Extensive investigation has demonstrated that the fibrotic process is due largely in part to the activation of effector cells, including fibroblasts, fibrocytes, and hepatic stellate cells (HSCs), which proliferate, release proinflammatory, profibrogenic, and promitogenic cytokines and produce extracellular matrix. This process is complex and is dependent on a variety of control pathways, growth factors, cytokines, vasoactive mediators, transcriptional regulators, epigenetic regulators, and immune system regulators, which modulate cellular fibrogenesis1.
Since cirrhosis is the end result of a variety of diseases affecting the liver, it follows that mechanisms underlying the fibrogenic process are likely to be common across these different disease states. Further, a variety of exogenous factors, such as obesity, alcohol abuse, and viral co-infection, contribute to the progression of fibrosis4,5. Yet, even after controlling for these factors, a large variation still exists in the propensity of developing cirrhosis between individuals6,7. For example, one study revealed a difference in cirrhosis mortality rates among various ethnicities8. This suggests that there are genetic and possibly inherited ethnic factors associated with the development of hepatic fibrosis and cirrhosis. Thus, we have hypothesized that individuals who develop cirrhosis at a young age may share common demographic and/or clinical features. We therefore aimed to better characterize clinically patients who develop cirrhosis under the age of 40, and here, we describe several key associations with age and ethnicity.
Methods
We performed a retrospective cross-sectional study of 2,048 patients admitted to Parkland Memorial Hospital with a documented clinical diagnosis of cirrhosis from 2001–2011. Patients were identified by an International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) search for causes of cirrhosis which included the following codes: 571 (chronic liver disease and cirrhosis), 571.2 (alcoholic cirrhosis), 571.5 (cirrhosis of liver without alcohol), 571.6 (biliary cirrhosis), 572.2 (hepatic encephalopathy), 572.3 (portal hypertension), 456.0 (esophageal varices with bleeding), and 456.1 (esophageal varices without documented bleeding). The EMR was manually reviewed in all patients to confirm the diagnosis of cirrhosis, defined as consistent histology or imaging showing a cirrhotic appearing liver with any associated signs of portal hypertension (ascites, encephalopathy, varices, or splenomegaly; see also below under definitions)9. Ethnicity was self-reported. The study was approved by the institutional review board at the University of Texas Southwestern Medical Center, St. Paul University Hospital, and the Parkland Health and Hospital System, and met all criteria for good clinical research.
A Microsoft Access database was created that included demographic, clinical and historical data for all patients. This included cause of cirrhosis, medical co-morbidities, complications of cirrhosis, and laboratory values. Clinical and laboratory variables reported were recorded at the time of hospital admission. The age at index hospitalization was taken to be the age at diagnosis of cirrhosis.
Patients for whom ethnicity or a cause of cirrhosis could not be identified were excluded from the final analysis. We separated all remaining patients (n=2,017) into two groups: those under the age of 40 (n=219) and those over the age of 40 (n=1,798). Deceased patients were identified by searching for the last documented encounter in the hospital system’s EMR (EPIC Systems Corporation, Verona, WI) after their index admission.
Definitions
Cirrhosis was defined based on clinical features, including a history consistent with chronic liver disease (CLD) as well as a documented complication of CLD (i.e. ascites, varices, hepatic encephalopathy)10 and/or imaging consistent with cirrhosis and/or liver histology consistent with cirrhosis11. The cause of cirrhosis was determined according to the following criteria: (1) hepatitis C cirrhosis was defined by the presence of cirrhosis in a person with hepatitis C virus (HCV) RNA, (2) hepatitis B cirrhosis was defined by the presence of cirrhosis in patients with hepatitis B virus (HBV) surface Ag, (3) alcoholic cirrhosis was determined by chart review in the presence of a history of prolonged alcohol consumption with abuse or dependence in the absence of other potential causes of liver disease, (4) non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH) cirrhosis was defined by biopsy proven or the presence of the metabolic syndrome without any other potential cause of liver disease, (5) patients without any known cause of primary liver disease were considered to have cryptogenic cirrhosis, and (6) other causes of cirrhosis were determined using standard diagnostic criteria (i.e. serologies, histology, iron studies). Cryptogenic cirrhosis was combined with NAFLD and NASH, consistent with previous studies showing that cryptogenic may be related to NAFLD/NASH12, 13, and current best practice. Finally, mortality was defined as death occurring during the hospital admission at Parkland Memorial Hospital and was determined for all patients.
Statistical Analysis
This study was a cross-sectional analysis of cirrhotic patients under the age of 40 compared to cirrhotic patients older than 40. Patient characteristics and clinical laboratory data were summarized using descriptive statistics. Categorical and continuous variables were compared using the chi-square test, Student's t-test and Fisher’s exact test; p-values of ≤ 0.05 level using a 2-sided t-test were considered statistically significant. A multiple regression analysis was performed with age as the dependent variable and ethnicity (African American, Hispanic and White), causes of cirrhosis (alcohol, HCV, cryptogenic/NASH/NAFLD, and all others listed above), co-morbid diabetes mellitus with and without end-organ damage, AIDS, and a history of esophageal varices (hepatocellular carcinoma and chronic kidney disease were not studied due to the small number of patients with these co-morbidities) as independent variables; p-values of ≤ 0.05 were considered statistically significant.
