Abstract
Carrying a short allele in the serotonin transporter polymorphism (5-HTTLPR) while experiencing stressful environments is linked to elevated risk for depression. What might offset this risky combination of genes and environment? We hypothesized that individual-level factors may play a protective role. Specifically, we examined whether individuals’ ability to decrease their stress responses via effective emotion regulation may be an important moderating factor and addressed this hypothesis in a socioeconomically-diverse sample of 205 children aged 9–15 years. At-risk children (short-allele carriers in high-stress contexts) exhibited more depressive symptoms than other groups. Importantly, at-risk children who used effective emotion regulation did not exhibit increased depressive symptoms. These results have important implications for the basic science of understanding risk and resilience: in addition to genes and environment, individuals’ agentic ability to self-regulate may need to be considered as a critical third factor. Given that emotion regulation is learnable, these results also have strong public-health implications.
Keywords: Depression, Emotion, Stress, Emotion Regulation, Genetics, 5-HTTLPR
Research supports a robust link between stress and depression (Hammen, 2005). This link is pernicious not only because depression remains one of the primary causes of disability and disease burden in the world (Murray & Lopez, 1997), but also because life stress is exceptionally common (Lazarus, 1993). The link between high stress and depression appears to be further exacerbated in people who carry a short allele in the serotonin transporter polymorphism (5-HTTLPR, linked to serotonin function) (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Caspi et al., 2003; Kim et al., 2007; Lesch et al., 1996; Manuck & McCaffery, 2014; Uher & McGuffin, 2010), a group that represents approximately 40–70% of the general population (Kim et al., 2007). These individuals – compared to those without a short allele – appear to be particularly sensitive to the quality of their environment, such that experiencing negative environments is associated with significantly worse psychological health (Belsky & Pluess, 2009; Hankin et al., 2011; Taylor et al., 2006). Given that genes and stressful environments are not always modifiable, how can people with this risky combination avoid depression? The present research examined this question by assessing the hypothesis that an individual’s agentic, self-regulatory behavior can moderate gene-by-environment risk.
Negative Emotional Reactivity and the Role of Emotion Regulation
The mechanisms by which gene-by-environment risk leads to depression may point to specific ways in which this risk may be attenuated. One such mechanism strongly suggested by a substantial body of research is increased emotional reactivity. Specifically, short-allele carriers compared to non-short-allele carriers demonstrate increased negative emotional reactivity to stressors, whether reactivity is measured using self-reported negative emotion (Gunthert et al., 2007), attentional biases to negative stimuli (Pergamin-Hight, Bakermans-Kranenburg, van Ijzendoorn, & Bar-Haim, 2012), central nervous system responses (e.g., amygdala activation) (Hariri et al., 2002), neuroendocrine responses (e.g., cortisol) (Miller, Wankerl, Stalder, Kirschbaum, & Alexander, 2013), or peripheral nervous system responses (e.g., heart rate) (McCaffery, Bleil, Pogue-Geile, Ferrell, & Manuck, 2003), and whether stress is measured using subjective assessment of daily stressors (Gunthert et al., 2007), fear-evoking stimuli (Hariri et al., 2002), or stressful laboratory tasks (McCaffery et al., 2003). Negative emotional reactivity to stress, in turn, has been linked with depression (Cohen, Gunthert, Butler, O’Neill, & Tolpin, 2005; Folkman & Lazarus, 1986; Nolen-Hoeksema & Morrow, 1991). Given these links, several models suggest that negative emotional reactivity is a mechanism linking gene-by-environment risk and depression (e.g., Caspi et al., 2010).
It follows, then, that individuals’ ability to reduce their own negative emotional reactivity, or emotion regulation (Gross, 1998), may offset the risk imposed by a short allele in stressful environments. One strategy shown to be particularly effective for reducing negative emotions is cognitive reappraisal (Gross & John, 2003; McRae, Ciesielski, & Gross, 2012; Ochsner, Bunge, Gross, & Gabrieli, 2002; Troy, Wilhelm, Shallcross, & Mauss, 2010), a strategy that involves reframing the meaning of an event (Gross, 1998). The effectiveness of cognitive reappraisal for reducing negative emotion has been demonstrated across several indicators of negative emotion: self-reported negative emotion (Gross & John, 2003; Ochsner et al., 2002; Troy et al., 2010), central nervous system responses (e.g., decreased amygdala activation) (Ochsner et al., 2002), and peripheral nervous system responses (e.g., decreased skin conductance level) (McRae, Ciesielski, et al., 2012). Cognitive reappraisal has also been found to predict decreased depressive symptoms, particularly in stressful environments (Troy et al., 2010). Thus, for people whose genes and environment put them at risk (i.e., stressed individuals who carry a short allele in the 5-HTTLPR genotype), using cognitive reappraisal may be a useful strategy to offset this risk.
Examining individual-level factors that might moderate the link between gene-by-environment interactions and depression – factors like cognitive reappraisal – may also help resolve inconsistent results regarding the interaction between 5-HTTLPR and stress in predicting depression. The hypothesized interaction between 5-HTTLPR and stress involves a pattern where carrying a short-allele predicts higher depressive symptoms in high-stress contexts, compared to low-stress contexts, and compared to individuals who do not carry a short-allele. Interestingly, this pattern has not been consistently demonstrated, with some (Karg, Burmeister, Shedden, & Sen, 2011) but not all (Risch et al., 2009) meta-analyses confirming the hypothesized pattern. Some of this inconsistency could be attributed to methodological heterogeneity; for example, studies that employ more objective assessments of stress are more likely to demonstrate the hypothesized gene-by-environment interaction, compared to those that employ more subjective assessments of stress (Uher & McGuffin, 2010). However, this inconsistency also points to the potential moderators of the link between genes, environment, and depression. We hypothesize that cognitive reappraisal may be such a moderator, and by taking into consideration such individual-level moderators, we may able to clarify the link between gene-by-environment interactions and psychological health.
