Abstract
Memories of drug experience and drug-associated environmental cues can elicit drug-seeking and taking behaviors in humans. Disruption of reconsolidation of drug memories dampens previous memories and therefore may provide a useful way to treat drug abuse. We and others previously demonstrated that dopamine D1 and D3 receptors play differential roles in acquiring cocaine-induced behaviors. Moreover, D3 receptors contribute to the reconsolidation of cocaine-induced conditioned place preference. In the present study, we examined effects of manipulating D1 or D3 receptors on reconsolidation of cocaine memories in mouse models of drug self-administration. We found that pharmacological blockade of D1 receptors or a genetic mutation of the D3 receptor gene attenuated reconsolidation that lasted for at least 1 week after the memory retrieval. In contrast, with no memory retrieval, pharmacological antagonism of D1 receptors or the D3 receptor gene mutation did not significantly affect reconsolidation of cocaine memories. Pharmacological blockade of D3 receptors also attenuated reconsolidation in wild-type mice that lasted for at least 1 week after the memory retrieval. These results suggest that D1 and D3 receptors and related signaling mechanisms play key roles in reconsolidation of cocaine memories in mice, and that these receptors may serve as novel targets for the treatment of cocaine abuse in humans.
Keywords: dopamine D1 and D3 receptors, reconsolidation, drug memory, antagonists, gene mutation, cocaine self-administration
INTRODUCTION
A central feature of drug addiction is the compulsive seeking and taking of drugs despite known negative consequences. Addicts experience drug craving after long periods of abstinence and are highly susceptible to relapse (O’Brien et al, 1998; Dackis and O’Brien, 2005). Memories of drug effects or learned associations between the rewarding properties of drugs and cues are thought to precipitate craving and relapse. Reconsolidation is a process in which memory undergoes a transiently labile stage after its retrieval and needs to be consolidated again in order to be maintained (Nader et al, 2000; Miller and Sweatt, 2006; Tronson and Tayler, 2007; Alberini, 2011). Pharmacological or molecular manipulations of reconsolidation of acquired drug memories have been shown to disrupt drug-seeking and relapsing behavior in animal models (Miller and Marshall, 2005; Lee et al, 2005; 2006; Valjent et al, 2006; Taylor et al, 2009; Sanchez et al, 2010; Yan et al, 2013) and in drug addicts (Xue et al, 2012). These studies suggest that understanding the molecular basis of reconsolidation of reward memory may help to develop new medications for the treatment of drug abuse (Sorg, 2012; Tronson and Taylor, 2013).
The mesolimbic dopamine (DA) projections are a major neural substrate for mediating actions of drugs of abuse that can increase synaptic levels of DA that is required for reward and reinforcement (Hyman et al., 2006; Kalivas and O’Brien, 2008; Koob and Volkow, 2010; Luscher and Malenka, 2011). Recent studies suggest that DA is involved in reward learning and that drugs of abuse can change related neuronal circuits in the mesolimbic DA system (Ito et al, 2000; Stuber et al, 2005; Hyman et al, 2006; Wise, 2008; Volkow et al, 2009; Schultz, 2010; Torregrossa et al, 2011; Milton and Everitt, 2012). DA binds to DA receptors to trigger many molecular, physiological and behavioral changes. Five DA receptors have been identified and classified into two subfamilies (Beaulieu and Gainetdinov, 2011). The D1-like family includes D1 and D5 receptors that interact with Gs proteins. The D2-like family includes D2, D3 and D4 receptors that interact with Gi or G0 proteins. Both D1 and D3 receptors are expressed in mesolimbic DA projection areas. We and others have shown that D1 (Xu et al, 1994a; 1994b; 2000; Anderson et al, 2003; Bachtell et al, 2005; Alleweireldt et al, 2006; Berglind et al, 2006; Caine et al, 2007; Chen and Xu, 2010) and D3 receptors (Xu et al, 1997; Pilla et al., 1999; Vorel et al., 2002; Di Ciano et al., 2003; Neisewander et al., 2004; Xi et al., 2004; 2005; 2006; Martelle et al., 2007; Micheli and Heidbreder, 2008; Heidbreder and Newman, 2010; Achat-Mendes, et al, 2010; Chen and Xu, 2010; Kong et al, 2011; Song et al, 2012a, b) mediate locomotor-stimulant and positive reinforcing effects of cocaine, as well as cue-induced reinstatement of cocaine-seeking.
D1 receptor-based medications have been tried to reduce euphoric effects of cocaine in addicts, and despite the D1 receptor’s key role in mediating cocaine actions in preclinical studies, the results have not been consistent (Haney et al, 1999; 2001; Romach et al, 1999; Nann-Vernotica et al, 2001). Manipulating D1 receptor activity during reconsolidation may provide a new time window to treat cocaine abuse. Many D3 receptor agonists and antagonists have been developed and tested and several D3 receptor antagonists show promise for attenuating reinstatement of drug-seeking in preclinical studies (Micheli and Heidbreder, 2008; Heidbreder and Newman, 2010; Newman et al, 2012). Among the many D3 receptor-preferring antagonists that have been developed and evaluated, PG01037 (N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide) selectively blocked D3-agonist induced yawning and attenuates reinstatement of drug-seeking via pharmacological antagonism of D3 receptors (Collins et al, 2005; 2007; Xi et al, 2006; Martelle et al, 2007, Achat-Mendes et al, 2010; Higley et al, 2011). We previously used both a D3 receptor mutant mouse model and PG01037 and found that D3 receptors play a key role in reconsolidation of cocaine-induced conditioned place preference (CPP) (Yan et al, 2013), implying the involvement of DA signaling in reconsolidation of cocaine memory. The rodent model of intravenous drug self-administration mimics voluntary drug intake in humans and is arguably the best available preclinical model to study the neurobiological basis of drug-seeking and taking (O’Brien and Gardner, 2005; Epstein et al, 2006; Kalivas et al, 2006). In the current study, we have used both pharmacological and genetic approaches to investigate the role of D1 receptors, and to further study the role of D3 receptors in reconsolidation of cocaine memories in mouse models of drug self-administration.