Results
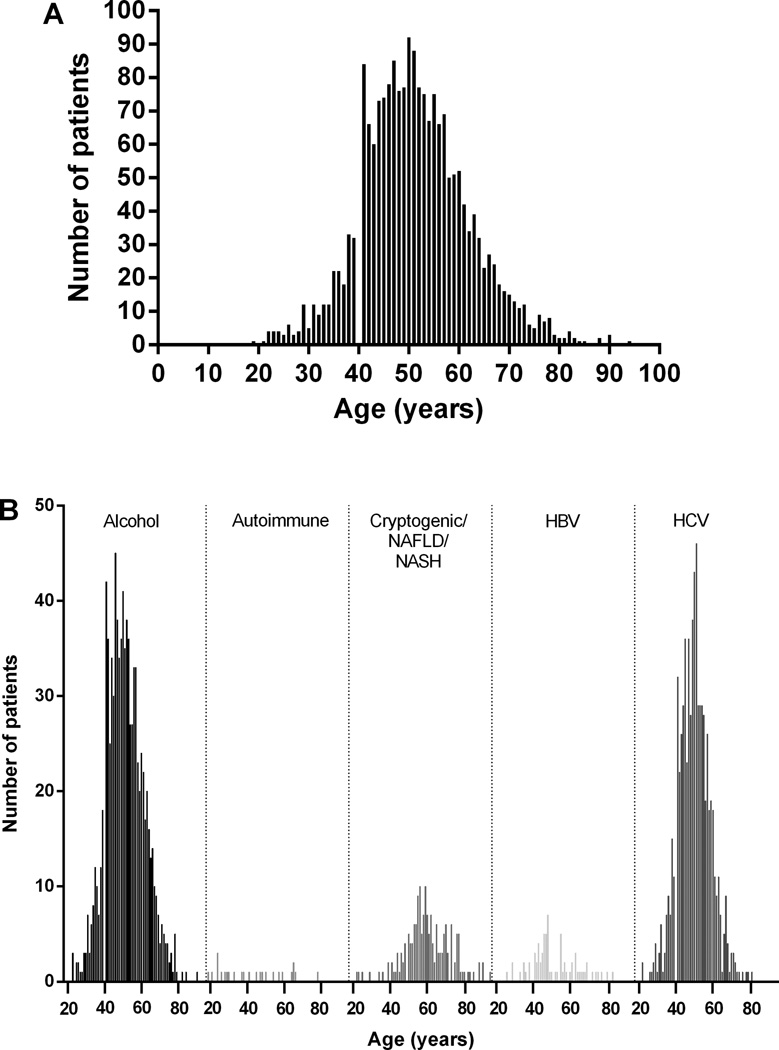
A total of 2,048 unique cirrhotic patients were identified. Thirty-one patients lacked data specifically as to ethnicity and/or a cause of cirrhosis, and were excluded. The final analysis was therefore performed on 2,017 cirrhotic patients, of who 219 were under the age of 40 and 1,798 were above the age of 40. The average age of all patients was 52 ± 11, however, patients with cryptogenic/NAFLD/NASH cirrhosis were older (age 60) and those with autoimmune cirrhosis were younger (age 43) (Table 1, Figure 1A). The mean MELD score for patients under 40 (17.0 ± 8.0, range 6 to 45, n = 213) was greater than that for patients over 40 (15.8 ± 7.2, range 6 to 52, n = 1224) (p=0.0275, 95% CI 0.132 to 2.268).
Table 1.
Age, ethnicity and cause of cirrhosis
| Ethnicity | Age |
|---|---|
| White/Caucasian | 51 ± 10 (n=802) |
| Black/African American | 52 ± 10 (n=550) |
| Hispanic | 51 ± 12 (n=550) |
| Asian/Pacific Islander/East Indian | 56 ± 13 (n=36) |
| American Indian/Alaska Native | 52 ± 11 (n=19) |
| Other | 51 ± 11 (n=60) |
| Cause of Cirrhosis | |
| Alcoholic | 51 ± 10 (n=921) |
| Autoimmune | 43 ± 17 (n=25) |
| Cryptogenic/NAFLD/NASH | 60 ± 12 (n=173) |
| HBV | 53 ± 16 (n=73) |
| HCV | 50 ± 9 (n=719) |
| HBV & HCV | 49 ± 8 (n=38) |
| PBC/PSC | 53 ± 15 (n=30) |
| Other* | 52 ± 15 (n=38) |
N=2,017. All values reported as mean ± SD (N).
Other includes cardiac, congenital, Sarcoidosis, Wilson’s disease, chronic granulomatous infection, and hemochromatosis.
HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis
Figure 1. Differences in cirrhosis.
