Abstract
Through synthetic lethality screening of isogenic cell lines with and without the oncogenic KRAS gene and through lead compound optimization, we recently developed a novel anticancer agent designated NSC-743380 (oncrasin-72) that has promising in vitro and in vivo anticancer activity in a subset of cancer cell lines, including KRAS-mutant cancer cells. However, NSC-743380 tends to form dimers, which dramatically reduces its anticancer activity. To improve the physicochemical properties of NSC-743380, we synthesized a prodrug of NSC-743380, designated oncrasin-266, by modifying NSC-743380 with cyclohexylacetic acid and evaluated its in vitro and in vivo properties. Oncrasin-266 spontaneously hydrolyzed in phosphate-buffered saline in a time-dependent manner and was more stable than NSC-743380 in powder or stock solutions. In vivo administration of oncrasin-266 in mice led to the release of NSC-743380 which improved the pharmacokinetics of NSC-743380. Tissue distribution analysis revealed that oncrasin-266 was deposited in liver, whereas released NSC-743380 was detected in liver, lung, kidney, and subcutaneous tumor. Oncrasin-266 was better tolerated in mice at a higher dose level treatment (150–300mg/kg, i.p.) than the parent agent was, suggesting that the prodrug reduced the acute toxicity of the parent agent. Our results demonstrated that the prodrug strategy could improve the stability, pharmacokinetic properties, and safety of NSC-743380.
Keywords: Cancer, drug development, prodrug, synthetic lethality
1. Introduction
Despite the advent of many new anticancer drugs, cancer remains a major public health problem worldwide and novel anticancer therapeutic agents are still urgently needed. In our efforts to develop novel agents, we have used synthetic lethality screening of a chemical library with a pair of isogenic cell lines with and without the oncogenic KRAS gene as well as lead compound optimization. Our results have led to reports of the development of a group of novel anticancer agents called oncrasins.1–3 In addition, in an in vitro study of the NCI-60 cancer cell line panel and other cancer cell lines, we demonstrated that structurally similar compounds elicited a similar anticancer spectrum in a subset of cancer cell lines, including KRAS-mutant cells.2, 4, 5 Mechanistic characterization revealed that this group of anticancer agents triggered apoptosis by modulating subcellular localization of PKC-iota,1, 6 disrupting mRNA processing machinery,7 inhibiting JNK dephosphorylation,2 inducing oxidative stress,6, 8 and inhibiting STAT3 phosphorylation,4, 5 suggesting that those agents modulate the functions of multiple cancer-related targets. An in vivo study revealed that the maximum tolerated doses of oncrasin-60 (NSC-741909) and NSC-743380 (oncrasin-72), two analogues of similar structure and in vitro antitumor activity, were quite different. NSC-743380 was much better tolerated, and it also had more effective anticancer activity in a xenograft tumor model derived from human renal cancer cell line A498.4 NSC-743380 also induced significant in vivo tumor suppression in xenografts derived from the KRAS-mutant lung cancer cell line H157.5 In vitro studies revealed that NSC-743380 was highly active (IC50 between 10 nM and 1 µM) in vitro in about 30% of the 102 cancer cell lines we tested, 4, 5 suggesting that a large subset of cancer patients might benefit from treatment with this agent. Therefore, we selected NSC-743380 for further preclinical development.
However, a preliminary study performed at the Developmental Therapeutics Program of the National Cancer Institute suggested that NSC-743380 is not stable in some clinically acceptable injection formulations (Dr. Joel Morris, personal communications). Our own study revealed that NSC-743380 tends to form dimers in solutions with water and loses activity, especially in low-pH conditions.3 We hypothesized that the stability of NSC-743380 can be improved by protecting the hydroxyl group of NSC-743380 from dimer formation. To test this hypothesis, we synthesized and evaluated 5 ester derivatives of NSC-743380, and evaluated their spontaneous release of NSC-743380 in phosphate buffered saline. One of the esterified derivatives, designated oncrasin-266, releases NSC-743380 relatively slowly and was further investigated for its in vitro and in vivo properties. Here we describe the synthesis of the prodrug oncrasin-266 and the results of in vitro and in vivo evaluation of its stability, pharmacokinetic features, and safety.
