Abstract
The impact of physical activity on cognitive function in older adults from minority and disadvantaged populations is not well understood. This study examined the longitudinal association between physical activity and cognition in older Mexican Americans. The study methodology included a prospective cohort with longitudinal analysis of data from the Hispanic Established Populations for the Epidemiological Study of the Elderly. General linear mixed models were used to assess the associations and interactions between physical activity and cognitive function over 14 years. Community-based assessments were performed in participants’ homes. Physical activity was recorded for 1,669 older Mexican Americans using the Physical Activity Scale for the Elderly. Cognition was measured by the Mini Mental State Exam, and separated into memory and non-memory components. A statistically significant positive association was observed between levels of physical activity and cognitive function after adjusting for age, sex, marital status, education and comorbid health conditions. The interaction between physical activity and time was significant for individuals in the third (β=0.11, SE=0.05) and fourth (β=0.10, SE=0.2) quartiles of physical activity compared to those in the first. The protective effect of physical activity on cognitive decline was evident for the memory component of the MMSE, but not the non-memory component after adjusting for covariates. Greater physical activity at baseline was associated with reduced cognitive decline over 14 years in older Mexican Americans. The reduction in cognitive decline appeared to be related to the memory components of cognitive function.
Keywords: aging, cognition, Hispanic Americans, minority health
INTRODUCTION
Research has demonstrated a positive relationship between physical activity and cognition in older adults.1–5 Meta-analyses6,7 have confirmed the positive effects of physical activity in adults with normal cognitive status, as well as those with cognitive impairment. Meta-analytic studies6,7 have also identified limitations in the research literature including the need for investigations to determine the level or intensity of physical activity required to produce positive cognitive outcomes, and the need for research to determine if various components of cognitive function are influenced in different ways by physical activity.
Studies investigating physical activity and cognition are frequently cross-sectional or include short-term follow-up periods.6,7 The long-term influence of physical activity on cognitive function remains unclear8,9, particularly in minority and underserved populations. Members of these populations often have lower educational levels and are less likely to engage in formal exercise or fitness programs. Data from the 2011 Behavioral Risk Factor Surveillance System indicates that only 15.7% of adults age 65 and older met the Centers for Disease Control and Prevention guidelines regarding physical activity, compared to 29.3% for 18–24 year olds.10 When all ages were stratified by race/ethnicity, Hispanics were least likely to meet physical activity recommendations. Eighteen percent of Hispanics met the guidelines compared to 21.0% for whites, 21.2% for Blacks, and 21.8% for others.10
The purpose of our study was to investigate the relationship between levels of physical activity and changes in cognition over time in a large cohort of Mexican American older adults with normal cognition (Mini Mental State Examination ≥ 21). Level of physical activity was examined to test for different effects in memory versus non-memory components of cognitive function. The hypothesis was that older Mexican Americans who reported greater physical activity at baseline would have less cognitive decline over the study period compared to participants who were sedentary.
METHODS
Sample and procedures
Participants were from the Hispanic Established Populations for the Epidemiological Study of the Elderly (EPESE), an ongoing longitudinal study of Mexican Americans aged 65 years and over and residing in Texas, New Mexico, Colorado, Arizona and California. The sampling plan and cohort characteristics have been described previously.11 Information and data for the Hispanic EPESE are available at the National Archive of Computerized Data on Aging.11
Seven waves of data have been collected for the Hispanic EPESE from 1993 through 2011. The present study used data obtained at the second through seventh waves (1995/96 to 2010/11). Information from the first wave was not used because the Physical Activity Scale for the Elderly (PASE)12 was not administered.
Of the 3,050 subjects interviewed at wave 1 (1993/1994) 2,438 participants were re-interviewed in 1995/96 (2,166 in person and 272 by proxy). At the two-year follow-up (wave 2), two hundred thirty-eight were confirmed dead through the National Death Index (NDI), 110 subjects refused to be re-interviewed, and 264 were lost to follow-up. Of the 2,438 participants interviewed at wave 2 (hereafter referred as baseline), those with a Mini Mental State Exam score < 21 (N=497)13 were excluded because the interest was in examining how physical activity influenced changes in cognitive function among those with normal and above cognition.