EXPERIMENTAL PROCEDURES
Mice and drugs
The engineering of the D3 receptor mutant mouse model, which resulted in a complete loss of D3 receptors, has been described in a previous report (Xu et al, 1997). Homozygous D3 receptor mutant mice and their wild-type littermates were produced by crossing D3 receptor heterozygous mutant mice. Wild-type mice were used to test the effects of the D1 receptor antagonist SCH23390 on reconsolidation of cocaine memories and they were bred by crossing wild-type mice. Genotypes of all mice were determined by genomic Southern blotting (Xu et al, 1994a; Xu et al, 1997). Mice were group housed under controlled temperature and humidity conditions with a 12-h light/dark cycle. Water and food were available ad libitum. Roughly equal numbers of male and female mice, 10 to 18 weeks of age, were used. Mice weighed around 25–30 g at the beginning of the experiments. All procedures followed National Institutes of Health Guide for the Care and Use of Laboratory Animal and were approved by the University of Chicago Institutional Animal Care and Use Committee.
Cocaine hydrochloride and SCH23390 were purchased from Sigma Chemical Co. (St. Louis, MO) and dissolved in sterile 0.9% saline. Cocaine was self-administered through catheterized tubing into the jugular vein. PG01037 was synthesized at the National Institute on Drug Abuse-Intramural Research Program (Baltimore, MD) using previously published methods (Grundt et al, 2005; 2007). PG01037 was initially described (Grundt et al, 2005; 2007) as a highly potent (Ki=0.7 nM) D3 receptor-selective antagonist (133-fold over D2 receptors). Although PD01037 also showed actions with low affinities at histamine H1, 5-HT1A, 2A, 2C, α1 and α2 adrenergic receptors (Kumar et al, 2009), it preferentially binds to D3 receptors. PG01037 rapidly penetrates the blood brain barrier and selectively localizes in D3 receptor-rich regions, such as the nucleus accumbens (NAc), Islets of Calleja and the hippocampus (Grunt et al, 2007). Therefore, it is appropriate for in vivo studies. PG01037 was first characterized as a D3 receptor-selective antagonist using a D3 receptor agonist yawning model in rats (Collins et al, 2005; 2007) and subsequently has been tested in numerous rodent and nonhuman primate models of psychostimulant abuse (Heidbreder and Newman, 2010). The selection of PD01037 dose in the present study was based on these behavioral studies and our recent report using a cocaine CPP paradigm (Yan et al, 2013) that demonstrated the 30 mg/kg dose was optimal for the antagonism of D3 receptors in in vivo studies. PG01037 was first dissolved in dimethyl sulfoxide (DMSO) and then diluted with sterile saline to 2% DMSO in saline (Yan et al, 2013). The selection of the SCH23390 doses was based on previous studies on acute locomotor activity (Xu et al, 1994a) and cocaine self-administration (Caine et al, 2007). SCH23390 and PG01037 were administered intraperitoneally (i.p.) in a volume of 10 ml/kg body weight.
Catheterization
The construction and implantation of tubing have been described in our previous reports (Yan et al, 2006; 2007; 2012). Indwelling catheters were constructed of micro-silicone tubing (inner diameter, 0.50 mm; outer diameter, 0.7 mm; Braintree Scientific Inc., Braintree, MA) and polyethylene tubing (inner diameter, 0.50 mm; outer diameter, 0.8 mm, Braintree Scientific Inc., Braintree, MA). D3 receptor mutant mice and different groups of wild-type mice were anesthetized with a combination of xylazine hydrochloride (10 mg/kg, i.p., Sigma-Aldrich, St Louis, MO) and ketamine hydrochloride (90 mg/kg, i.p., Sigma-Aldrich, St Louis, MO). Incisions were made on the skin of the head and ventral neck, and the right jugular vein was externalized. The end of the catheter was inserted into the jugular vein via a small incision and was secured to the vein and surrounding tissues with silk sutures (South Pointe Surgical Supply Inc., Coral Springs, FL). The exit port of the catheter passed subcutaneously to the top of the skull where it was attached to a modified 24-gauge cannula (Braintree Scientific Inc., Braintree, MA), which was secured to the mouse’s skull with all-purpose Instant Krazy Glue (Walgreens, Chicago). Buprenorphine (Sigma-Aldrich, St Louis, MO) was subcutaneously administered (0.10 mg/kg) for postoperative analgesia once a day for at least 3 days. To extend catheter patency, the catheters were flushed once a day immediately after surgery or cocaine self-administration training with 0.05 ml of heparin in saline (30 Unit/ml; Fisher Scientific, Pittsburgh, PA).