Present Study
The present study examined whether the use of cognitive reappraisal attenuates the risk for increased depressive symptoms observed in highly stressed individuals with a 5-HTTLPR short allele. We examined this question in a sample of socioeconomically-diverse children aged 9–15, an age range when depression first develops, and thus an age range in which assessing risk for depression is particularly relevant (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003). It is worth noting that gene-by-environment interactions have been examined within child samples and yielded results similar to those from adult samples. While there is some speculation that gene-by-environment interactions may operate differently within children compared to adults (e.g., Chipman et al., 2007), some studies have found the expected interaction between genotype and stressful environment on emotional outcomes like depression (Cicchetti, Rogosch, & Sturge-Apple, 2007; Nobile et al., 2009; Petersen et al., 2012), and others have not (Araya et al., 2009; Chipman et al., 2007). These inconsistent results parallel the findings from adult findings, and it is possible that these inconsistent results in the developmental literature may too be clarified by taking into account individual-level variables as moderators of gene-by-environment interactions.
We used well-validated, standardized, and widely-used self-report measures to assess current severity of depressive symptoms (Kovacs, 1981) and the severity of stress experienced over the last 3 months (Hankin & Abramson, 2002). This measure of stress assesses the experience of objectively-defined stressful events, which is important given that many of the studies reporting inconsistent gene-by-environment interactions employed more subjective measures of stress (Uher & McGuffin, 2010). Furthermore, we assessed cognitive reappraisal using an adaptation of a widely-used measure (Gross & John, 2003). Prior research confirms that young children understand the contingencies of their emotions and understand that it is possible to change how you feel by changing how you think (i.e., the core of cognitive reappraisal) (Bamford & Lagattuta, 2012; Lagattuta & Wellman, 2001). Furthermore, recent research confirms that young children can reliably self-report the frequency with which they use emotion regulation strategies like reappraisal (Gullone & Taffe, 2012). Children are less likely to use reappraisal than adults (Garnefski, Legerstee, Kraaij, Van Den Kommer, & Teerds, 2002), and demonstrate lower reappraisal ability than adults (McRae, Gross, et al., 2012). Crucially, however, children who use reappraisal more (vs. less) frequently have fewer depressive symptoms (Gullone & Taffe, 2012). Given that childhood is a time when depression first develops, it is critical to know whether individual-level factors like reappraisal can moderate the link between gene-by-environment risk and depressive outcomes in children.
Participants were genotyped using standard protocols (Anchordoquy, McGeary, Liu, Krauter, & Smolen, 2003) to ascertain who were short-allele carriers (those with either one or two short alleles of 5-HTTLPR) and who were non-carriers (those with two long alleles of 5-HTTLPR). This grouping is consistent with prior research (Lenze et al., 2005; Otte, McCaffery, Ali, & Whooley, 2007; Ramasubbu, Tobia, Buchan, & Bech-Hansen, 2006) and with theoretical support for the dominant genetic effect of the short allele on outcomes (Greenberg et al., 1999; Lesch et al., 1996), whether there are one or two short alleles. We predicted a three-way interaction in which short-allele carriers would experience more depressive symptoms under stress compared to their non-carrier counterparts, but that stressed short-allele carriers should experience fewer depressive symptoms if they also use cognitive reappraisal more (vs. less) often.
Methods
Participants
A sample of 205 children aged 9–15 (M = 12.09; 62% female) from the Denver, CO area was recruited as part of a larger study. The sample was largely ethnically homogeneous (74% Caucasian; 7% African American/Black; 4% Latino/Hispanic; 4% Asian/Island Pacific; 11% other/multiracial) and was socioeconomically heterogeneous with regard to yearly family income (7% <$20,000; 10% $20,001–40,000; 13% $40,001–60,000; 20% $60,001–80,000; 20% $80,001–100,000; 30% >$100,000). Parents of these children were recruited to be the primary caregiver of the child (96% of parents completing the study reported being the primary caregiver). The parent sample consisted of 173 mothers (including 1 step-mother and 1 grand-mother) and 28 fathers (including 1 step-father and 1 grand-father) (4 parents did not report their gender).
Participants were recruited by brief information letters sent home directly by the participating school districts to families with a child in 3rd, 6th, or 9th grades of public schools in the Denver, CO area (approximately 2,000 families). The short letter stated that the experimenters were conducting a study on social and emotional development and requested that interested participants call the laboratory to receive more detailed information on the study. Four-hundred and ninety-three families called the laboratory for more information. During this phone call, parents responded to a brief set of questions establishing that both the parent and child were fluent in English, that the child did not carry an autism spectrum or psychotic disorder, and that the child had an IQ > 70. Of the 493 families initially interviewed, 350 met these criteria, and completed the first laboratory assessment (including genotyping). Of the 350 children who began the study, 241 completed the 18-month follow-up (31% attrition).
We examined this attrition by comparing the children who began the study and were also assessed at the 18-month follow-up (N = 241) to the children who began the study but were not assessed at the 18-month follow-up (N = 109). These two samples of children did not significantly differ in age (p = .90), levels of stress (p = .59), or genotype (p = .78). Compared to the sample that did not complete the 18-month follow-up, the sample that completed the 18-month follow-up were marginally more likely to be female (p = .10), marginally more likely to have a higher family income (p = .11), significantly more likely to be Caucasian (p = .03), and had significantly fewer depressive symptoms (p = .03). Thus, there exists some non-random drop-out from the initial time point to the 18-month follow-up assessment that consists largely of demographic differences. It also appears that participants with more depressive symptoms may have self-selected out of the study. This indicates that our data yields a more conservative test of our hypothesis given that the range of depression scores would be reduced.