2. Results and Discussion
2.1. Prodrug Synthesis
Prodrugs are chemically modified derivatives of a pharmacologically active compound that can release the active parent compound through chemical or enzymatic transformation. Prodrug approaches are commonly used in drug development to improve physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically active compounds. About 5–10% of drugs approved worldwide can be considered prodrugs, and there is an increased trend in implementing prodrugs in the early stages of drug development.9, 10 Indeed, about 20% of all small-molecule drugs approved between 2000 and 2008 were prodrugs.11 In anticancer drug development, prodrugs are intensively explored for their usefulness in targeted delivery or activation of anticancer therapeutics to improve bioavailability, reduce adverse effects, and maximize clinical efficacy compared with conventional methods.12, 13, 14
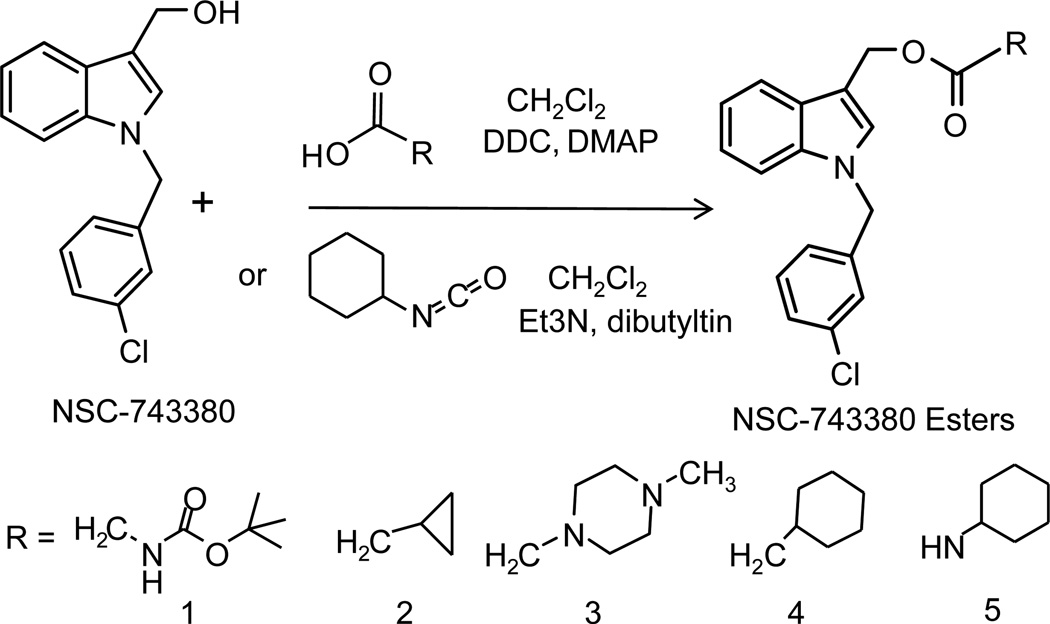
To determine whether an ester derivative of NSC-743380 could improve the stability of this anticancer agent, we esterified NSC743380 with 5 organic acids with or without cyclic groups (Fig 1). The purity and molecular weight of the final products were determined by HPLC/MS with ultraviolet detection at 280 nm. All compounds used in the study were ≥98% pure. The chemical structure of the final product was confirmed by nuclear magnetic resonance spectrum analyses.
Figure 1.
Synthesis of esterified NSC-743380 derivatives. Dichloromethane (CH2Cl2) is used as solvent. 1,3-dicyclohexylcarbodiimide (DCC), 4-(dimethylamine)pyridine (DMAP), triethylamine (Et3N) and dibutyltin diactetate were used as catalysts.
2.2. Release of NSC-743380 from esterified derivatives
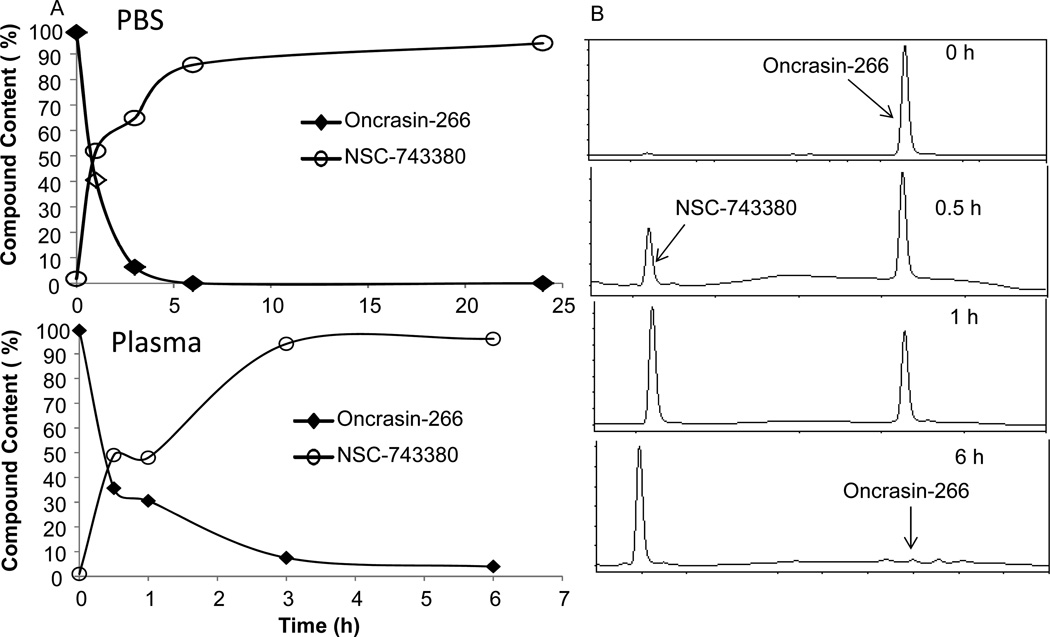
To determine whether NSC-743380 can be released from the esterified derivatives in physiological conditions, we dissolved the esterified products in phosphate buffered solution (PBS, pH 7.4) at a concentration of 0.5 mg/ml. An aliquot of the solution was taken at 0, 0.5, 1, 3, 6, and 24 h for HPLC analysis. The esterified derivatives from acid groups of 1, 2, 3, and 5 shown in Figure 1 hydrolyzed completely within 30 min, whereas the esterified derivative from acid group 4, designated oncrasin-266, released NSC-743380 relatively slowly in a time-dependent manner, completing the release in about 6 hours (Figure 2). The similar results was observed for the NSC-743380 release from oncrasin-266 when it was incubated in human plasma. This result demonstrated that oncrasin-266 can spontaneously hydrolyze to release NSC-743380 in a physiological buffer. Like many other ester prodrugs in use,15 parent drug can be released from the prodrugs by hydrolysis of the ester bond spontaneously or through various esterases present in body fluids and organs.16 The release of NSC-743380 from oncrasin-266 in the PBS solution suggested that the ester bond in oncrasin-266 can hydrolyze spontaneously. However, whether this process can be accelerated in vivo through esterases is not yet clear.