Intravenous cocaine self-administration and extinction
Intravenous self-administration (IVSA) was conducted in standard mouse operant conditioning chambers (ENV-307A, Med Associates, Georgia, VT) located in a behavioral procedure room (Yan et al, 2006; 2007; 2012). The chambers were equipped with nose-poke sensors (ENV-313M, Med Associates) in two holes located on one side of the chamber 1.0 cm above the floor, and cue- and hole-lamps located, respectively, above and in each hole, and a house light located on the top of the chamber opposite the holes. During cocaine self-administration training, one hole was set as active and the other inactive. Nose-pokes in the active hole triggered pump (PHM-100, Med Associates) infusions (3 s) and turned on both cue-lamp and hole-lamp (10 s). Nose-pokes in the inactive hole and active hole during the timeout period (30 s) had no programmed consequences but were recorded. The components of the infusion line were connected from the injector to the exit port of the mouse’s catheter by PE20 tubing (Instech, Plymouth Meeting, PA), which was encased in steel spring leashes (Instech, Plymouth Meeting, PA). Swivels were suspended above the chamber. One pump/syringe set was located inside of each cubicle.
After recovery from the catheterization (3–7 days), mice were initially subjected to 3 h daily sessions of cocaine self-administration under a fixed ratio (FR) 1 schedule for 5 days, and the cocaine reinforcement schedule was then changed to an FR2 for an additional 9–10 days. A combination of an FR1 and FR2 schedules was selected for cocaine self-administration training in the current study because this schedule of reinforcement may facilitate self-administration training and shorten extinction training (Yan and Nabeshima, 2009). Based on previous reports (Caine et al, 2007; 2012) and our preliminary data, the unit dose of cocaine was used at 0.6 mg/kg/infusion over 3 s (infusion volume, 6.6 μl) for IVSA in our study.
Once stable cocaine self-administration, defined as deviations of less than 20% of the mean active responses in 3 consecutive training sessions, was established, mice were subjected to 3 h daily sessions of extinction training. Throughout the extinction session, the house light was on. The cocaine-associated cue- and hole-lamps, and the pump for cocaine infusions, were turned off. Therefore, nose-pokes into the previously active hole resulted in neither an infusion of cocaine nor cocaine-associated cues (cue- and hole-lamps) though responding was recorded. The extinction criterion was met when there were less than 15 active responses or 20% of active responses in the stable phase of self-administration in 2 consecutive sessions. In the current study, mice met the extinction criterion after 4–10 days of training.
Reconsolidation
Once the extinction criterion was met, mice were subjected to reconsolidation testing or no-retrieval control testing. For groups with the memory retrieval, mice were connected to self-administration manipulates for 10 min. Based on our preliminary data and previous reports (Sanchez et al, 2010; Wells et al, 2013), a 10-min interval was selected for the retrieval. During this period, mice were exposed to cocaine-associated cues, but with no infusion of cocaine, after active nose-pokes. If there was no active nose-poke response made in the first 6 min, non-contingent cocaine-associated cues were presented 3 times with 1-min intervals. SCH23390, PG01037, or their vehicles were i.p. administered immediately after the 10-min retrieval. All mice were returned to their home cages afterwards. 24 h, 48 h or 1 week after the 10-min retrieval, mice were tested for reconsolidation of cocaine self-administration under FR2 schedule for 3 h, during which there were cocaine-associated cues but no cocaine infusions after active nose-poke responses were presented.
For control groups with no memory retrieval, mice were taken to the behavioral testing room and they were not connected to self-administration manipulates. Mice were given an i.p. injection of SCH23390, or its vehicles, and they were returned to their home cages in the housing room. 24 h, 48 h or 1 week after the treatment of drugs or vehicles, mice were tested for reconsolidation of cocaine self-administration under FR2 schedule for 3 h, during which there were cocaine-associated cues but no cocaine infusions after active nose-poke responses were presented.
For effects of the D3 receptor gene mutation on reconsolidation of cocaine self-administration, D3 receptor mutant mice and wild-type littermates were connected to self-administration manipulates for 10 min, but were without any drug or vehicle treatment after the 10-min retrieval. Mice were returned to their home cages afterwards. No-retrieval groups of D3 receptor mutant mice and wild-type littermates were taken to the behavioral room and they were not connected to self-administration manipulates. Mice were not given drug or vehicle treatment and they were returned to their home cages. 24 h, 48 h or 1 week afterwards, mice were subjected to testing for reconsolidation of cocaine self-administration under FR2 schedule for 3 h, during which there were cocaine-associated cues but no cocaine infusions after active nose-poke responses were presented.
Data analysis
All data were expressed as the mean ± SEM. A repeated measures ANOVA was used to analyze the data from acquisition of IVSA in wild-type mice for D1 receptor antagonist treatment. A two-way ANOVA with repeated measures was used to analyze the data from acquisition of IVSA for wild-type and D3 receptor mutant mice, effects of the D1 receptor antagonist SCH23390 and D3 receptor antagonist PG01037, and the D3 receptor gene mutation on reconsolidation of cocaine self-administration. All post hoc ANOVAs were followed by Bonferroni multiple comparisons (Yan et al, 2013). In all cases, a significant difference was set at P<0.05.
RESULTS
Effects of the D1 receptor antagonist SCH23390 on reconsolidation of cocaine memory
After recovery from the catheterization, wild-type mice were subjected to 3 h daily sessions of cocaine self-administration training for 5 days under an FR1 schedule of reinforcement and then for 9 additional days under an FR2 schedule of reinforcement. A repeated measures ANOVA analysis revealed that mice started to discriminate active from inactive nose-pokes on day 4 and acquired stable cocaine self-administration after 14 days of training [Fig 1, F (31, 403) = 9.88, P< 0.001].