In (A), all patients are shown grouped by age. Ages ranged from 19 to 94 with a mean of 51.5 ± 10.7. In (B), all patients with cirrhosis due to alcohol, autoimmune causes, cryptogenic or NAFLD/NASH, HBV, or HCV are shown grouped by age. Ages ranged from 23 to 90 for alcohol (n=921), 19 to 78 for autoimmune (n=25), 22 to 94 for cryptogenic/NAFLD/NASH (n=173), 26 to 83 for HBV (n=73), and 22 to 81 for HCV (n=719).
The major causes of cirrhosis were due to HCV and alcohol (Table 1/2, Figure 1B/2). Other prominent causes of cirrhosis included the following disorders: cryptogenic/NAFLD/NASH liver disease, HBV, autoimmune hepatitis, primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC) (Table 1/2, Figure 1B/2). The average age at presentation of cirrhosis due to alcoholic liver disease was 51 ± 10 years, significantly greater than that for HCV (p < 0.001) and less than that for cryptogenic/NAFLD/NASH (p < 0.001), and nearly 90% of these patients (n=823/921) was over 40. Similarly, the average age at presentation of cirrhosis due to HCV was 50 ± 9, and again the majority of these patients (n=646/719) was over 40. The average age for presentation of cirrhosis due to cryptogenic/NAFLD/NASH causes was 60 ± 12, significantly greater than that for HCV (p < 0.001).
Table 2.
Patient characteristics
| All patients (n=2,017) |
Under 40 (n=219) |
Over 40 (n=1,798) |
p | |
|---|---|---|---|---|
| Age (mean ± SD) | 51.5 ± 10.7 | 34.0 ± 4.7 | 53.7 ± 9.2 | <0.001 |
| Female | 679 | 68 (10%) | 611 (90%) | 0.405 |
| Male | 1338 | 151 (13%) | 1187 (87%) | 0.257 |
| Ethnicity | ||||
| White | 802 | 87 (11%) | 715 (89%) | 1 |
| African American | 550 | 31 (6%) | 519 (94%) | <0.001 |
| Hispanic | 550 | 89 (16%) | 461 (84%) | <0.001 |
| Asian/Pacific Islander/East Indian | 36 | 3 (8%) | 33 (92%) | 0.791 |
| American Indian/Alaska Native | 19 | 2 (11%) | 17 (89%) | 1 |
| Other | 60 | 7 (12%) | 53 (88%) | 0.832 |
| Cause of Cirrhosis | ||||
| Alcoholic | 921 | 98 (11%) | 823 (89%) | 0.706 |
| Autoimmune | 25 | 12 (48%) | 13 (52%) | <0.001 |
| Cryptogenic/NAFLD/NASH | 173 | 8 (5%) | 165 (95%) | 0.003 |
| HBV | 73 | 9 (12%) | 64 (88%) | 0.108 |
| HCV | 719 | 73 (10%) | 646 (90%) | <0.001 |
| HBV & HCV | 38 | 5 (13%) | 33 (87%) | 0.598 |
| PBC/PSC | 30 | 5 (17%) | 25 (83%) | 0.366 |
| Other* | 38 | 9 (24%) | 29 (76%) | 0.037 |
| Medical History | ||||
| Diabetes Mellitus | 437 | 18 (4%) | 419 (96%) | <0.001 |
| Diabetes Mellitus w Organ Damage | 50 | 1 (2%) | 49 (98%) | 0.037 |
| Hepatocellular Carcinoma | 41 | 0 (0%) | 41 (100%) | 0.019 |
| Chronic Kidney Disease | 36 | 8 (28%) | 28 (72%) | 0.0502 |
| AIDS | 68 | 20 (29%) | 48 (71%) | <0.001 |
| Esophageal Varices | 340 | 53 (16%) | 287 (84%) | 0.003 |
All values reported as N or N (%).
Other includes cardiac, congenital, Sarcoidosis, Wilson’s disease, chronic granulomatous infection, and hemochromatosis.
HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis
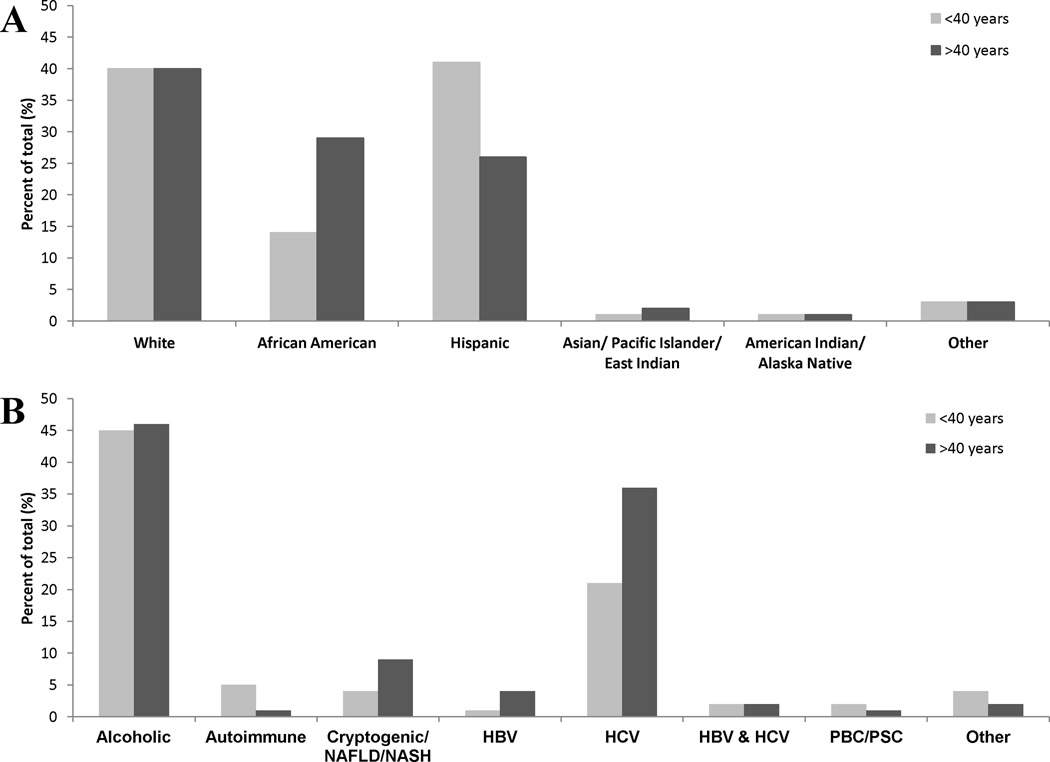
Figure 2. Differences in cirrhosis by age group according to ethnicity and etiology of cirrhosis.
In (A), the frequency with which patients in different ethnic groups were identified is shown. African Americans were more likely to present with cirrhosis at age >40 (p<0.001), while Hispanics were more likely to present with cirrhosis at age <40 (p<0.001). In (B), the frequency of patients with different etiologies in each age group is shown. Percent is out of the total for ages <40 (n=219) or >40 (n=1,798). Autoimmune liver disease was identified more often at age <40 than >40 (p<0.001). Cirrhosis due to cryptogenic/NAFLD/NASH was identified more often at age >40 (p=0.008).
African Americans were significantly more likely to be older, with 94% over the age of 40 (p<0.001, Table 2), while Hispanic patients were less likely to be older (p<0.001, Table 2). The average age of all African American patients was significantly greater than that of Hispanic (p <0.001) and White (p = 0.031) patients. There was no difference in average age between Hispanic and White patients (p = 0.181). The mean MELD score for African American patients over 40 (15.2 ± 8.0, range 6 to 52, n=318) was no different from that of other patients over 40 (p=0.215). The mean MELD score for Hispanic patients under 40 (13.5 ± 7.5, range 6 to 33, n=88) was significantly less than that of other patients under 40 (p<0.001). Hispanic (89/550) and White (87/802) patients together (176/1352, 13%) were more than twice as likely to be diagnosed with cirrhosis at an age under 40 as African American patients (31/550, 6%) (p<0.001). When considering all patients specifically under or over the age of 40 (Figure 2), the proportion of African American patients over the age of 40 (519/1798, 29%) was much greater than the proportion under the age of 40 (31/219, 14%) (p<0.001). In contrast, Hispanic patients were comparatively younger at diagnosis; 461/1798 (26%) of patients were over 40, while 89/219 (41%) were under 40 (p<0.001). White patients had equal proportions of cirrhotics in the younger and older (40% for each) age groups.
A multiple regression analysis was performed to examine the relationship between several different variables and age as in Methods. A significant correlation was identified with ethnicity (African American, Hispanic, and White) (p < 0.001), all causes of cirrhosis (p < 0.001), co-morbid diabetes mellitus without end-organ damage (p < 0.001), and co-morbid AIDS (p < 0.001) on admission.