Figure 2.
Release of NSC-743380 from oncrasin-266 in PBS and human plasma. A) Time-dependent curve of NSC-743380 release from oncrasin-266. B) Examples of HPLC profiles of oncrasin-266 in PBS at different time points. Data shown are from one of two experiments with similar results.
To test whether oncrasin-266 has similar anticancer activity as NSC-743380, we compared dose-response of NSC743380 and oncrasin-266 in four lung cancer cell lines (H157, H460, Calu3 and H1299). Our previous study revealed that H157, H460 and Calu3 cells are highly sensitive while H1299 cell line is resistant to NSC743380 therapy.5 The in vitro study revealed oncrasin-266 has equivalent anticancer activity as NSC743380 for H157, H460 and Calu3 cells. The NSC743380 resistant H1299 cells are also resistant to oncrasin-26 (Figure 3), demonstrating that oncrasin-266 and NSC-743380 have similar anticancer activity and pattern.
Figure 3.
Anticancer activity of NSC-743380 (A) and oncrasin-266 (B) in lung cancer cells. Cells were treated with different concentrations of the compounds as indicated. Cell viability was determined 3 days after treatment. Control cells were treated with DMSO and their value was set as 100. The values represent mean ± SD of a quadruplet assay.
2.3. Stability and Formulation
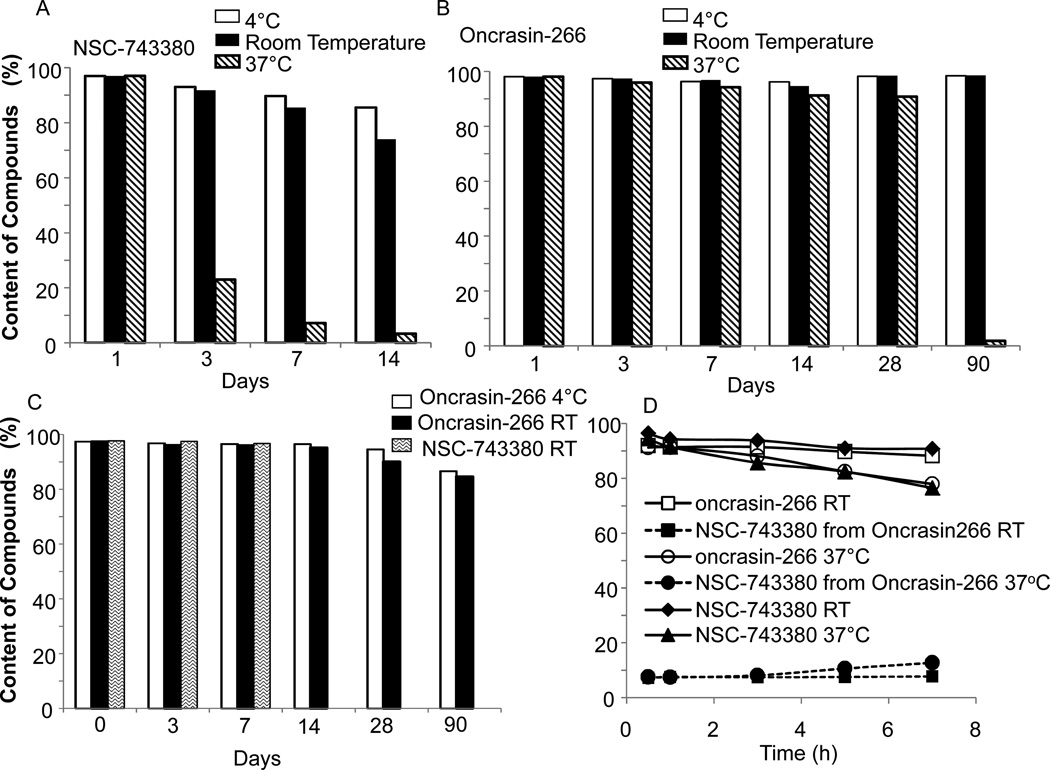
We then evaluated the stability of NSC-743380 and oncrasin-266 before and after formulation. The powders were stored at 4°C, room temperature, or 37°C. Aliquots of those powders were taken for up to 90 days to assess for changes in purity during storage. We found that although NSC-743380 was relatively stable in an evacuated container for long times (no data shown), its chemical nature changed relatively quickly in a non-evacuated container, especially at 37°C (Fig 4A). In contrast, oncrasin-266 was more stable than NSC-743380 in a non-evacuated container (Fig 4B). Powdered oncrasin-266 was stable at room temperature or at 4°C for at least 90 days, without detectable changes. At 37°C, the stability of powdered oncrasin-266 dropped slowly for 28 days but then plummeted to <5% by day 90. Powdered NSC-743380 was <5% over 14 days at 37°C. In the stock solution with Solutol HS15 and ethyl alcohol as solvent, NSC-743380 was rather stable for at least 1 week at room temperature and oncrasin-266 was stable for at least 2 weeks. At the 4°C, oncrasin-266 was stable for at least 4 weeks (Fig 4C). In the injectable solution with Solutol HS15, ethyl alcohol and 0.9% NaCl, about 10% of oncrasin-266 was hydrolyzed to release NSC-743380 within 7 hours at room temperature. The release of NSC-743380 was slightly higher than 10% at 37°C. NSC-743380 prepared in the same formulation had little change at room temperature for 7 h but lost more than 20% at 37°C (Fig 4D). Our results indicated that the prodrug improved the stability of the parent drug in both powder and formulated versions.
Figure 4.
Stability of NSC-743380 and oncrasin-266 in powder and after formulation. A) and B) powders; C) Stock solution; D) Injectable solution. The temperatures for storages are indicated in each graph. The stock solution is formulated with 75%(w/v) Solutol HS15, 20%(v/v) ethanol. The injectable solution is formulated with 9.4% (w/v) Solutol HS15, 12.5% (v/v) ethanol, and 79% (v/v) normal saline (0.9% NaCl)