Because the present manuscript reports data from a larger study and the 18-month assessment was the only assessment that included reappraisal, this is the time point at which we examined the three-way interaction between genotype, stress, and reappraisal on depressive symptoms. All analyses included only participants who had complete data for all key assessments (genotype, stress, reappraisal, and depression; N = 205).
Materials
Cognitive reappraisal
Children’s use of cognitive reappraisal was assessed using an adapted version of the Emotion Regulation Questionnaire with simpler language more appropriate for children (Gross & John, 2003). This scale includes 6 items rated on a scale of 1 (strongly disagree) to 7 (strongly agree) measuring the extent to which the participant engages in cognitive reappraisal (e.g., I control my feelings by changing the way I think about the situation I’m in), α = .82.
Stress
Children’s stress levels were assessed using the Adolescent Life Events Questionnaire (Hankin & Abramson, 2002), which lists 37 stressful life events. Children indicated how often each event occurred in the past 3 months on a scale of 1 (never) to 5 (always), and responses were summed to create a composite stress severity score.
Depressive symptoms
Children’s depressive symptoms were assessed using the Children’s Depression Inventory (Kovacs, 1981), which contains 27 items rated on a scale of 0 (e.g., I am sad once in awhile) to 2 (e.g., I am sad all of the time) assessing the severity of psychological, social and somatic symptoms of depression. Responses were summed to create a composite score, α = .82.
Genotyping
Children provided saliva cells for DNA collection via Oragene™ kits from DNA Genotek (Ottawa, Ontario, Canada) and genomic DNA was collected and isolated using standard salting out and solvent precipitation methods. The 5-HTTLPR alleles were assayed (Anchordoquy et al., 2003) and modified by using primers reported by (Hu et al., 2005). Samples were analyzed on an ABI PRISM® 3130xl Sequencer. Trichotomous groups of SS (n = 38), SL (n = 99), and LL (n = 68) genotypes were formed. These genotypes were distributed according to Hardy-Weinberg equilibrium. While we conducted our primary analyses examining the 5-HTTPLR genotype and not the functional variants of the long allele, rs25531 (i.e., LA and LG), because the majority of studies focus on 5-HTTLPR (Caspi et al., 2010), we also report parallel results in our “supplementary analyses” section that examined the functional variants (short-allele carriers plus LG carriers, versus LA carriers).
Control variables
Several potential confounds were assessed and controlled for. Specifically, parents’ use of cognitive reappraisal was assessed using the Emotion Regulation Questionnaire (ERQ) (Gross & John, 2003). This scale includes 6 items rated on a scale of 1 (strongly disagree) to 7 (strongly agree) targeting the extent to which the parent engages in cognitive reappraisal, α = .84. Parents’ stress levels were assessed using the Life Events Inventory (Cochrane & Robertson, 1973), which lists 36 stressful life events. Parents indicated whether each event occurred or not in the past 3 months, and the number of affirmative responses were summed to create a composite score. Finally, parents’ depressive symptoms were assessed using the Beck Depression Inventory (BDI-II) (Beck, Steer, & Brown, 1996), which contains 21 items rated on a scale of 0 to 3 assessing the severity of psychological, social and somatic symptoms of depression. Responses were summed to create a composite score, α = .93.
Procedure
The parent and child visited the laboratory for the first assessment. The parent provided informed written consent for their participation and for their child; youth provided written assent. At the first assessment, children gave a DNA sample via saliva and their parents reported on the child’s demographic information (sex, age, race) as well as their own socioeconomic status (as indicated by their household income). Eighteen months later, children and their parent completed a series of questionnaires assessing reappraisal, stress, and depressive symptoms. The Institutional Review Board approved all procedures. Parents and children were reimbursed for participation at each time point.
Results
We first verified that the three predictors (genotype, stress, and reappraisal) were statistically independent of each other, as evidenced by non-significant zero-order correlations between genotype and stress (r = .11, p = .11), genotype and reappraisal (r = −.06, p = .42), and stress and reappraisal (r = −.13, p = .06). See Table 1 for descriptive statistics and inter-correlations for all study variables.
Table 1.
Descriptive statistics and inter-correlations between study variables.