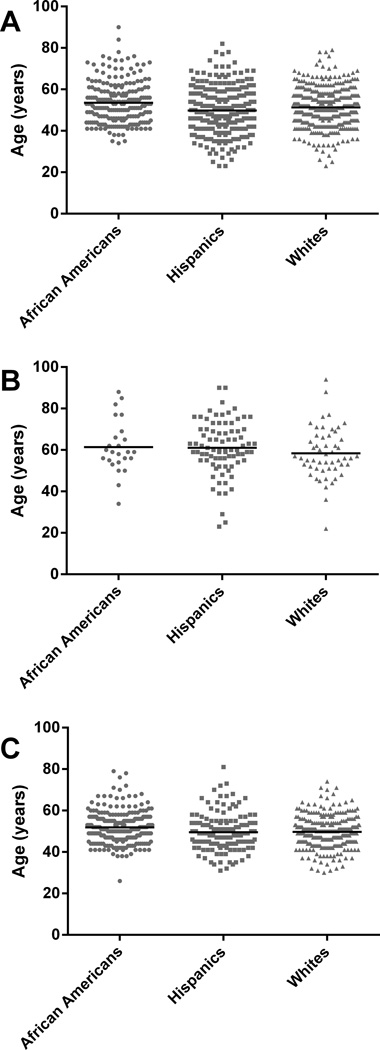
The age of presentation in patients with certain causes of cirrhosis differed among the different ethnic groups. African American patients with alcoholic cirrhosis had an older average age (54 ± 10) than both Hispanics (50 ± 11, p<0.001) and Whites (51 ± 9, p=<0.001) (Figure 3A). A greater proportion of patients with alcoholic cirrhosis were Hispanic (291/921, 32%, p<0.001) or White (374/921, 41%, p<0.001) than African American (218/921, 24%) (Figure 3A); this was the case for both the under and over 40 age groups. Among patients over 40, Hispanic patients (78/1798, 4%) were more likely than White patients (52/1798, 3%, p<0.001), and White patients were more likely than African American patients (23/1798, 1%, p<0.001), to present with cirrhosis due to cryptogenic/NAFLD/NASH (Figure 3B). African American patients were significantly older (52 ± 8) when presenting with cirrhosis due to HCV than either Hispanic patients (49 ± 10, p=0.003) or White patients (49 ± 8, p<0.001) (Figure 3C). Among patients over 40, African American patients (235/1798, 13%, p<0.001) and White patients (258/1798, 14%, p<0.001) were more likely than Hispanic patients (122/1798, 7%) to present with cirrhosis due to HCV (Figure 3C). Among patients under 40, Hispanic (23/219, 11%, p=0.048) and White (34/219, 16%, p<0.001) patients were more likely than African American patients (11/219, 5%) to present with cirrhosis due to HCV (Figure 3C). African American patients composed a larger percentage of patients with cirrhosis due to autoimmune disease under 40 than Hispanic patients (p=0.012). Cirrhosis due to HBV in patients over 40 was more common in African American and White patients than in Hispanic patients (p<0.001).
Figure 3. Cirrhosis in African Americans, Hispanics, and Whites.
Individual patients as a function of their age and ethnicity are shown. The black line indicates average age. In (A), in patients with alcoholic cirrhosis, it was 53.5 ± 10.0 for African Americans (n=218); 49.8 ± 10.7 for Hispanics (n=291); 51.3 ± 9.3 for Whites (n=374) (there were differences in age between African Americans and either Hispanics (p<0.001) or Whites (p=0.001)). In (B), in patients with NASH, NAFLD, or cryptogenic cirrhosis, average age was 61.4 ± 13.0 for African Americans (n=24); 61.1 ± 13.0 for Hispanics (n=83); 58.4 ± 12.2 for Whites (n=54). In (C), in patients with HCV cirrhosis, average age was 51.5 ± 8.1 for African Americans (n=246); 48.7 ± 9.5 for Hispanics (n=145); 49.1 ± 8.0 for Whites (n=292) (there were differences in age between African Americans compared to Hispanics (p=0.003) or Whites (p<0.001)).
A variety of medical disorders, including diabetes mellitus with and without end organ damage, hepatocellular carcinoma, chronic kidney disease, and AIDS were also noted (Table 2). Diabetes mellitus with and without end-organ damage and hepatocellular carcinoma were identified more commonly in patients over 40 (p=0.037, p<0.001 and p=0.019, respectively). AIDS was more prevalent in cirrhotics under 40 (p<0.001). Varices were more prevalent in patients over 40 (p = 0.003). As explained above, diabetes mellitus and AIDS had a significant correlation with age.
Mortality among patients was 32% (70/219) in the under 40 age group (34 with alcoholic, 24 with HCV, 3 with autoimmune, 2 with cryptogenic/NAFLD/NASH, and 7 with other causes of cirrhosis), significantly greater than 11% (202/1798) in the over 40 age group (141 with alcoholic, 36 with HCV, 16 with cryptogenic/NAFLD/NASH, and 9 with other causes of cirrhosis) (p<0.001). Of deceased patients under 40, 33/87 (38%) were White, 12/31 (39%) were African American, and 22/89 (25%) were Hispanic (p=0.07 and p=0.17 compared to White and African American patients, respectively). Of those over 40, 89/715 (12%) were White, 61/519 (12%) were African American, and 46/461 (10%) were Hispanic.
Discussion
Here, we have studied a uniquely diverse cohort of patients with cirrhosis, and identified associations between the age at which cirrhosis develops and ethnicity. We found that Hispanic patients were more likely to be younger when presenting with cirrhosis while African American patients were more likely to be older when presenting with cirrhosis. Another novel finding was that alcoholic cirrhosis presented later in life in African American patients compared to Hispanics and Whites.