2.4. Pharmacokinetics and tissue distribution
To determine whether NSC-743380 can be released from oncrasin-266 in vivo, we injected oncrasin-266 formulated in Solutol HS15 and ethanol into Balb/c mice through the tail vein at a dose of 25 mg/kg. The blood samples were collected from 3 animals/group at 0, 1, 2, 3.5, 7, and 24 hour and used to determine the presence of NSC-743380 and oncrasin-266 in plasma by HPLC/MS analysis. Animals treated with 30 mg/kg of NSC-743380 were used to compare the pharmacokinetics of those agents. As we expected, NSC-743380 was detected in NSC-743380-treated animals, while both NSC-743380 and oncrasin-266 were detected in the blood samples of animals treated with oncrasin-266, suggesting that NSC-743380 is effectively released in vivo. The half-life for NSC-743380, oncrasin-266 and NSC-743380 released from oncrasin-266 were about 30 min, 60 min and 75 min, respectively. The elimination kinetics for these compounds were similar, although the half-life of NSC-743380 released from oncrasin-266 is longer than that of NSC-743380 itself. The NSC-743380 concentration was much higher in oncrasin-266-treated animals than in NSC-743380-treated animals (Fig 5), which suggested that using the prodrug would improve the pharmacokinetics of NSC-743380.
Figure 5.
Pharmacokinetics of NSC-743380 and oncrasin-266 in mice after intravenous administration. The data are from 3 mice/group/time point.
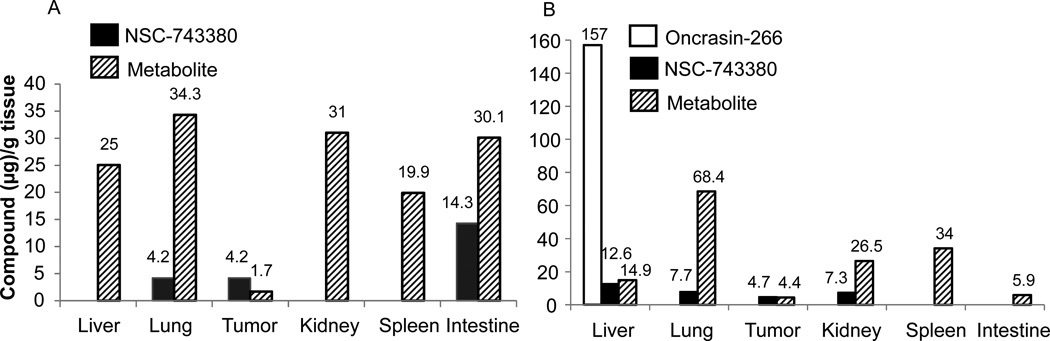
We then assessed the distribution of NSC-743380 and oncrasin-266 in major organs and in subcutaneous tumor tissue in nude mice. For this purpose, xenograft tumors derived from human lung cancer cell line Calu3 were established in nude mice. The animals were then intravenously injected with either NSC-743380 or oncrasin-266. Twenty-four hours after the treatment, animals were euthanized and the liver, lungs, kidneys, spleen, intestine, and subcutaneous tumor tissues were collected. The samples were homogenized and the presence of NSC-743380 or oncrasin-266 was determined by HPLC/MS analysis as described in Materials and Methods. In mice treated with NSC-743380, this agent was detected in the lungs, intestine, and subcutaneous tumor tissue (Figure 6A). In addition, a relatively high amount of a metabolite identified with HPLC/MS as the aldehyde derivative of NSC-743380 was detectable in all the tissues tested. Our previous study showed that the compounds with an aldehyde group or a hydorxymethyl group at the 3-position of the indole had anticancer activity whereas compounds with carboxylic acid group lost the activity,3 indicating that the major metabolite detected in those tissue may have antitumor activity. In contrast, in animals treated with oncrasin-266, NSC-743380 was detectable in the liver, lungs, kidneys, and subcutaneous tumor tissue, and the metabolite was similarly detected in all the tissues tested. A high concentration of oncrasin-266 was detected in the liver, suggesting that the prodrug was enriched or stored in liver tissue (Figure 6B). Nevertheless, why oncrasin-266 accumulate in liver and whether oncrasin-266 is a good candidate for primary or metastatic tumors in liver remain to be determined.