| Descriptive statistics | Intercorrelations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| N | M | SD | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| 1. Genotype | 205 | 67% short-allele carriers | .11 | −.06 | .09 | .05 | −.01 | .08 | .03 | .02 | −.02 | .13 | |
| 2. Stress | 205 | 58.48 | 14.07 | −.12† | .56* | .11 | .42* | −.04 | .00 | .17† | −.01 | .33* | |
| 3. Reappraisal | 205 | 4.86 | 1.21 | −.32* | −.08 | .09 | −.08 | .08 | −.06 | −.08 | −.05 | ||
| 4. Depressive symptoms | 205 | 4.75 | 4.96 | .11 | .14† | −.12† | −.03 | .14 | .00 | .16† | |||
| 5. Child’s gender | 202 | 62% female | .04 | .03 | −.04 | −.23* | .15 | .03 | |||||
| 6. Child’s age | 205 | 12.09 | 2.24 | .04 | .22* | .07 | −.06 | .15 | |||||
| 7. Child’s ethnicity | 205 | 75% Caucasian | .23* | −.10 | −.01 | −.19* | |||||||
| 8. Family income | 194 | $100,041 | $87, 156 | −.15 | .07 | −.22* | |||||||
| 9. Parent’s stress | 113 | 3.02 | 2.65 | −.06 | .47* | ||||||||
| 10. Parent’s reappraisal | 114 | 5.18 | 1.03 | −.16† | |||||||||
| 11. Parent’s depressive symptoms | 117 | 4.72 | 7.10 | ||||||||||
To test the hypothesis that reappraisal moderates the interactive effect of genotype and stress on depression, we entered the three-way interaction between genotype, stress, and reappraisal, all main effects, and two-way interactions in a regression analysis as predictors of depressive symptoms (all continuous variables were mean-centered and genotype was effect coded as short-allele carrier = .5, non-carrier = −.5). See Table 2 for a summary of the regression analyses. There was a significant main effect of stress, β = .38, t(197) = 6.22, p < .001, such that participants with higher (vs. lower) stress reported more depressive symptoms, and a significant main effect of reappraisal, β = −.27, t(197) = 4.77, p < .001, such that participants higher (vs. lower) in reappraisal reported fewer depressive symptoms. Furthermore, we replicated the two-way interaction between genotype and stress often hypothesized in gene-by-environment interactions, β = .21, t(197) = 3.44, p = .001, such that greater stress was associated with more depressive symptoms in short-allele carriers, β = .64, t(149) = 10.09, p < .001, compared to non-carriers, β = .25, t(77) = 2.24, p = .028.
Table 2.
Results from the primary regression analyses examining the 3-way interaction between genotype, stress, and reappraisal in predicting depressive symptoms.
| Interaction | |||
|---|---|---|---|
|
| |||
| β | t | p | |
| Genotype | .02 | < 1 | .70 |
| Stress | .38 | 6.22 | < .001 |
| Reappraisal | −.27 | 4.77 | < .001 |
| Genotype x stress | .21 | 3.44 | .001 |
| Genotype x reappraisal | .04 | < 1 | .45 |
| Stress x reappraisal | .002 | < 1 | .97 |
| Genotype x stress x reappraisal | −.20 | 3.68 | < .001 |
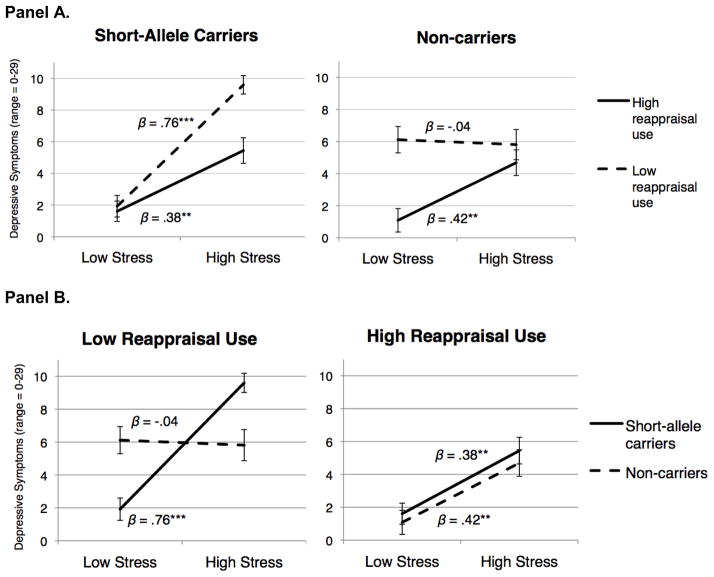
Finally, there was a significant three-way interaction between genotype, stress, and reappraisal, β = −.20, t(197) = 3.68, p < .001. As displayed in Figure 1, reappraisal moderated the interaction between genotype and stress. Specifically, simple-slopes analyses were used to examine values at +/− 1SD from the mean (Aiken & West, 1991) and revealed that highly stressed short-allele carriers reported significantly fewer depressive symptoms when they were high on reappraisal, compared to when they were low on reappraisal, β = −.39, t(132) = 3.94, p < .001 (see Figure 1, left-side of panel A). Indeed, short-allele carriers high on reappraisal reported the same number of depressive symptoms as non-carriers, whether the high-reappraisal short-allele carriers were experiencing high levels of stress, β = .07, t(197) < 1, p = .52, or low levels of stress, β = .05, t(197) < 1, p = .61 (see Figure 1, right-side of panel B). Thus, using reappraisal completely buffered the risk associated with carrying a short allele in the context of elevated stress. See Table 1 for complete results of simple effects analyses.
Fig. 1.
Figure depicts two ways of viewing the three-way interaction between 5-HTTLPR genotype (short-allele carriers vs. non-carriers), low vs. high environmental stress (−/+ 1SD from the mean), and low vs. high use of cognitive reappraisal (−/+ 1SD from the mean) in predicting depressive symptoms. Panel A displays the interaction separated by genotype (short-allele varriers vs. non-carriers); Panel B displays the interaction separated by use of cognitive reappraisal (low vs. high). Error bars represent one standard error of the mean. (N=205; nshort-allele carriers=137; nnon-carriers=68).