Various correlations between ethnicity and causes of cirrhosis in young and old patients were apparent in our large and diverse patient sample, consistent with several epidemiological associations reported smaller studies14,15. While the data raise the speculative possibility that there may be inherent genetic factors that could predispose to more rapid fibrosis progression in certain groups of patients, it is important to emphasize that hepatic fibrosis and cirrhosis are complex and that there are multiple potential levels of intrinsic cell and molecular interactions. Further, environmental factors almost certainly play a critical role in the development of the cirrhosis phenotype, and likely exhibit critical interplay with host factors. For example, Hispanic and White patients were more likely to have cirrhosis due to alcohol than African American patients. Bearing in mind environmental factors, it has been reported that among college students, Hispanic students were more than twice as likely as African American students to report episodic heavy drinking16, which in theory could lead to earlier development of cirrhosis. Additionally, African American patients constituted the majority of autoimmune cases of cirrhosis in younger patients. Previous studies have reported that African American patients have a poorer outcome and higher mortality due to autoimmune hepatitis compared with other ethnicities14.
Among older patients, cryptogenic causes of cirrhosis were greatest in Hispanic patients, followed by White, and then African American patients, consistent with previous studies15. The prevalence of type 2 diabetes and average body mass index is similar in Hispanic patients and African American patients, but the former have the greatest annual increase in body mass index of all ethnicities in the United States, and Hispanic patients also have greater hepatic steatosis17. Thus, genetic factors are likely to play a role in making Hispanic patients more susceptible and/or African American patients less susceptible to NAFLD, and consequently cryptogenic/NAFLD/NASH cirrhosis18,19.
The prevalence of HCV appears to be the greatest in African American patients, compared to all other ethnic groups in the United States20, yet in our study, cirrhosis caused by HCV presented at an older age in African American patients than in either Hispanic or White patients. It is possible that environmental factors may explain this finding, such as differential age at exposure to HCV, but epidemiologic data supporting this possibility are lacking. Rather, while previous data has demonstrated that African American patients have decreased viral clearance and decreased response to antiviral therapy, previous data also suggests slower progression to cirrhosis20,21. On the other hand, Hispanic patients presenting with cirrhosis due to HCV at a younger age may be due to more aggressive and faster disease progression in this ethnic group22.
In addition, the presence of medical co-morbidities varied with age. For example, it was remarkable that patients with AIDS were more likely to have cirrhosis at a young age (Table 1). This may be related to the biology of fibrogenesis in these patients, in which it has been demonstrated that patients coinfected with a hepatitis virus have a more accelerated fibrosing process23. Diabetes mellitus both with and without end-organ damage, as well as hepatocellular carcinoma, were more often identified in older cirrhotics; the finding of hepatocellular carcinoma at an older age would be expected since hepatocellular carcinoma takes time to develop.
Interestingly, the in-hospital mortality rate was higher among younger patients than in older patients, although mortality did not appear to differ in different ethnic groups. Although this finding is surprising, it is consistent with the concept that younger patients had more advanced disease at the time of hospital admission than older patients. Further, the finding that MELD scores were higher in younger patients than in older patients is consistent with this possibility. We are unable to explain this surprising finding, but speculate that either the biology of their disease is different than that of older patients or that they come to hospital admission later in the course of their disease.
Several gene association studies in humans have uncovered genes contributing to hepatic fibrosis24. In studies focused on hepatitis C, the LPS receptor TLR4 was identified to be a pro-fibrotic protein; there was no association with progression of other forms of chronic liver disease1. Mice with a targeted deletion of the TLR4 are largely protected from experimental fibrosis25. More significantly, a genome-wide screen identified seven SNPs, termed the ‘cirrhosis risk score,’ to be associated with the progression of liver disease26. Recently, studies have demonstrated an inverse correlation between 25(OH)-vitamin D levels and the commencement of fibrosis27. The seven SNPs, representing seven genes (AP3S2, AQP2, AZIN1, DEGS1, STXBP5L, TLR4 and TRPM5), is the best tool for genetic prediction of liver fibrosis in hepatitis C infection28,29. Whether this score can predict fibrosis progression in non-HCV liver disease is unclear. Further, two large-scale genome-wide association studies identified three novel markers showing strong association with liver fibrosis. These were the DEAD box polypeptide 5 (DDX5) and carnitine palmitoyltransferase 1A (CPT1A) proteins, involved in the development of liver fibrosis in subjects with HCV30, and the integrin beta 5 (ITGB5) protein, associated with liver fibrosis in HCV and HCV/HIV patients31. Complement factor 5 may also be a cause for the development of liver fibrosis32. Clearly, there are genetic links to the development of cirrhosis in some patients.
Additional studies in mice have identified genes or gene families in which mutations are associated with the development of hepatic fibrosis. Included are a phospholipid transporter ABCB4 (MDR2/MDR3) necessary for proper bile formation33, an overexpressed proliferative protein of the PDGF family (Pdgf-A, Pdgf-B, Pdgf-C)34,35,36, TGF-B1 a promoter of ECM protein synthesis in hepatic stellate cells37, LIM homeobox protein LHX238, the taurine transport protein TAUT39, and the anti-apoptotic protein Bcl-xl40. Further, a locus has been mapped to chromosome 15 for susceptibility to hepatic fibrosis41.