Figure 6.
Tissue distribution of NSC-743380 and oncrasin-266. Nude mice bearing subcutaneous xenograft tumors derived from the human lung cancer line Calu3 were intravenously injected with NSC-743380 or oncrasin-266 at a dose of 60 mg/kg. Organs and subcutaneous tumors were harvested 24 h after treatment. Values represent data from one animal/agent.
It is not uncommon that a prodrug changes the tissue distribution, pharmacokinetics, and toxicity profile of the parent drug. Because primary liver cancer is a major cause of cancer-related death worldwide and the liver is a major organ site of cancer metastasis,17, 18 the enrichment of oncrasin-266 in liver that we observed raises an intriguing question as to whether it can be used to treat liver cancer or liver metastasis of other cancers.
2.5. Oncrasin-266 is better tolerated than NSC-743380 is
The relatively higher concentration of NSC-743380 in blood and tissues in oncrasin-266-treated animals than in animals treated directly with NSC-743380 raised the question of whether the animals could tolerate the same dose of oncrasin-266 as NSC-743380. To address this question, we intraperitoneally injected 3 Balb/c mice/treatment group with 300 mg/kg of oncrasin-266 or NSC-743380 on day 1 and again on day 2. All three mice injected with NSC-743380 died on day 3. Of the mice treated with oncrasin-266, one died on day 4 and the remaining two survived for at least 2 weeks, when the experiment ended.
We then conducted the same experiment but with a daily dose of 150 mg/kg for 3 days (Figure 7A). Two mice in the NSC-743380-treated group died on day 5, whereas none of the oncrasin-266-treated mice died. We collected blood samples on day 6 from the four surviving mice for liver enzyme assays. The mouse treated with NSC-743380 had blood alanine transaminase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels about 1936, 4045, and 3 Units/L, respectively (Figure 7A). The corresponding values in mice treated with oncrasin-266 were about 115, 325 and 50 Units/L respectively. The normal ranges of ALT, AST and ALP levels in mouse plasma are 17–77, 54–298 and 35–96 (Unit/L), respectively. Histopathological analysis on livers harvested 48 h after treatment with 150 mg/kg of NSC-743380 or oncrasin-266 revealed that liver cell damage (necrosis) was obvious in mice treated with NSC-743380, but no obvious changes were observed in livers of oncrasin-266 treated mice (Figure 7C). Blood cell analysis harvested at the 48 h after treatment showed no abnormality in all groups. These results showed that oncrasin-266 was better tolerated than NSC-743380 in vivo, although the reason for this is not yet clear. When mice were treated with a dose of 25 mg/kd/day, the serum liver enzyme activities were mostly in normal ranges. Nevertheless, further toxicological analysis will be needed to document the safety of oncrasin-266.
Figure 7.
Effects on liver functions. Serum liver enzyme levels in mice that had been treated with (A) 150 mg/kg NSC-743380 or oncrasin-266 each day for 3 days or (B) 25 mg/kg NSC-743380 or oncrasin-266 each day for 8 days. Blood was collected 3 days after the last treatment. The numbers indicate actual or mean values for each bar. ALT: alanine transaminase; AST: aspartate aminotransferase; ALP, alkaline phosphatase. (C) Histopathological changes in liver. Mice treated once with 150 mg/kg NSC-743380 or oncrasin-266 and liver tissues were harvested at 48 h. The sections represent one of two mice/group with the similar results. Note, the liver cell damage at the low left corner of the liver section from NSC-743380 treated mice.
3. Conclusion
Our results showed that NSC-743380 can be spontaneously released from oncrasin-266 in vitro in PBS and in vivo in mice and that oncrasin-266 and NSC-743380 have similar anticancer activities and pattern, demonstrating that oncrasin-266 could serve as a prodrug of NSC-743380. Moreover, we found that, in comparison with NSC-743380, oncrasin-266 was more stable in vitro and had better pharmacokinetic properties and was better tolerated in vivo. Thus, our results demonstrated the feasibility of improving the stability, pharmacokinetics, and safety of NSC-743380 with a prodrug.