Fig. 1.
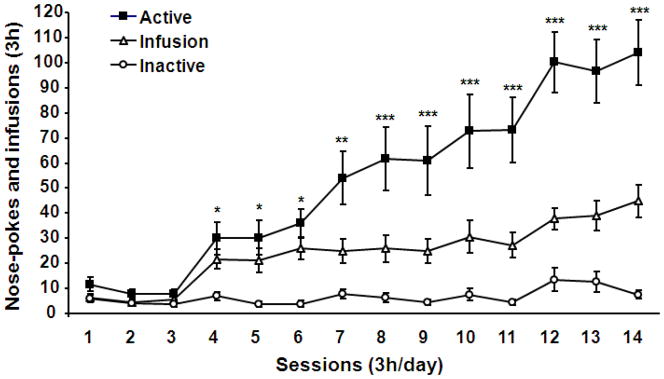
Acquisition of intravenous cocaine self-administration in wild-type mice. Day 1–5: FR1 schedule of cocaine reinforcement, and day 6–14: FR2 schedule of cocaine reinforcement. N = 16 mice. Data represent mean±SEM. *P<0.05, **P<0.01, and ***P<0.001 active versus inactive nose-pokes.
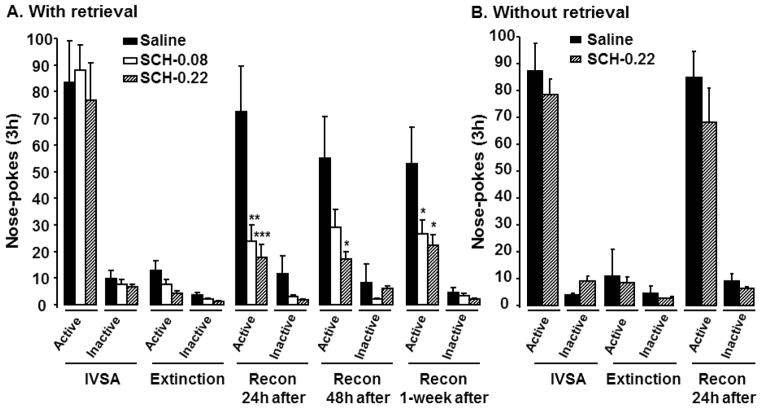
Starting on day 15, mice were subjected to 3 h daily sessions of extinction training for 4–10 days. Once the extinction criterion was met (Fig 2A), mice were then divided into three sub-groups. On the next day, the three sub-groups of mice were subjected to a 10-min memory retrieval followed immediately by an i.p. injection of saline or SCH23390 (0.08 or 0.22 mg/kg). 24 h, 48 h, and 1 week after the 10-min retrieval, the three sub-groups of mice were subjected to reconsolidation testing. Two-way ANOVA analysis with different SCH23390 doses and behavioral testing as fixed factors indicated that SCH23390 attenuated reconsolidation of cocaine self-administration in mice [Fig 2A, F (2, 21) = 3.70, P= 0.04]. This attenuation lasted for at least 1 week (Fig 2A). Without the 10-min memory retrieval, two-way ANOVA analysis with SCH23390 treatment and behavioral testing as fixed factors indicated that there was no significant difference in cocaine self-administration between the saline- and SCH23390-treated groups (0.22 mg/kg, i.p. administered 24 h prior to the reconsolidation testing) [Fig 2B, F (1, 7) = 0.55, P= 0.48]. Together, these results demonstrate that the blockade of D1 receptors with SCH23390 attenuated reconsolidation of cocaine memory in mouse models of drug self-administration.
Fig. 2.
Effects of the D1 receptor antagonist SCH23390 on reconsolidation of cocaine self-administration in wild-type mice. Wild-type mice were subjected to 3 h daily self-administration training and showed stable IVSA. Mice then went through 3 h extinction training daily. Extinction data were from the last day of training. Once the extinction criterion was met, mice were subjected to a 10-min retrieval. Reconsolidation was tested 24 h, 48 h or 1 week after the retrieval (A), or 24 h after no retrieval manipulations (B). SCH23390 was administered i.p. immediately after the retrieval or during no retrieval manipulations. N = 6–8 mice for each group. Data represent mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001 SCH23390 versus saline treatment. Recon: reconsolidation. SCH-0.08: SCH23390-0.08 mg/kg. SCH-0.22: SCH23390-0.22 mg/kg.