Supplementary Analyses
Controlling for demographic and parent variables
It is possible that these findings are due not to reappraisal but other factors potentially confounded with reappraisal (e.g., age, socioeconomic status). These alternative explanations can be ruled out because the three-way interaction (between genotype, stress, and reappraisal) remained significant when controlling for the child’s age, sex, and race (Caucasian vs. other), and when controlling for the child’s parents’ socioeconomic status, use of cognitive reappraisal, stress level, and depressive symptoms by including these variables simultaneously as covariates in the primary regression analysis, β = −.24, t(88) = 2.86, p = .005. Other significant findings from this regression analysis include replicating a main effect of stress, β = .50, t(88) = 4.92, p < .001, and replicating a main effect of reappraisal, β = −.32, t(88) = 3.50, p = .001. While in the same direction, the two-way interaction between genotype and stress is no longer significant, β = .13, t(88) = 1.42, p = .16.
Controlling for functional variants of the long-allele
To ensure that the three-way interaction between genotype, stress, and reappraisal in predicting depressive symptoms is robust whether we examine the 5-HTTPLR genotype or the functional variants of the long allele, rs25531, we conducted an additional three-way interaction comparing the short-allele carriers plus the LG carriers (n = 152) with the LA carriers (n = 49). This analysis replicated the pattern of results from the primary analyses. Specifically, we replicated the main effect of stress, β = .34, t(193) = 4.05, p < .001, the main effect of reappraisal, β = −.24, t(193) = 3.25, p = .001, the two-way genotype by stress interaction, β = .22, t(193) = 2.67, p = .008, and the three-way genotype by stress by reappraisal interaction, β = −.16, t(193) = 1.74, p = .084.
We note that – while the direction of the interaction remains the same – the three-way interaction’s p-value is reduced from a significant to a statistically marginal one. This may be due to the loss of power when an additional 9% of the population is moved from the high expressing to the low expressing group. This may also suggest that LG carriers are less liable to high stress situations, since the maximum depressive symptom response to stress is muted when they are included in the “short-allele carrier” group. Finally, all simple effects that were significant when examining the 5-HTTLPR genotype remain significant when taking into account the functional variants of the long allele (all ps < .043), and all simple effects that were not significant when examining the 5-HTTLPR genotype remain not significant when taking into account the functional variants (all ps > .11).
Discussion
As predicted, the present investigation supports the notion that the mental-health risk associated with being a highly stressed short-allele carrier is attenuated for individuals who use adaptive emotion regulation, namely cognitive reappraisal. The fact that these results were obtained in a sample of children is particularly meaningful because depression experienced in adolescence substantially increases risk for depression in adulthood. Understanding mechanisms for depression experienced in children and adolescents may thus inform prevention efforts (Rutter, Moffitt, & Caspi, 2006). Finally, the socioeconomically diverse sample enhances the generalizability of the findings. Establishing cognitive reappraisal as a moderator of gene-by-environment risk has important theoretical and practical implications.
Theoretical Implications
The present results provide evidence that individuals are not necessarily at increased risk for experiencing depression in the context of a stressful environment and a 5-HTTLPR short allele. While some studies have provided evidence for this risk, our findings suggest that this effect is moderated by individual differences such that children low in reappraisal exhibit this risk, but children high in reappraisal do not exhibit this risk. Thus, by taking into account individual-level factors in a three-way interaction with genotype and environment, we observe that the risk conveyed by gene-by-environment interactions can be offset by a factor that is under the control of the individual. Given the compelling empirical evidence that the 5-HTTLPR-by-stress interaction promotes heightened negative emotional reactivity to high stress environments, we propose that individuals who can counteract that reactivity may experience better psychological health outcomes.
More broadly, the present findings suggest that to fully understand risk and resilience, in addition to genes and environment, a third type of factor needs to be considered: individuals’ agentic, self-regulatory behavior can profoundly alter the effects of gene-by-environment interactions on health. By taking into account these third factors (e.g., reappraisal) and examining three-way interactions between these individual-level factors in addition to genes and the environment, we may also help resolve inconsistent results regarding the interaction between 5-HTTLPR and stress in predicting depression (e.g., Karg et al., 2011; Risch et al., 2009). These inconsistent findings suggest that there may be additional moderators of the effects of genes and environment on depression, and one might expect these results to remain inconsistent until the moderators are adequately assessed and included in the model.
The present results also provide support for models of gene-by-environment interactions that emphasize the risk but also the potential rewards associated with the short allele (Belsky & Pluess, 2009; Boyce & Ellis, 2005; Hankin et al., 2011; Taylor et al., 2006). The differential susceptibility model proposes that certain individuals (e.g., those with a short allele) are more sensitive to their environment ‘for better or worse’, such that they experience worse psychological health outcomes in negative environments, yet better psychological health outcomes in positive environments, compared to their less sensitive counterparts (e.g., those without a short allele) (Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2007). Specifically, for individuals with low reappraisal use, we observe the standard susceptibility pattern. As can be seen in Figure 1, for individuals who use reappraisal less frequently, short-allele carriers experience worse health in negative environments (i.e., high stress), but experience better health in positive environments (i.e., low stress), compared to non-carriers, who do not appear to be susceptible to the quality of their environment (see left-side of panel B).
Conversely, individuals who use reappraisal more frequently do not demonstrate the pattern of differential susceptibility. Rather, both short-allele carriers and non-carriers alike report moderately low levels of depression in low stress environments, and somewhat higher levels of depression in higher stress environments. Using reappraisal relatively frequently, therefore, appears to promote an individual’s ability to reap the benefits of positive environments, yet not suffer much from negative environments – regardless of their genetic composition. In other words, reappraisal may provide the best of both worlds as it buffers individuals from gene-by-environment risk while still enabling individuals to be sensitive to, and benefit from, their positive environments.