4. Materials and Methods
4.1. Chemicals and reagents
All reagents were purchased from Sigma-Aldrich at the highest commercially available quality. Solvents were reagent grade, high-performance liquid chromatography (HPLC) grade, or mass spectrometry (MS) grade depending on whether they were to be used for synthesis or chemical analysis.
4.2. Chemistry
NSC-743380 and its esterified products were synthesized and purified as we reported previously.3 Esterified NSC-743380 derivatives were synthesized as in as described in the Figure 1. For syntheses of derivatives 1 – 4, 10 mmol NSC-743380 was dissolved in 40 ml of anhydrous dichloromethane by stirring. Then, 12 mmol acids, for example cyclopropylacetic acid, 12 mmol 1,3-dicyclohexylcarbodiimide (DDC), and 2 mmol 4-(dimethylamine) pyridine (DMAP) were added into the solution. The mixture was stirred at room temperature for 5–6 hours. The product was filtered through Celite 521. The residue remaining in Celite 521 was washed with 20 ml of diethyl ether and then concentrated by evaporation under reduced pressure. The concentrated product was kept in a freezer overnight at −20°C to get a white solid product, which was washed with 20 ml of hexane to obtain the final product. For synthesis of derivative 5, 2 mmol NSC-743380 and 3 mmol cyclohexyl isocynate were dissolved in anhydrous dichloromethane. 1.5 mmol triethylamine and a catalytic amount of dibutyltin diactetate were added to the solution. After stirring the mixture at room temperature for 1 hour, the solvent was evaporated to stop the reaction. The residue was dissolved in chloroform/methanol (40/1), and the product was separated on silica gel column. The purified product was concentrated, then dissolved in diethyl ether, and crystallized with hexane.
For characterization of the product, proton nuclear magnetic resonance spectra (1H NMR) were recorded on Bruker Avance DPX 300-MHz NMR spectrometer as reported.3 Chemical shifts for 1H NMR spectra are reported as δ in units of parts per million (ppm) relative to tetramethylsilane (δ 0.0) using the residual solvent as an internal standard: chloroform-d (δ 7.26), DMSO-d6 (δ 2.50).
[1-(3-chlorobenzyl)-1H-indol-3-yl]methyl 2-(tert-butoxycarbonyl)-glycinate (1)
1H NMR (300MHz, CDCl3) δ 7.75 (m, 2H, C4-, C7-indole), δ 7.02–7.21 (m, 1H, indole and 4H, phenyl), δ 6.69 (m, 1H, C2-indole), δ 5.25 (s, 2H,-N-CH2), δ 5.02 (s, 2H, indole-3-CH2-O-), δ 3.98 (m,2H,CO-CH2-NH), δ 1.55 (s, 9H, CH(CH3)3). did not see –NH–. ESIMS m/z 371.8[M-C4H9+H].
[1-(3-chlorobenzyl)-1H-indol-3-yl] methyl 2-cyclopropylacetate (2)
1H NMR (300MHz, CDCl3) δ 7.73 (m, 1H, C4-indole), δ 7.00–7.21 (s, 3H, indole and 4H, phenyl), δ 6.69 (m, 1H, C2-indole), δ 5.34 (s, 2H,-N-CH2), δ 5.24 (s, 2H, indole-3-CH2-O-), δ 2.88 (m,2H,CO-CH2-), δ 1.01–0.21 (s, 5H, cyclopropyl group). ESI-MS m/z 353.8[M+H].
[1-(3-chlorobenzyl)-1H-indol-3-yl]methyl 2-(4-methyl-piperazin-1-yl)acetate (3)
1H NMR (300MHz, CDCl3) δ 7.78 (m, 1H, C4-indole), δ 7.00–7.21 (s, 3H, indole and 4H, phenyl), δ 6.65 (m, 1H, C2-indole), δ 5.36 (s, 2H,-N-CH2), δ 5.06 (s, 2H, indole-3-CH2-O-), δ 3.12 (s,2H,CO-CH2-N), δ 2.05–2.20 (s,11H, CH3-piperazinyl ring). ESI-MS m/z 411.9[M+H].
[1-(3-chlorobenzyl)-1H-indol-3-yl]methyl 2-cyclohexylacetate (4)
Oncrasin-266. 1H NMR (300MHz, CDCl3) δ 7.75 (m, 1H, C4-indole), δ 7.00–7.21 (s, 3H, indole and 4H, phenyl), δ 6.69 (m, 1H, C2-indole), δ 5.36 (s, 2H,-N-CH2), δ 5.26 (s, 2H, indole-3-CH2-O-), δ 2.09–2.18 (m,3H,CO-CH2-CH(CH2)5), δ 1.51–1.65 (m,10H, hexyl ring). ESI-MS m/z 395.8[M+H].