Effects of a D3 receptor gene mutation on reconsolidation of cocaine memory
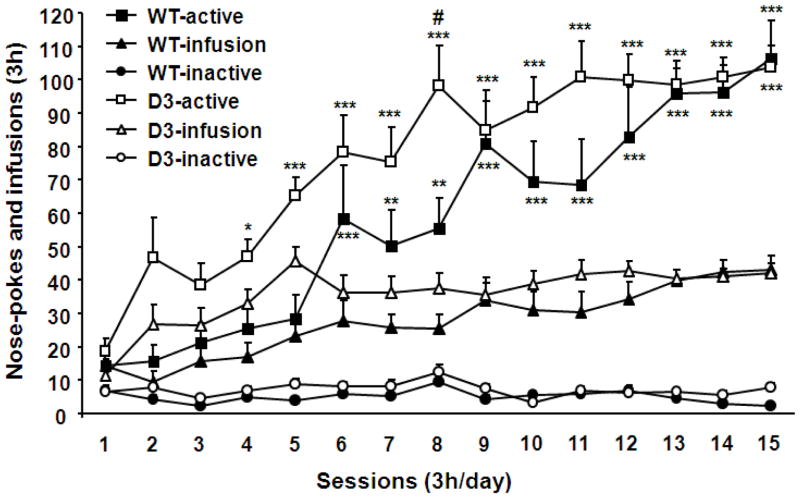
To further study the role of D3 receptors in reconsolidation of cocaine memory, we first used D3 receptor mutant mice and wild-type littermates. These mice were subjected to 3 h daily sessions of cocaine self-administration training for 5 days under an FR1 schedule of reinforcement and then for 10 additional days under an FR2 schedule of reinforcement. As shown in Fig 3, wild-type mice started to discriminate active from inactive nose-pokes on day 6 and D3 receptor mutant mice on day 4. Both groups of mice acquired stable cocaine self-administration following the 15 day training [F (14, 840) =11.18, P<0.001]. Two-way ANOVA analysis with genotypes and behavioral testing as fixed factors suggested that there was a significant difference in active nose-pokes between D3 receptor mutant mice and their wild-type littermates [F (3, 60) = 41.48, P< 0.001]. The post-hoc analysis with Bonferroni multiple comparisons indicated that D3 receptor mutant mice did more active nose-pokes than their wild-type littermates on day 8 (P<0.05). In contrast, there was no significant difference in inactive nose-pokes between D3 receptor mutant mice and wild-type littermates (P>0.05).
Fig. 3.
Acquisition of intravenous cocaine self-administration in D3 receptor mutant mice and wild-type littermates. Day 1–5: FR1 schedule of cocaine reinforcement, and day 6–15: FR2 schedule of cocaine reinforcement. N = 14–18 mice for each group. Data represent mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001 active versus inactive nose-pokes. #P<0.05 active nose-pokes in D3 receptor mutant versus those in wild-type mice.
From day 16 on, D3 receptor mutant mice and wild-type littermates were subjected to 3 h daily sessions of extinction training until the extinction criterion was met (Fig 4A). On the next day, both groups of mice were subjected to a 10-min memory retrieval in operant chambers. 24 h, 48 h and 1 week after the 10-min retrieval, these mice were tested for reconsolidation. Two-way ANOVA analysis with genotypes and behavioral testing as fixed factors suggested that the mutation of D3 receptor gene in mice disrupted the reconsolidation of cocaine memory [Fig 4A, F (1, 23) = 9.89, P= 0.005]. This disruption lasted for at least 1 week (Fig 4A). In the absence of the 10-min memory retrieval, although the D3 receptor gene mutation showed a tendency toward reduced active nose-pokes for cocaine-seeking behavior when tested 24 h later, two-way ANOVA analysis with genotypes and testing as fixed factors indicated that D3 receptor mutation did not affect cocaine self-administration with no memory retrieval [Fig 4B, F (1, 20) = 0.94, P= 0.34]. These results suggest that the mutation of the D3 receptor gene disrupted reconsolidation of cocaine memories.
Fig. 4.
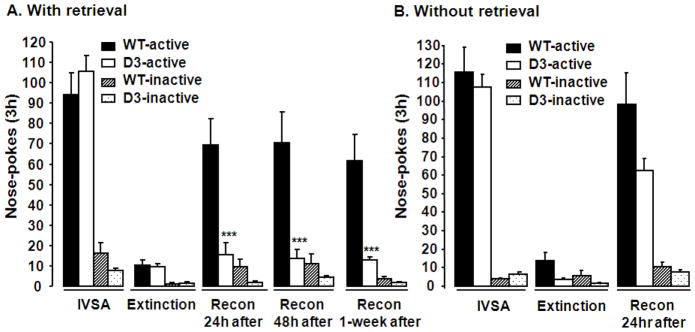
Effects of a D3 receptor gene mutation on reconsolidation of cocaine self-administration. D3 receptor mutant mice and wild-type littermates were trained to stably self-administer cocaine. Mice were then subjected to extinction training. Extinction data were from the last day of training. Once the extinction criterion was met, mice were connected to IVSA manipulates for 10 min, during which mice could trigger the presentation of drug-associated cues, but were with no cocaine infusions. Reconsolidation was tested 24 h, 48 h or 1 week after the retrieval (A), or 24 h after no retrieval manipulations (B). N = 8–13 for each group. Data represent mean ± SEM. ***P<0.001 active nose-pokes in D3 receptor mutant versus those in wild-type mice. Recon: reconsolidation.
Effects of the D3 receptor selective antagonist PG01037 on reconsolidation of cocaine memory
To further confirm the role of D3 receptors in reconsolidation of cocaine memories, we examined the effects of the selective D3 receptor antagonist PG01037 on reconsolidation in wild-type mice and D3 receptor mutant littermates. Both groups of mice were subjected to 3 h daily sessions of cocaine self-administration training for 5 days under an FR1 schedule of reinforcement and then for 10 additional days under an FR2 schedule of reinforcement (Fig 5A). Starting on day 16, mice were subjected to 3 h daily sessions of extinction training. Once the extinction criterion was met (Fig 5A), each group of mice was divided into two sub-groups. On the next day, all groups of mice were subjected to a 10-min memory retrieval followed immediately by an i.p. injection of PG01037 (30 mg/kg) or its vehicle. 24 h, 48 h and 1 week after the 10-min retrieval, these mice were tested for reconsolidation. Two-way ANOVA analysis with PG01037 treatment and behavioral testing as fixed factors indicated that PG01037 at 30 mg/kg (i.p.) blocked the reconsolidation of cocaine memories in wild-type mice [Fig 5B, F (1, 22) = 12.27, P= 0.0004], and that reconsolidation in D3 receptor mutant mice was attenuated after vehicle treatment [F (1, 23) = 13.50, P= 0.0013]. In contrast, the PG01037 treatment in D3 receptor mutant mice did not significantly affect the attenuation of the reconsolidation of cocaine memories caused by the D3 receptor gene mutation. Taken together, these results suggest that D3 receptors play a critical role in reconsolidation of cocaine memory in mouse models of drug self-administration.