Finally, while reappraisal is, on average, associated with fewer depressive symptoms across both genotype and stress level, the benefit of using reappraisal appears to be asymmetrical for short-allele carriers versus non-carriers. Specifically, short-allele carriers report fewer depressive symptoms when they use reappraisal more (vs. less) frequently, but only in high-stress environments; meanwhile, non-carriers report fewer depressive symptoms when they use reappraisal more (vs. less) frequently, but only in low-stress environments. These findings suggest that the relative benefit of using reappraisal may vary by both genotype and stress. Overall, these results provide evidence that differential susceptibility may account for interactions between genotype and stress for individuals who do not use reappraisal frequently, but that both short-allele carriers and non-carriers can benefit from using reappraisal more frequently.
Practical Implications
Practically, the present results suggest a promising and cost-effective avenue for intervention and prevention, because individuals’ agentic self-regulatory behavior is likely to be more amenable to deliberate change than genes or a stressful environment. For example, changing how one thinks about a stressful environment (i.e., using cognitive reappraisal) may be easier than changing the stressful environment itself. This may be particularly true for children, who are even less likely to have control over their stressful environments than adults. Prior research confirms that young children’s sense of agency can be increased with interventions (Blackwell, Trzesniewski, & Dweck, 2007). Cognitive reappraisal, specifically, is a learnable skill, as evidenced by experimental interventions among adults (Gross & John, 2003) and children as young as 10 years (McRae, Gross, et al., 2012). The fact that the present results were obtained in children further enhances their implications for prevention for two reasons. First, promoting self-regulation in children who are at increased genetic or environmental risk may be particularly useful because children who are better at regulating themselves are more likely to become more socially, emotionally, and scholastically successful as they grow older from adolescence through adulthood (Ayduk et al., 2000; Mischel, Shoda, & Peake, 1988; Mischel, Shoda, & Rodriguez, 1989; Shoda, Mischel, & Peake, 1990). Second, avoiding the first episode of depression could have considerable cumulative benefits because most individuals with depression experience their first depressive episode in adolescence (Costello et al., 2003) and adolescent-onset depression substantially increases risk for depression in adulthood (Rutter et al., 2006).
Overall, the present findings suggest that gene-by-environment interactions can be modulated by specific individual-level factors. These findings were also able to rule out several alternative hypotheses by accounting for important potential confounds (e.g., age, sex, and socioeconomic status). These cross-sectional results are an important first step toward a causal model in which cognitive reappraisal protects individuals from the risk that unfolds overtime as genes interact with the environment. However, it could be argued that depressive symptoms, stress, or genes influence cognitive reappraisal, rather than the other way around. We believe this hypothesis is unlikely for both theoretical and empirical reasons. Theoretically, although cognitive reappraisal can be improved through training, it is not conceptualized as a characteristic that results from an individual’s present symptoms or stress levels (i.e., symptom and stress levels are not thought to cause reappraisal levels; Gross & John, 2003). Empirically, if we change the statistical model such that reappraisal is the outcome, and examine the three-way interaction among depressive symptoms, stress, or genes, or any of the two-way interactions therein, we find that none of these interactions predict cognitive reappraisal. These empirical findings suggest that reappraisal is not simply a side effect of these other constructs or their interactions with one another.
Furthermore, while some recent evidence suggests that reappraisal may act as a mediator in the link between 5-HTTLPR and psychological health (Miu, Vulturar, Chis, Ungureanu, & Gross, 2013), the present investigation focused on reappraisal as a moderator – rather than a mediator – in the link between gene-by-environment risk and depression for both conceptual and empirical reasons. Conceptually, we have hypothesized that reappraisal is an individual-level factor that is largely independent from the interaction between genes and the environment. Empirically, we do not find support for a moderated mediation where the gene x environment interaction predicts depression via reappraisal as a mediator. Specifically, there is no link between the gene x environment interaction on reappraisal whether we control for depressive symptoms (p = .20) or not (p = .23). This indicates that reappraisal is not likely to be a mediator in the link between the gene x environment interaction and depressive symptoms. Thus, reappraisal may be better conceptualized as a trait-level individual difference that can alter the links between children’s gene x environment experiences and depressive symptoms. As such, the present results highlight the important protective role that emotion regulation can have in avoiding serious psychopathology (Kovacs, Joormann, & Gotlib, 2008).
Limitations and Future Directions
The current investigation is an initial step in a hopefully fruitful line of research examining how emotion regulation – and other individual-level factors – may be able to offset gene-by-environment risk. Given that this is a preliminary investigation, there are limitations to the present study that provide opportunities for exciting future directions.
First, based on prior research, we propose that emotion regulation can causally protect individuals from gene-by-environment risk. While our results are consistent with this interpretation, and we have been able to rule out several alternative models (e.g., that children’s demographics or their parent’s stress, reappraisal, or depression account for the observed pattern; that reappraisal is the outcome of genotype, stress, and depression), our data do not directly speak to this causal model. Two lines of future research would better address this causal model: (a) longitudinal assessments of all variables would enable lagged analyses to explore which factors protect against future depressive symptoms and (b) experimental laboratory manipulations or training-programs designed to improve reappraisal would allow researchers to test the causal role of reappraisal per se in preventing or attenuating depression.
Second, although we propose and find evidence that three-way interactions with individual-level factors may be able to explain the inconsistent gene-by-environment interactions within prior research, it is important to begin replicating these three-way interactions. There are at least two statistically-based reasons why we believe the present results are reliable within the relatively smaller sample size we obtained. First, if the present sample were underpowered, we would have expected not to find a robust three-way interaction, especially when simultaneously including several control variables in the model. Second, a power analysis on the present data reveals that we had adequate power to detect the three-way interaction (.73 two-tailed, .82 one-tailed). However, to further support the reliability and robustness of the present results, it will be important to replicate them in additional studies with larger sample sizes (see Duncan & Keller, 2011), as well as with additional measures of stress (e.g., early adversity), reappraisal (e.g., ability assessments), and depression (e.g., clinical diagnoses, or parent-reports of symptoms).