Although a remarkable strength of this study was that we examined an extremely large and ethnically diverse population, we recognize several limitations of this work. First, our study was retrospective, and certain data elements could not be identified. However, patients lacking key data (ethnicity and cause of cirrhosis) were excluded from the study. Additionally, as emphasized above, we cannot exclude the possibility that certain ethnic groups had specific environmental influences (s) that may have predisposed them to liver disease at a younger age. However, with regard to ethanol consumption and the acquisition of hepatitis C virus, the most common causes of liver disease in our cohort, we are not aware of definite epidemiologic factors in the US that predispose certain groups to develop cirrhosis at a younger age42,43.
A further consideration with the current study is that we focused on patients admitted to the hospital. While the vast majority of these patients were also followed in outpatient clinics, it is possible that inpatients had more advanced disease than outpatients. If anything, the focus on inpatients would have skewed the results toward finding patients with cirrhosis at an older age or at a more decompensated stage. It should also be emphasized that by virtue of the safety net status of the Parkland Health and Hospital System, there do not appear to be disparities to access to care among ethnic groups. Thus, we doubt that there is bias toward one ethnic group or another with regard to hospital admission and inclusion in our patient cohort. Finally, the study was performed in a single institution, raising the concern that our findings may not be generalizable to other healthcare systems. However, the wide diversity of the population studied here is a unique strength of the study and this element raises the possibility that our study findings are likely in fact to be more generalizable to the entire US population than studies from only tertiary or other focused healthcare systems.
In conclusion, our data suggest that ethnicity may play an important role in the fibrogenic process leading to cirrhosis, and raises new questions regarding ethnicity and age in patients with cirrhosis. The correlations here may become crucial in identifying risk factors for cirrhosis in certain patient populations. We speculate that further studies may identify ethnic and perhaps genetic factors that are important in the fibrogenic response and development of cirrhosis.
Acknowledgements
The authors thank Changxing Chen from Southern Methodist University for statistical analysis assistance. The work was supported in part by the NIH (grant DK 098819 to DCR).
Financial disclosure:
This project was supported in part by the NIH Grant R01 DK 57830 to DCR and an institutional grant, number 5T35DK066141).
Footnotes
Author contributions:
Krishna Sajja - study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
Desh Mohan - study concept and design; acquisition of data
Don Rockey - study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Conflict of interest disclosure:
The authors certify that we have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, major honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product.
References
- 1.Friedman SL, Rockey DC, Bissell DM. Hepatic fibrosis 2006: report of the Third AASLD Single Topic Conference. Hepatology. 2007;45:242–249. doi: 10.1002/hep.21459. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi RS, Rockey DC. Complications of cirrhosis. Curr Opin Gastroenterol. 2012;28:223–229. doi: 10.1097/MOG.0b013e328351d003. [DOI] [PubMed] [Google Scholar]
- 3.Starr SP, Raines D. Cirrhosis: diagnosis, management, prevention. Am Fam Physician. 2011;84:1353–1359. [PubMed] [Google Scholar]
- 4.Mallat A, Hezode C, Lotersztajn S. Environmental factors as disease accelerators during chronic hepatitis C. J Hepatol. 2008;48:657–665. doi: 10.1016/j.jhep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 7.Wright M, Goldin R, Fabre A, Lloyd J, Thomas H, et al. Measurement and determinants of the natural history of liver fibrosis in hepatitis C virus infection: a cross sectional and longitudinal study. Gut. 2003;52:574–579. doi: 10.1136/gut.52.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stinson FS, Grant BF, Dufour MC. The critical dimension of ethnicity in liver cirrhosis mortality statistics. Alcohol Clin Exp Res. 2001;25:1181–1187. [PubMed] [Google Scholar]
- 9.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Alimentary Pharmacology and Therapeutics. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi RS, Rockey DC. Complications and outcomes in chronic liver disease. Curr Opin Gastroenterol. 2011;27:204–209. doi: 10.1097/MOG.0b013e3283460c7d. [DOI] [PubMed] [Google Scholar]
- 11.Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell SH, Lee VD, Kleiner DE, Al-Osaimi AM, Argo CK, Northup PG, Berg CL. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8:436–352. [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell S. Cryptogenic cirrhosis: what are we missing? Curr Gastroenterol Rep. 2010;12:40–48. doi: 10.1007/s11894-009-0082-7. [DOI] [PubMed] [Google Scholar]
- 14.Verma S, Torbenson M, Thuluvath PJ. The impact of ethnicity on the natural history of autoimmune hepatitis. Hepatology. 2007;46:1828–1835. doi: 10.1002/hep.21884. [DOI] [PubMed] [Google Scholar]