Fig. 5.
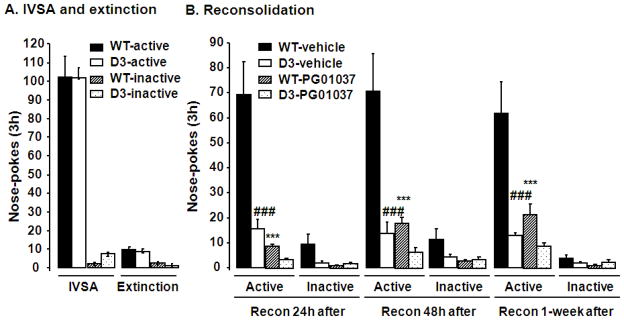
Effects of the D3 receptor antagonist PG01037 on reconsolidation of cocaine self-administration. Wild-type and D3 receptor mutant mice were subjected to daily IVSA training and showed stable acquisition (A). Once the extinction criterion was met following daily extinction training (A), mice were subjected to a memory retrieval for 6–10 min. Reconsolidation was tested 24 h, 48 h or 1 week after the retrieval (B). PG01037 (30 mg/kg) or its vehicle was administered i.p. immediately after the retrieval. N = 8–13 for each group. Data represent mean ± SEM. ***P<0.001 PG01037 versus vehicle treatment in wild-type mice. ###P<0.001 D3 receptor mutant versus wild-type mice with vehicle treatment. Recon: reconsolidation. Pharmacological blockade of D1 or D3 receptors disrupts reconsolidation of cocaine memory Genetic mutations in D3 receptors attenuate reconsolidation of cocaine-induced reward memory Dopamine D1 and D3 receptors may serve as new targets for combating cocaine abuse
DISCUSSION
Memories of drug experience and drug-associated cues can elicit drug craving and relapse in humans. Emerging studies demonstrated that molecular manipulations of the N-methyl-D-aspartate receptor, and the extracellular signal-regulated kinase and protein kinase A-mediated signaling events in the context of reconsolidation of drug-induced reward memory can reduce drug craving and seeking behavior in animal models (Lee et al, 2005; 2006; Miller and Marshall, 2005; Valjent et al, 2006; Taylor et al, 2009; Sanchez et al, 2010) and in drug addicts (Xue et al, 2012). These findings suggest that identifying novel molecular targets of reconsolidation may aid the treatment of drug abuse (Sorg, 2012; Tronson and Taylor, 2013). We and others have demonstrated that DA D1 and D3 receptors play differential roles in acquiring cocaine-induced behaviors (Xu et al, 1994a; 1994b, 2007; Caine et al, 2007; Chen and Xu, 2010; Kong et al, 2011). Moreover, D3 receptors contribute to the reconsolidation of cocaine-induced CPP (Yan et al, 2013). In this study, we further studied dopaminergic mechanisms of reconsolidation cocaine memories. Our findings demonstrate that D1 and D3 receptors play critical roles in reconsolidation of cocaine memories, and that these receptors may serve as novel targets for the treatment of cocaine abuse in humans.
Role of D1 receptors in reconsolidation of cocaine memory
We previously used D1 receptor mutant mice and found that they do not acquire cocaine-induced CPP at several doses (Chen and Xu, 2010) and acquire little cocaine self-administration even after extended training (Caine et al, 2007). Although the D1 receptor mutant mouse model was very useful in studying functions of D1 receptors in the acquisition of cocaine-induced CPP and operant behaviors as well as underlying molecular mechanisms, this mouse model cannot be used to evaluate the role of D1 receptors in reconsolidation of cocaine memory. Consequently, we used a pharmacological approach to address this issue in the current study. We first trained wild-type mice to acquire stable cocaine self-administration behavior (Fig 1). Following extinction training and after the extinction criterion has been met (Fig 2A), we administered i.p. SCH23390 immediately following the 10-min memory retrieval in these mice. We found that SCH23390, at both the 0.08 and 0.22 mg/kg doses, attenuated reconsolidation of cocaine self-administration (Fig 2A). Such attenuation in reconsolidation remained at least 1 week after the memory retrieval (Fig 2A). In the absence of the 10-min retrieval, mice in the control group showed normal levels of reconsolidation (Fig 2B). These results suggest that D1 receptors contribute to the reconsolidation of cocaine self-administration in mice. To the best of our knowledge, this is the first demonstration that D1 receptors contribute to reconsolidation of cocaine-induced reward memory. D1 receptor-based medications have been tried to treat cocaine addicts yet the results have not been consistent (Haney et al, 1999; 2001; Romach et al, 1999; Nann-Vernotica et al, 2001). Our current results provide preclinical evidence for a potential new time window for developing D1 receptor-based treatment for cocaine abuse.