Finally, the present investigation assessed individual differences in the frequency with which individuals use reappraisal. While there is evidence that training reappraisal improves emotional outcomes both in the short run (e.g., in laboratory experiments; Gross & John, 2003; McRae, Gross, et al., 2012) and in the long run (e.g., clinical interventions that train emotion regulation; Mennin, 2004), there may be something unique about individuals who naturally use reappraisal versus those who require training in the strategy. Thus, to be able to confirm that individuals who improve their cognitive reappraisal through training would reap the same benefits as those who naturally reappraise (as we measured in the current investigation), it is necessary to manipulate reappraisal skill and assess subsequent outcomes.
Concluding comment
While preliminary, the present results demonstrate that children who use reappraisal more frequently are less likely to experience increased depression in contexts of gene-by-environment risk. These results suggest that assessing individual differences like emotion regulation will play an important role in untangling the psychological health correlates of gene-by-environment interactions.
Table 3.
Summary of the simple-effect analyses decomposing the significant three-way interaction among genotype, stress, and reappraisal in predicting depressive symptoms. High and low values of stress and reappraisal were determined using values +/− 1 SD from the mean.
| Stress level | Reappraisal level | Simple effect result | β | t-statistic | df | p-value |
|---|---|---|---|---|---|---|
| Comparing short allele carriers to non-carriers (N = 201) | ||||||
| High | High | Short-allele carriers did not differ from non-carriers | .07 | < 1 | 197 | .52 |
| High | Low | Short-allele carriers reported more depressive symptoms than non-carriers | .36 | 3.21 | 197 | .002 |
| Low | High | Short-allele carriers did not differ from non-carriers | .05 | < 1 | 197 | .61 |
| Low | Low | Short-allele carriers reported fewer depressive symptoms than non-carriers | −.40 | 3.75 | 197 | <.001 |
|
| ||||||
| Comparing stress levels or reappraisal levels within short-allele carriers (n = 137) | ||||||
| High | Reported fewer depressive symptoms when they were high (vs. low) in reappraisal | −.39 | 3.94 | 133 | <.001 | |
| Low | Reported the same number of depressive symptoms whether they were high or low in reappraisal | −.03 | < 1 | 133 | .73 | |
| High | Reported increased depressive symptoms when they were high (vs. low) in stress | .38 | 3.55 | 133 | .001 | |
| Low | Reported increased depressive symptoms when they were high (vs. low) in stress | .76 | 9.26 | 133 | <.001 | |
|
| ||||||
| Comparing stress levels or reappraisal levels within non-carriers (n = 68) | ||||||
| High | Reported the same number of depressive symptoms whether they were high or low in reappraisal | −.15 | 1.02 | 64 | .31 | |
| Low | Reported fewer depressive symptoms when they were high (vs. low) in reappraisal | −.65 | 4.65 | 64 | <.001 | |
| High | Reported increased depressive symptoms when they were high (vs. low) in stress | .42 | 3.38 | 64 | .001 | |
| Low | Reported the same number of depressive symptoms whether they were high or low in stress | −.04 | < 1 | 64 | .81 | |
Acknowledgments
This research was supported by NIMH grant 5R01 MH077195. The authors thank Igor Grossmann for his feedback on an earlier draft of this paper.
Footnotes
Author Contributions
B.Q.F. analyzed data and wrote the paper. A.S.T. and I.B.M. wrote the paper. A.S. performed research. B.H. designed research, performed research, and wrote the paper.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Anchordoquy H, McGeary C, Liu L, Krauter K, Smolen A. Genotyping of three candidate genes after whole-genome preamplification of DNA collected from buccal cells. Behavior Genetics. 2003;33(1):71–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- Araya R, Hu X, Heron J, Enoch MA, Evans J, Lewis G, Goldman D. Effects of stressful life events, maternal depression and 5-HTTLPR genotype on emotional symptoms in pre-adolescent children. Americal Journal of Medical Genetics B: Neuropsychiatric Genetics. 2009;150B(5):670–682. doi: 10.1002/ajmg.b.30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: Strategic self-regulation for coping with rejection sensitivity. Journal of Personality and Social Psychology. 2000;79(5):776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- Bamford C, Lagattuta KH. Looking on the bright side: children’s knowledge about the benefits of positive versus negative thinking. Child Development. 2012;83(2):667–682. doi: 10.1111/j.1467-8624.2011.01706.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. manual. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. For Better and For Worse: Differential Susceptibility to Environmental Influences. Current Directions in Psychological Science. 2007;16(6):300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Blackwell LS, Trzesniewski KH, Dweck CS. Implicit theories of intelligence predict achivement across an adolescent transition: A longitudinal study and an intervention. Child Development. 2007;78(1):246–263. doi: 10.1111/j.1467-8624.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chipman P, Jorm AF, Prior M, Sanson A, Smart D, Tan X, Easteal S. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: results from two community surveys. Americal Journal of Medical Genetics B: Neuropsychiatric Genetics. 2007;144B(4):561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and Psychopathology. 2007;19(4):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Cochrane R, Robertson A. The life events inventory: A measure of the relative severity of psychosocial stressors. Journal of Psychosomatic Research. 1973;17:135–139. doi: 10.1016/0022-3999(73)90014-7. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Gunthert KC, Butler AC, O’Neill SC, Tolpin LH. Daily affective reactivity as a prospective predictor of depressive symptoms. Journal of Personality. 2005;73(6):1687–1714. doi: 10.1111/j.1467-6494.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Americal Journal of Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS. Stress processes and depressive symptomatology. Journal of Abnormal Psychology. 1986;95(2):107–113. doi: 10.1037//0021-843x.95.2.107. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Legerstee J, Kraaij V, Van Den Kommer T, Teerds JAN. Cognitive coping strategies and symptoms of depression and anxiety: a comparison between adolescents and adults. Journal of Adolescence. 2002;25(6):603–611. doi: 10.1006/jado.2002.0507. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1999;88:83–87. [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gullone E, Taffe J. The Emotion Regulation Questionnaire for Children and Adolescents (ERQ-CA): A psychometric evaluation. Psychological Assessment. 2012;24(2):409–417. doi: 10.1037/a0025777. [DOI] [PubMed] [Google Scholar]