The final cohort included 1,669 subjects age >67 years who had a MMSE score >21 with complete information on the PASE and the MMSE (see descriptions below). At the end of the 14-year follow-up (2011), 427 were re-interviewed in person, 264 refused to be re-interviewed or were lost to follow-up, and 978 were confirmed dead through the National Death Index and reports from relatives. The average follow-up was 11.2 years (SD =1.3) for those who were re-interviewed, 7.4 (SD=4.6) years for those who were lost to follow-up, and 7.5 (SD=4.0) years for those who died.
Excluded participants (N=769) were significantly more likely to be older, to be unmarried, to have low levels of education and lower MMSE scores, and to report more heart attacks, strokes, and high depressive symptoms than those included in the analysis.
Measures
Independent Variable
Physical activity was assessed by the PASE, a validated instrument used to assess activity levels in older adults.12,14 Frequency, duration, and intensity of activity are measured in the PASE using three subscales: leisure, household, and occupational activity (described elsewhere).12 Levels of physical activity are calculated based on an empirically derived weighting system that provides a score for each subscale and a total physical activity score.14 The PASE asks respondents to recall activities from the previous week, and calculates a score ranging from 0 to 360.12,14 Higher scores represent greater levels of physical activity. To improve interpretation of the data, and consistency with previous studies,15,16 total PASE scores were converted into quartiles to represent gradients of activity: Q I (0 to < 33.6), Q II (33.6 to < 85), Q III (85 to 132.4), and Q IV (≥ 132.4). Categorizing PASE scores into quartiles is common, and has been performed in previous research evaluating the association between physical activity and stroke severity, bone strength, urinary symptoms, and functional limitations in knee osteoarthritis.15,16
Covariates
Baseline sociodemographic variables included age, sex, marital status, and years of education. The presence of medical conditions was assessed by asking whether a doctor had ever told participants that they had diabetes mellitus, heart attack, or stroke. Depressive symptomatology was measured using the Center for Epidemiologic Study –Depression Scale (CES-D),17 with scores ranging from 0 to 60. Participants with a score of 16 or greater were considered to have high depressive symptoms.
Outcome
Cognitive function was assessed with the 30-item MMSE.18 The English and Spanish versions of the MMSE were adopted from the Diagnostic Interview Scale.18 Similar to previous studies in populations with low educational attainment and low English literacy,13 only participants with MMSE scores of 21 or higher at baseline were included in the analyses. MMSE was used as a total score (range 0–30) and also divided into the following sub-domains: orientation (score 0–10), working memory (score 0–3), attention (score 0–5), delayed memory (score 0–3), and language (score 0–9). Based on previous research demonstrating that episodic memory predicts time to mild cognitive impairment19, and the fact that several studies have observed an association between physical activity and memory,20,21 the working and delayed memory subdomains of the MMSE were combined into one global “memory domain” (score 0–6) to assess this relationship in the Hispanic EPESE participants. The remaining sub-domains (orientation, attention, and language) were combined into a global “non-memory” domain (score 0–24).