- 15.Bambha K, Belt P, Abraham M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Youth risk behavior surveillance: national college health risk behavior survery – United States, 1995. MMWR CDC Surveill Summ. 1997;46:1–56. [PubMed] [Google Scholar]
- 17.Browning JD, Szcepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 18.Puppala J, Siddapuram SP, Akka J, Munshi A. Genetics of Nonalcoholic fatty liver disease: an overview. J Genet Genomics. 2013;40:15–22. doi: 10.1016/j.jgg.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Krueger PM, Coleman-Minahan K, Rooks RN. Race/ethnicity, nativity, and trends in body mass index among U. S. adults. Obesity Advance online publication. 2014 doi: 10.1002/oby.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearlman BL. Hepatitis C virus infection in African Americans. Clin Infect Dis. 2006;42:82–91. doi: 10.1086/498512. [DOI] [PubMed] [Google Scholar]
- 21.Pyrsopoulos N, Jeffers L. Chronic hepatitis C in African Americans. Clin Liver Dis. 2005;9:427–438. doi: 10.1016/j.cld.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Bonacini M, Govinarajan S, Kanel G, Lindsay KL, Redeker A. More advanced hepatic fibrosis in Hispanics with chronic hepatitis C infection: Role of patient demographics, hepatic necroinflammation, and steatosis. Am J Gastroenterol. 2006;101:1817–1823. doi: 10.1111/j.1572-0241.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 23.Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J. HIV, age, the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med. 2013;158:658–666. doi: 10.7326/0003-4819-158-9-201305070-00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bataller R, North KE, Brenner DA. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003;37:493–503. doi: 10.1053/jhep.2003.50127. [DOI] [PubMed] [Google Scholar]
- 25.Guo J, Loke J, Zheng F, Hong F, Yea S, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 27.Grunhage F, Hochrath K, Krawczyk M, Hoblinger A, Obermayer-Pietsch B, et al. Common genetic variation in vitamin D metabolism is associated with liver stiffness. Hepatology. 2012;56:1883–1891. doi: 10.1002/hep.25830. [DOI] [PubMed] [Google Scholar]
- 28.Marcolongo M, Young B, Dal Pero F, Fattovich G, Peraro L, et al. A seven-gene signature (cirrhosis risk score) predicts liver fibrosis progression in patients with initially mild chronic hepatitis C. Hepatology. 2009;50:1038–1044. doi: 10.1002/hep.23111. [DOI] [PubMed] [Google Scholar]
- 29.Pradat P, Trepo E, Potthoff A, Bakshi R, Young B, et al. The cirrhosis risk score predicts liver fibrosis progression in patients with initially mild chronic hepatitis C. Hepatology. 2009;51:356–357. doi: 10.1002/hep.23223. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Shiffman ML, Cheung RC, Layden TJ, Friedman S, et al. Identification of two gene variants associated with risk of advanced fibrosis in patients with chronic hepatitis C. Gastroenterology. 2006;130:1679–1687. doi: 10.1053/j.gastro.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Lempicki RA, Schlaak JF, Murphy A, Kottilil S. Genome-wide single nucleotide polymorphism analysis identifies unique integrin beta 5 (ITGB5) haplotype in advanced liver fibrosis patients. Hepatology. 2009;50:942A. [Google Scholar]
- 32.Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, et al. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet. 2005;37:835–843. doi: 10.1038/ng1599. [DOI] [PubMed] [Google Scholar]
- 33.Skill N, Wu J, Xu Y, Zhao Z, Maluccio M. Lysophosphatidic acid aberrancies and hepatocellular carcinoma: studies in the MDR2 gene knockout mouse. Cancer Invest. 2013;31:145–155. doi: 10.3109/07357907.2012.762779. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–428. doi: 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Thieringer F, Maass T, Czochra P, Klopcic B, Conrad I, et al. Spontaneous hepatic fibrosis in transgenic mice overexpressing PDGF-A. Gene. 2008;423:23–28. doi: 10.1016/j.gene.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Jia D, Duan F, Peng P, Sun L, Liu X, et al. Up-regulation of RACK1 by TGF-β1 promotes hepatic fibrosis in mice. PLoS One. 2013 doi: 10.1371/journal.pone.0060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wandzioch E, Kolterud A, Jacobsson M, Friedman SL, Carlsson L. Lhx2−/− mice develop liver fibrosis. Proc Natl Acad Sci U S A. 2004;101:16549–16554. doi: 10.1073/pnas.0404678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warskulat U, Borsch E, Reinehr R, Heller-Stilb B, Monnighoff I, et al. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006;20:574–576. doi: 10.1096/fj.05-5016fje. [DOI] [PubMed] [Google Scholar]
- 40.Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Hillebrandt S, Goos C, Matern S, Lammert F. Genome-wide analysis of hepatic fibrosis in inbred mice identifies the susceptibility locus Hfib1 on chromosome 15. Gastroenterology. 2002;123:2041–2051. doi: 10.1053/gast.2002.37069. [DOI] [PubMed] [Google Scholar]
- 42.Bell BP, Manos MM, Zaman A, Terrault N, Thomas A, et al. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol. 2008;103:2727–2736. doi: 10.1111/j.1572-0241.2008.02071.x. [DOI] [PubMed] [Google Scholar]
- 43.Sofair AN, Barry V, Manos MM, Thomas A, Zaman A, et al. The epidemiology and clinical characteristics of patients with newly diagnosed alcohol-related liver disease: results from population-based surveillance. J Clin Gastroenterol. 2010;44:301–307. doi: 10.1097/MCG.0b013e3181b3f760. [DOI] [PubMed] [Google Scholar]