Role of D3 receptors in reconsolidation of cocaine memory
We previously used D3 receptor mutant mice and found that they exhibit potentiated acquisition of cocaine-induced CPP at lower, but not higher doses of cocaine compared to their wild-type littermates (Chen and Xu, 2010; Kong et al, 2011). Others have shown that D3 receptor mutant mice exhibit enhanced (Song et al, 2012a) or relatively normal acquisition of cocaine self-administration (Caine et al, 2012). In the current study, we found that D3 receptor mutant mice did more active nose-pokes than their wild-type littermates during acquisition training (Fig 3). Following extinction training and after the extinction criterion has been met, we found that the mutation of the D3 receptor gene in mice reduced reconsolidation of cocaine self-administration (Fig 4A). Such reduction in reconsolidation lasted for at least 1 week after the memory retrieval (Fig 4A). Moreover, in the absence of the retrieval, there was no significant difference in cocaine-seeking between wild-type and D3 receptor mutant mice (Fig 4B). This is consistent with our previous studies using the D3 receptor mutant mice and a CPP paradigm (Yan et al, 2013), and it further suggests that D3 receptors participate in mechanisms related to reconsolidation of cocaine-induced reward memory.
PG01037 is an antagonist which shows high affinity and selectivity for D3 receptors in vitro and in vivo (Grundt et al, 2005; 2007; Micheli and Heidbreder, 2008; Heidbreder and Newman, 2010). We previously found that PG01037 administration immediately following a 3-min memory retrieval disrupted reconsolidation of cocaine-induced CPP in wild-type mice and such disruption remained at least 1 week after the retrieval (Yan et al, 2013). With no memory retrieval, PG01037 did not affect cocaine-induced CPP (Yan et al, 2013). To expand this finding and those using the D3 receptor mutant mice, following acquisition and extinction training, we administered PG01037 immediately following the 10-min memory retrieval in wild-type and D3 receptor mutant mice. PG01037 but not vehicle attenuated reconsolidation of cocaine self-administration at the 30 mg/kg dose in wild-type mice (Fig 5). Moreover, such attenuation in reconsolidation remained at least 1 week after the retrieval (Fig 5). PG01037 treatment in D3 receptor mutant mice did not significantly affect the attenuation of the reconsolidation of cocaine memories caused by the D3 receptor gene mutation, although it does appear that there was some minor pharmacological effect when reconsolidation was tested at 48 h time point, possibly due to non-specific antagonism of PG01037 at DA D2 and other receptors.
We note that under no retrieval conditions, D3 receptor mutant mice showed a tendency toward a reduction in nose-pokes compared to that exhibited by wild-type littermates (Fig 4B). One possible explanation is that, due to the lack of D3 receptors, these mutant mice show deficits in memory retrieval for cocaine-seeking. Supporting this possibility is the fact that a D3 receptor antagonist, YQA14, reduces cocaine-seeking behavior in mice (Song et al, 2012b). The fact that D3 receptor mutant mice showed a loss of reconsolidation after memory retrieval (Fig 4A) and a non-significant reduction in nose-pokes without the retrieval (Fig 4B), combined with results from the pharmacological studies described above, is consistent with the interpretation that D3 receptors contribute to reconsolidation of cocaine-induced reward memory as analyzed in mouse models of drug self-administration.
Potential mechanisms and conclusions
Our current studies using both pharmacological and genetic approaches suggest that D1 and D3 receptors play key roles in reconsolidation of cocaine-induced reward memories. We and others have demonstrated that D1 and D3 receptors play differential roles in acquiring cocaine-induced behaviors (Xu et al, 1994b; 1997; 2000; Caine et al, 2007; Chen and Xu, 2010; Kong et al, 2011) and that underlying molecular mechanisms are also different (Zhang et al, 2002; Zhang et al, 2004; Jiao et al, 2007; Liu et al, 2009; Chen and Xu, 2010). D3 receptors are known to have higher affinities for DA than D1 receptors (Sokoloff et al, 1992). It has been thought that D2 family receptors including D3 receptors can respond to tonic DA release while D1 receptors are activated when there is phasic DA release (Goto et al., 2007; Grace et al., 2007). A large percentage of D3 receptor-bearing neurons co-express D1 receptors, especially in the NAc (Surmeier et al, 1996; Schwartz et al, 1998). D3 receptors are activated by basal levels of DA to inhibit adenylyl cyclase and related signaling including extracellular signal-regulated kinase and Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα). When DA release is enhanced and DA levels increase, CaMKIIα phosphorylates D3 receptors at the serine 229 site in the third intracellular loop (Liu et al, 2009). As a result, the inhibitory tone of D3 receptors on cocaine-induced behavior and related signaling is removed. This allows other DA receptors including D1 receptors to fully mediate behavioral activation and related signaling induced by cocaine. We found that inhibition of functions of either D1 or D3 receptors attenuates reconsolidation of cocaine memories. This result suggests that responses to both tonic and phasic DA release are needed for the reconsolidation process.
D1 and D3 receptors are expressed in brain reward circuits including the basolateral amygdala, the prefrontal cortex, and NAc (Beaulieu and Gainetdinov, 2011). These different brain regions play an important role in reconsolidation of drug memories (Théberge et al, 2010; Otis et al, 2013). D1 and D3 receptors expressed in these brain regions may participate in the reconsolidation of cocaine-induced reward memories. Indeed, abnormal levels of D1 receptor availability have been linked to an increased risk of cocaine taking in cocaine-dependent subjects (Martinez et al, 2009). Moreover, D3 receptors may be upregulated in these places in the brains of cocaine and methamphetamine abusers (Staley and Mash, 1996; Boileau et al, 2012). It is also intriguing to consider that D1–D3 receptor heteromers (Marcellino et al, 2008, Ferre et al, 2014) in these discrete brain regions may play a role in drug memory reconsolidation. Despite a need for additional studies, our current results suggest that pharmacological blockade of either D1 or D3 receptors in the context of drug memory reconsolidation may be therapeutic for the treatment of cocaine craving and relapse in clinical settings.