[1-(3-chlorobenzyl)-1H-indol-3-yl]methyl cyclohexylcarbamate (5)
1H NMR (300MHz, CDCl3) δ 7.75 (m, 1H, C4-indole), δ 7.00–7.24 (s, 3H, indole and 4H, phenyl), δ 6.63 (m, 1H, C2-indole), δ 5.38 (s, 2H,-N-CH2), δ 5.03 (s, 2H, indole-3-CH2-O-), δ 3.42 (m,1H, C-H in hexyl ring), δ 1.25–1.88 (s,10H, (CH2)5 in hexyl ring). ESI-MS m/z 397.1 [M+H].
4.3. High performance liquid chromatography (HPLC)/Mass spectrometer (MS) analysis
HPLC analysis was performed using the Agilent Technologies 1200 Series equipped with a C-18 bounded-phase column (Agilent, Zorbax Elipse Plus C18, 4.6×50 mm, 3.5 μm; or Poroshell 120 C18, 50×2.1 mm, 2.7 μm). A gradient elution was performed with acetonitrile and water as a mobile phase (0.2% formic acid) and was monitored with ultraviolet detection at 280 nm. All compounds were ≥98% pure. The chemical entities of the compounds were determined by liquid chromatography/MS with ultraviolet detection at 280 nm. Electrospray ionization MS spectra were recorded with an Agilent LC/MSD Trap XCT Ultra spectrometer.3
4.4 Cell viability assay
The in vitro anticancer activity of oncrasin-266 and NSC-743380 in lung cancer cells were determined by using the sulforhodamine B assay as we previously described.1
4.5. Compound stability analysis
Hydrolysis of oncrasin-266 in phosphate-buffered solution (PBS) was analyzed by diluting oncrasin-266 dissolved in DMSO/acetonitrile with PBS (pH 7.4) to reach the desired concentration of oncrasin-266 in 0.5 mg/ml, 12% DMSO and 18% acetonitrile. The sample solution was kept in 37°C water for up to 24 h. An aliquot of each sample was taken at 0.5, 1, 3, 6 and 24 hour and kept in a refrigerator cooled to −20°C for further testing. Samples were diluted 50-fold with acetonitrile before HPLC analysis.
The stabilities of NSC-743380 and oncrasin-266 were determined by storing them as powder, stock solution, or injectable solution at 4°C, room temperature (21°C), or 37°C. An aliquot was taken from those samples at various time points and placed in a refrigerator cooled to −20°C until analysis by HPLC. The powder samples were dissolved in DMSO and then diluted with 9 volumes of acetonitrile before HPLC analysis. To determine the stability of the stock solution, 70 mg of oncrasin-266 or 60 mg of NSC-743380 was completely dissolved in 1 ml of solvent containing 75% (w/v) Solutol HS15 dissolved in ethanol [final ethanol concentration is about 20% (v/v) in the solvent]. A total of 50 μl of the stock solution was stored in a brown glass tube with a tight cap and kept at room temperature until the time analysis. Ten microliters of each sample was diluted to 1 ml with acetonitrile/water (4/1) for HPLC analysis. To assess the stability of the injectable solution, the compounds were formulated in the solvent containing 9.0% (w/v) Solutol HS15, 4.8% (v/v) ethyl alcohol, and 89% (v/v) normal saline (0.9% NaCl). The final concentrations of oncrasin-266 and NSC-743380 were 8 mg/ml and 5 mg/ml, respectively.
4.6. Animal study
Animal experiments were carried out in accordance with Guidelines for the Care and Use of Laboratory Animals (NIH publication number 85–23) and the institutional guidelines of The University of Texas MD Anderson Cancer Center.
Compounds were dissolved in Solutol HS15/ethanol and diluted with 0.9% NaCl before intraperitoneal or intravenous injection into the mice 7–8 weeks old nude and/or Balb/c mice from Charles River (Raleigh, North Carolina). The final concentrations of the solvent components used for a higher dose (>150mg/kg) injection were 9.4% (w/v) of Solutol HS15, 8.3% (v/v) of ethanol, 8.3% (v/v) PEG-400, 0.2% 2-hydroxypropyl β-cyclodextrin and 70% (v/v) and normal saline (0.9% NaCl). For all other studies, the injectable solvent contains 9.4% (w/v) Solutol HS15, 12.5% (v/v) ethyl alcohol, and 79% (v/v) normal saline (0.9% NaCl). The pharmacokinetic properties of NSC-743380 and oncrasin-266 were determined by intraperitoneal injecting the agents at doses of 30 mg/kg and 25 mg/kg, respectively. Blood was collected from the tail vein of 3 mice/treatment group/time point in a plasma separator tube with lithium heparin. Plasma was obtained by centrifuging the tubes at 13000 rpm for 10 min. A 50-µl plasma sample was mixed with 150 µl of acetonitrile containing 0.2% formic acid and 8 ng/μl of 1-(3-chlorobenzyl)-1H-indole, the latter of which served as the internal standard. After incubation on ice for 15 min and centrifugation for 10 min, the supernatant was used for HPLC analysis to determine the levels of NSC-743380 and oncrasin-266. To assess the tissue distribution of the compounds, we injected a nude mouse (1 mouse/treatment group) bearing a xenograft tumor established from the human lung cancer cell line Calu3 through the tail vein with 60 mg/kg NSC-743380 or oncrasin-266. The mouse was euthanized with carbon monoxide gas after 24 h of this injection, at which time the liver, lungs, kidneys, spleen, small intestine, and subcutaneous tumor were harvested and kept at −80°C until analysis. The organs were homogenized in 50 mg/ml methanol on an ice bath. A total of 200 µl of each homogenate was mixed with 200 µl of acetonitrile containing 0.2% formic acid and 10 ng/μl 1-(3-chlorobenzyl)-1H-indole as the internal standard. After incubation at room temperature for 5 min and then placement on ice for 10 min, the mixture was centrifuged at 13000 rmp for 15min at 4°C. The supernatant was used for HPLC analysis as described for the pharmacokinetics analysis.