Statistical Analysis
Chi-square and analysis of variance tests were used to examine the distribution of covariates for subjects according to PASE quartiles at baseline (1995/96). General linear mixed models using the MIXED procedure in SAS (SAS Institute, Cary, NC) were used to estimate change in cognitive function over a 14-year period as a function of PASE at baseline. Mixed models provide a general approach to the analysis of repeated measures. This approach uses all available data on each subject, is not affected by random missing data, and allows usage of realistic variance and correlation patterns.22 Mixed models also allow for modeling of time-dependent change in variables as well as time-dependent change in the magnitude of association between the variables.22
Two mixed models were constructed to test the relationship between PASE at baseline and change in cognitive function over a 14-year period. Model 1 included age, sex, marital status, years of formal education, PASE quartiles, time, and an interaction term including PASE x time [to estimate the effect of PASE quartiles at baseline on the rate of change in cognitive function (slope) during the study period]. In Model 2, stroke, heart attack, diabetes mellitus, and depressive symptoms were added to the variables in Model 1. All variables were analyzed as time-dependent covariates, except for age, sex, education, and PASE scale. PASE was also analyzed by category of activity; specifically leisure, household, and occupation. Analyses were performed using the SAS System for Windows, Version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
At baseline the mean age for the 1,669 participants was 74.3 years (SD=5.4). Sixty percent of the participants were female, 56.9% were married, and the mean years of education was 5.5 (SD=3.9). The mean total PASE was 97.8 (SD=63.8). Mean MMSE for the cohort at baseline was 25.6 (SD=3.2) with a mean for the memory component of 5.5 (SD=0.8), and 20.2 (SD=3.1) for the non-memory component. Forty-seven percent of the participants reported hypertension, 27.6% diabetes, 8.8% heart attack, 6.8% stroke, and 10.5% indicated high depressive symptoms at baseline.
Table 1 presents the descriptive characteristics of the sample by PASE quartiles. Mean PASE scores were 17.5 (SD=13.3) for Q I, 61.3 (SD=12.2) for Q II, 108.5 (SD=14.6) for Q III, and 177.5 (SD=40.1) for Q IV. Participants in Q IV were significantly more likely to be younger, male, married, to report less depressive symptoms and less medical conditions (hypertension, diabetes, heart attack or stroke), and to have higher scores in the total MMSE, and in the non-memory component of the MMSE.
Table 1.
Descriptive baseline characteristics of the sample by physical activity quartile (N=1,669).
| Explanatory variables | Total N=1669 |
Q1 (PASE: 0 to < 33.6) N=341 |
Q2 (PASE: 33.6 to < 85) N=416 |
Q3 (PASE: 85 to < 132.4) N=437 |
Q4 (PASE ≥ 132.4) N=475 |
p-value |
|---|---|---|---|---|---|---|
| PASE, mean ± SDa | 1669 | 17.5 ± 13.3 | 61.3 ± 12.2 | 108.5 ± 14.6 | 177.5 ± 40.1 | < 0.001 |
| Age, mean ± SD | 1669 | 76.6 ± 6.5 | 74.5 ± 5.6 | 73.7 ± 5.2 | 72.9 ± 4.5 | < 0.001 |
| Gender, n (%) | < 0.001 | |||||
| Male | 662 | 107 (31.4) | 107 (25.7) | 154 (35.2) | 294 (61.9) | |
| Female | 1007 | 234 (68.6) | 309 (74.3) | 283 (64.8) | 181 (38.1) | |
| Education (yrs), mean ± SD | 1649 | 5.2 ± 3.8 | 5.6 ± 3.9 | 5.3 ± 3.9 | 5.9 ± 4.2 | 0.049 |
| Marital status, n (%) | < 0.001 | |||||
| Not married | 720 | 163 (47.8) | 210 (50.5) | 188 (43.0) | 159 (33.5) | |
| Married | 949 | 178 (68.6) | 206 (49.5) | 249 (57.0) | 316 (66.5) | |
| Hypertension, n (%) | < 0.001 | |||||
| Yes | 774 | 197 (58.5) | 199 (48.1) | 193 (44.4) | 185 (39.0) | |
| No | 886 | 140 (41.5) | 215 (51.9) | 242 (55.6) | 289 (70.0) | |
| Heart attack, n (%) | 0.005 | |||||
| Yes | 146 | 48 (14.2) | 32 (7.7) | 25 (5.7) | 41 (8.7) | |
| No | 1519 | 291(85.8) | 384 (92.3) | 411 (94.3) | 433 (91.4) | |