- Gunthert KC, Conner TS, Armeli S, Tennen H, Covault J, Kranzler HR. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: a daily process approach to gene-environment interaction. Psychosomatic medicine. 2007;69(8):762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of Clinical Child and Adolescent Psychology. 2002;31(4):491–504. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JRZ, Oldehinkel AJ. Differential susceptibility in youth: evidence that 5-HTTLPR x positive parenting is associated with positive affect ‘for better and worse’. Translational Psychiatry. 2011;1(10):e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An Expanded Evaluation of the Relationship of Four Alleles to the Level of Response to Alcohol and the Alcoholism Risk. Alcoholism: Clinical & Experimental Research. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biological Psychiatry. 2007;62(5):423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica: International Journal of Child & Adolescent Psychiatry. 1981;46(5–6):305–315. [PubMed] [Google Scholar]
- Kovacs M, Joormann J, Gotlib IH. Emotion (dys)regulation and links to depressive disorders. Child Development Perspectives. 2008;2(3):149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagattuta KH, Wellman HM. Thinking about the past: Early knowledge about links between prior experience, thinking, and emotion. Child Development. 2001;72(1):82–102. doi: 10.1111/1467-8624.00267. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. From psychological stress to the emotions: A history of changing outlooks. Annual Review of Psychology. 1993;44:1–21. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Munin MC, Ferrell RE, Pollock BG, Skidmore E, Lotrich F, Reynolds CF. Association of the serotonin transporter gene-linked polymorphic region (5-HTTLPR) genotype with depression in elderly persons after hip fracture. The American Journal of Geriatric Psychiatry. 2005;13(5):428–432. doi: 10.1176/appi.ajgp.13.5.428. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Murphy DL. Association of Anxiety-Related Traits with a Polymorphism in the Serotonin Transporter Gene Regulatory Region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Manuck SB, McCaffery JM. Gene-environment interaction. Annual Review of Psychology. 2014;65:41–70. doi: 10.1146/annurev-psych-010213-115100. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Bleil M, Pogue-Geile MF, Ferrell RE, Manuck SB. Allelic Variation in the Serotonin Transporter Gene-Linked Polymorphic Region (5-HTTLPR) and Cardiovascular Reactivity in Young Adult Male and Female Twins of European-American Descent. Psychosomatic Medicine. 2003;65(5):721–728. doi: 10.1097/01.psy.0000088585.67365.1d. [DOI] [PubMed] [Google Scholar]
- McRae K, Ciesielski B, Gross JJ. Unpacking cognitive reappraisal: Goals, tactics, and outcomes. Emotion. 2012;12(2):250–255. doi: 10.1037/a0026351. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS. Emotion regulation therapy for generalized anxiety disorder. Clinical Psychology & Psychotherapy. 2004;11(1):17–29. doi: 10.1002/cpp.389. [DOI] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: a meta-analysis. Molecular Psychiatry. 2013;18(9):1018–1024. doi: 10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. Journal of Personality and Social Psychology. 1988;54(4):687–696. doi: 10.1037//0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Miu AC, Vulturar R, Chis A, Ungureanu L, Gross JJ. Reappraisal as a mediator in the link between 5-HTTLPR and social anxiety symptoms. Emotion. 2013;13(6):1012–1022. doi: 10.1037/a0033383. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. The Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, Battaglia M. The influence of family structure, the TPH2 G-703T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. Journal of Child Psychology and Psychiatry. 2009;50(3):317–325. doi: 10.1111/j.1469-7610.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61(1):115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: The heart and soul study. American Journal of Psychiatry. 2007;164(9):1379–1384. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biological Psychiatry. 2012;71(4):373–379. doi: 10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, Goodnight JA, Dodge KA, Lansford JE, Pettit GS, Dick DM. Interaction between serotonin transporter polymorphism (5-HTTLPR) and stressful life events in adolescents’ trajectories of anxious/depressed symptoms. Developmental Psychology. 2012;48(5):1463–1475. doi: 10.1037/a0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Tobia R, Buchan A, Bech-Hansen NT. Serotonin transporter gene promoter region polymorphism associated with poststroke major depression. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(1):96–99. doi: 10.1176/jnp.18.1.96. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk for depression: A meta-analysis. Journal of the American Medical Association. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Shoda Y, Mischel W, Peake PK. Predicting adolescent cognitive and self-regulatory competencies from preschool delay of gratification: Identifying diagnostic conditions. Developmental Psychology. 1990;26(6):978–986. [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60(7):671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Troy AS, Wilhelm FH, Shallcross AJ, Mauss IB. Seeing the silver lining: cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emotion. 2010;10(6):783–795. doi: 10.1037/a0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15(1):18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]