For the acute toxicity study, 3 mice/treatment-group were injected intraperitoneally with NSC-743380 or oncrasin-266 at 300 mg/kg/day for 2 days or 150 mg/kg/day for 3 days. The solvent used was the same as that described for the pharmacokinetic and tissue distribution study. The mice were monitored for surviving status up to 2 weeks.
Acknowledgements
We thank Elizabeth L. Hess of the Department of Scientific Publication at The University of Texas MD Anderson Cancer Center for editorial review of this manuscript.
Funding
This work was supported in part by the National Institutes of Health R01 grant CA124951 to B.F.; The University of Texas MD Anderson Cancer Center support grant CA-016672 (Pharmaceutical and Cellular Imaging Core Facility); and endowed funds to The University of Texas MD Anderson Cancer Center, including Moon Shot Program, the Homer Flower Gene Therapy Research Fund, the Charles Rogers Gene Therapy Fund, the Flora & Stuart Mason Lung Cancer Research Fund, the Charles B. Swank Memorial Fund for Esophageal Cancer Research, Stading Lung Cancer Research Fund, and the M.W. Elkins Endowed Fund for Thoracic Surgical Oncology
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guo W, Wu S, Liu J, Fang B. Cancer Res. 2008;68:7403. doi: 10.1158/0008-5472.CAN-08-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei X, Guo W, Wu S, Wang L, Lu Y, Xu B, Liu J, Fang B. J. Biol. Chemistry. 2009;284:16948. doi: 10.1074/jbc.M109.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S, Wang L, Guo W, Liu X, Liu J, Wei X, Fang B. J. Med. Chem. 2011;54:2668. doi: 10.1021/jm101417n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo W, Wu S, Wang L, Wei X, Liu X, Wang J, Lu Z, Hollingshead M, Fang B. PLoS ONE. 2011;6:e28487. doi: 10.1371/journal.pone.0028487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Guo W, Wu S, Wang L, Wang J, Dai B, Kim ES, Heymach JV, Wang M, Girard L, Minna J, Roth JA, Swisher SG, Fang B. Biochem. Pharmacol. 2012;83:1456. doi: 10.1016/j.bcp.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei X, Guo W, Wu S, Wang L, Huang P, Liu J, Fang B. J Translat. Med. 2010;8:37. doi: 10.1186/1479-5876-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo W, Wu S, Wang L, Wang R, Wei L, Liu J, Fang B. Mol. Cancer Ther. 2009;8:441. doi: 10.1158/1535-7163.MCT-08-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Wei X, Wu S, Wang L, Peng H, Wang J, Fang B. Eur. J. Pharmacol. 2010;649:51. doi: 10.1016/j.ejphar.2010.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Nat. Rev. Drug Discov. 2008;7:255. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 10.Zawilska JB, Wojcieszak J, Olejniczak AB. Pharmacol. Reports: PR. 2013;65:1. doi: 10.1016/s1734-1140(13)70959-9. [DOI] [PubMed] [Google Scholar]
- 11.Huttunen KM, Raunio H, Rautio J. Pharmacol. Rev. 2011;63:750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- 12.Du C, Deng D, Shan L, Wan S, Cao J, Tian J, Achilefu S, Gu Y. Biomaterials. 2013;34:3087–3097. doi: 10.1016/j.biomaterials.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Khan ZA, Tripathi R, Mishra B. Expert Opin. Drug Deliv. 2012;9:151. doi: 10.1517/17425247.2012.642362. [DOI] [PubMed] [Google Scholar]
- 14.Mahato R, Tai W, Cheng K. Adv. Drug Deliv. Rev. 2011;63:659. doi: 10.1016/j.addr.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettmayer P, Amidon GL, Clement B, Testa B. J. Med. Chem. 2004;47:2393. doi: 10.1021/jm0303812. [DOI] [PubMed] [Google Scholar]
- 16.Liederer BM, Borchardt RT. J. Pharmaceut. Sci. 2006;95:1177. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- 17.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Cancer. 2006;106:1624. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 18.Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, Giuliano R, Archie V, Calvin D, Mensha L, Shivers S, Cox C, Werner JA, Kitagawa Y, Kitajima M. Cancer Metastasis Rev. 2006;25:221. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]