| Diabetes, n (%) | < 0.001 | |||||
| Yes | 460 | 133 (39.0) | 108 (26.0) | 101 (23.1) | 118 (24.8) | |
| No | 1209 | 208 (61.0) | 308 (74.0) | 336 (76.9) | 357 (75.2) | |
| Stroke, n (%) | < 0.001 | |||||
| Yes | 163 | 49 (14.4) | 23 (5.5) | 19 (4.4) | 22 (4.6) | |
| No | 1554 | 292 (85.6) | 392 (94.5) | 417 (95.6) | 453 (95.4) | |
| High depressive symptoms (CES-D ≥ 16), n (%) | < 0.001 | |||||
| Yes | 175 | 74 (21.7) | 36 (8.7) | 38 (8.7) | 27 (5.7) | |
| No | 1490 | 267 (78.3) | 379 (91.3) | 399 (91.3) | 445 (94.3) | |
| Total MMSE scoreb, mean ± SD | 1669 | 24.7 ± 2.9 | 25.5 ± 3.2 | 25.9 ± 3.2 | 26.2 ± 3.2 | < 0.001 |
| MMSE memory domainc mean ± SD | 1623 | 5.4 ± 0.9 | 5.4 ± 0.9 | 5.4 ± 0.9 | 5.5 ± 0.8 | 0.071 |
| MMSE non-memory scored, mean ± SD | 1625 | 21.2 ± 2.9 | 21.7 ± 2.7 | 22.1 ± 2.5 | 22.3 ± 2.4 | < 0.001 |
PASE= Physical Activity Scale for the Elderly; CES-D=Center for Epidemiologic Studies Depression Scale
PASE ranges from 0 to 360
MMSE total score ranges from 0 to 30
MMSE memory domain score ranges from 0–6
MMSE non-memory score ranges from 0–24
Note: “N” varies because of missing data in some covariates
Table 2 shows the general linear mixed model estimates for total MMSE score and for memory and non-memory components as a function of PASE quartiles over the 14-year period. The adjusted rate of decline in the total MMSE score was −0.38 points per-year (Table 2). The association between PASE quartiles and total MMSE score at baseline (intercept of total MMSE score) after adjusting for age, sex, marital status, education, and time was statistically significant for Quartile II [β=0.47, SE=0.21], Quartile III (β=0.95, SE=0.21), and Quartile IV (β=1.10, SE=0.22). The interaction term between PASE quartiles and time of follow-up (slope of total MMSE score over 14-years) was statistically significant for Q III (β=0.11, SE=0.05) and for Q IV (β=0.10, SE=0.05) suggesting that participants in the higher activity quartiles experienced slower rates of decline in MMSE score over 14-years than did those subjects in the less active quartiles. In Model 2, when medical conditions and depressive symptoms were added to the variables in Model 1, the interaction term between Q III and time remained significant (β=0.11, SE=0.05).
Table 2.
General linear mixed models estimates for total MMSE, memory domain, and non-memory domain scores as a function of PASE quartiles over 14-year period in older Mexican Americans (N= 1,669).
| Total MMSE | Memory domain | Non-memory domain | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor variables | Model 1 β (SE) | Model 2 β (SE) | Model 1 β (SE) | Model 2 β (SE) | Model 1 β (SE) | Model 2 β (SE) |
| Intercept | 28.60 (1.04) * | 28.43 (1.05) * | 6.77 (0.27)* | 6.72 (0.27)* | 22.48 (1.00)* | 22.86 (1.00)* |
| Time | −0.33 (0.04) * | −0.38 (0.05) * | −0.08 (0.01)* | −0.07 (0.01)* | −0.47 (0.05)* | −0.29 (0.04) * |
| PASE quartiles | ||||||
| Q I | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q II | 0.47 (0.21) ‡ | 0.51 (0.22) ‡ | 0.02 (0.06) | 0.01 (0.06) | 0.39 (0.20) | 0.30 (0.21) |
| Q III | 0.95 (0.21) * | 1.00 (0.22) * | 0.05 (0.06) | 0.05 (0.06) | 0.85 (0.20)* | 0.76 (0.21) † |
| Q IV | 1.10 (0.22) * | 1.14 (0.22) * | 0.13 (0.06)‡ | 0.12 (0.06)‡ | 0.96 (0.21)* | 0.90 (0.21)* |
| PASE x time | ||||||
| Q I x time | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q II x time | 0.03 (0.05) | 0.03 (0.05) | 0.01 (0.01) | 0.006 (0.01) | 0.08 (0.05) | 0.03 (0.04) |
| Q III x time | 0.11 (0.05) ‡ | 0.11 (0.05) ‡ | 0.03 (0.01)‡ | 0.03 (0.01)‡ | 0.12 (0.05) ‡ | 0.04 (0.04) |
| Q IV x time | 0.10 (0.05) ‡ | 0.08 (0.05) | 0.01 (0.01) | 0.008 (0.01) | 0.10 (0.05) ‡ | 0.04 (0.04) |
PASE = Physical Activity Scale for the Elderly
< 0.0001,
<0.001,
<0.01
QI = (PASE: 0 to < 33.6); QII = (PASE: 33.6 to < 85.0); QIII = (PASE: 85.0 to < 132.4); QIV = (PASE: ≥ 132.4)
Model 1 included time, age, gender, education, and marital status.