Pharmacological blockade of D1 or D3 receptors disrupts reconsolidation of cocaine memory
Genetic mutations in D3 receptors attenuate reconsolidation of cocaine-induced reward memory
Dopamine D1 and D3 receptors may serve as new targets for combating cocaine abuse
Acknowledgments
We greatly appreciate Qin Zheng for genotyping mice, and J. Cao for synthesizing PG01037. M.X. was supported by a grant from NIDA (DA025088), and from CTSA UL1 TR000430. A.H.N was supported by the NIDA Intramural Research Program.
ABBREVIATIONS
- CaMKIIα
Ca2+/calmodulin-dependent protein kinase IIα
- CPP
conditioned place preference
- DMSO
dimethyl sulfoxide
- DA
dopamine
- FR
fixed ratio
- i.p
intraperitoneally
- IVSA
intravenous self-administration
- PG01037
N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide
- NAc
nucleus accumbens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J Pharmacol Exp Ther. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5:12–21. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharm. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharm. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharm. 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neurosci. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of cocaine self-administration in dopamine D1 receptor knockout mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, Butler P, Xu M. The dopamine D3 receptor is not necessary for cocaine self-administration: studies in D3 knockout mice. Exp Clinical Psychopharm. 2012;20:352–363. doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LP, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue- elicited cocaine seeking. J Neurochem. 2010;114:530–541. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Phar Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharm. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis C, O’Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharm. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharm. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. Pharmacol Rev. 2014;66:413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The yin and yang of dopamine release: a new perspective. Neuropharm. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharm. 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharm. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2011;25:263–73. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. Dopamine D1 and D3 receptors oppositely regulate NMDA- and cocaine-induced MARK signaling via NMDA receptor phosphorylation. J Neurochem. 2007;103:840–848. doi: 10.1111/j.1471-4159.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharm. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kong H, Kuang W, Li S, Xu M. Activation of dopamine D3 receptors inhibits reward-related learning induced by cocaine. Neurosci. 2011;176:152–161. doi: 10.1016/j.neuroscience.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharm. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Riddle L, Grifffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-DOPA dependent abnormal involuntary movements in rats. Neuropharm. 2009;56:677–686. doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Mao L, Zhang G, Papasian CJ, Fibuch EE, Lan H, Zhou HF, Xu M, Wang JQ. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1–D3 receptor heteromers. Indications for a role of synergistic D1–D3 receptor interactions in the striatum. J Biol Chem. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4- (2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Phar Exp Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, Perez A, Abi-Dargham A, Fischman MW, Kleber HD, Laruelle M. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharm. 2009;34:1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F, Heidbreder C. Selective dopamine D3 receptor antagonists 1997–2007: a decade of progress. Expert Opin Ther Patents. 2008;18:821–840. [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learn Mem. 2006;5:498–505. doi: 10.1101/lm.304606. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subject effects of cocaine. Psychopharm. 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharm. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: Translating the dopamine D3 receptor hypothesis. Biochemical Pharmacol. 2012;84:882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: Human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Otis JM, Dashew KB, Mueller D. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci. 2013;33:1271–1281. doi: 10.1523/JNEUROSCI.3463-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O’Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychi. 1999;56:1101–1106. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci. 2010;30:4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: Basic and recent data. Behavioral and Brain Functions (BBF) 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P. Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Res Brain Res Rev. 1998;26:236–242. doi: 10.1016/s0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Martres MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci USA. 2012a;109:17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaál J, Xi ZX, Gardner EL. YQA14: a novel dopamine D(3) receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D(3) receptor-knockout mice. Addict Biol. 2012b;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev. 2012;36:1400–1417. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharm. 2009;56:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge FR, Milton AL, Belin D, Lee JL, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Addiction: a drug-induced disorder of memory reconsolidation. Current Opion Neurobio. 2013;23:1–8. doi: 10.1016/j.conb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharm. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharm. 2013;38:753–762. doi: 10.1038/npp.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharm. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharm. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiro N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994a;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994b;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Tirado G, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine in D1 dopamine receptor mutant mice. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizoguchi H, Yamada K, Nabeshima T. Relapse of methamphetamine-seeking behavior in C57BL/6J mice demonstrated by a reinstatement procedure involving intravenous self-administration. Behav Brain Res. 2006;168:137–143. doi: 10.1016/j.bbr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Nitta A, Nabeshima T. Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res. 2007;177:261–268. doi: 10.1016/j.bbr.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Yan Y, Nabeshima T. Mouse model of relapse to the abuse of drugs: procedural considerations and characterizations. Behav Brain Res. 2009;196:1–10. doi: 10.1016/j.bbr.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Yan Y, Pushparaj A, Gamaleddin I, Steiner RC, Picciotto MR, Roder J, Le Foll B. Nicotine-taking and nicotine-seeking in C57Bl/6J mice without prior operant training or food restriction. Behav Brain Res. 2012;230:34–39. doi: 10.1016/j.bbr.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Kong H, Wu EJ, Newman AH, Xu M. Dopamine D3 receptors regulate reconsolidation of cocaine memory. Neurosci. 2013;241:32–40. doi: 10.1016/j.neuroscience.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Ying X, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]