Model 2 included hypertension, diabetes, heart attack, stroke, high depressive symptoms and variables in Model 1.
Moderate levels of physical activity (QIII) were associated with a reduced decline over time in the memory component of the MMSE compared to lower levels (Q I and Q II). No significant association was found between physical activity and cognitive decline for the non-memory component of cognitive function in the fully adjusted model (see Table 2). There was no significant association between the individual components of the PASE (leisure activity/household activity or occupation) and total MMSE or the memory and non-memory components over time (data not shown).
When PASE scores were analyzed as a continuous variable instead of quartiles, the parameter estimates for MMSE scores at baseline (intercept of MMSE score) were statistically significant (β= 0.006, SE=0.001) and the interaction term between PASE and time of follow-up (slope of MMSE score over 14-years) was also significant (β=0.0005, SE=0.0002) after adjusting for all covariates. This finding suggests that a higher PASE score at baseline is associated with slower rates of decline in MMSE over time. Non-significant associations were observed between the continuous PASE measure, and the memory and non-memory components (data not shown).
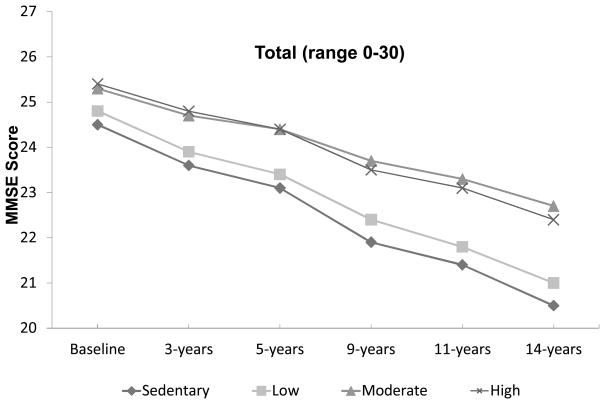
Figure 1 shows the adjusted mean distribution for the total MMSE score over the 14-year period by PASE quartiles at baseline. Subjects in quartiles I and II had lower total MMSE scores than subjects in quartiles III and IV over the entire follow-up period.
Figure 1.
Adjusted means for total Mini Mental State Exam (MMSE) score as a function of Physical Activity Scale for the Elderly quartiles at baseline (N=1,669) over 14 year follow-up period.
DISCUSSION
The findings support our hypothesis that Mexican American older adults who reported greater physical activity at baseline would display reduced cognitive decline over time. The results can be summarized as follows. The rate of decline in MMSE scores for participants in our cohort averaged −0.38 points per year over the 14-year study period after adjusting for personal, sociodemographic and health characteristics. The highest levels of activity (quartiles III and IV), as measured by the PASE at baseline, were associated with a protective effect on MMSE decline over the study period. The protective effect of moderate physical activity (quartile III) was also observed in the memory component of cognitive function.
Our results are consistent with a previous study of 544 older adults with a similar age range and using the PASE.23 The authors reported that more physical activity was associated with better cognitive status.23 This association was strongest for executive function, as measured by the MMSE.23 Intensity of activity was also positively associated with cognition among older adults wearing an accelerometer and completing the Trail Making Test.5
Our findings contribute new information to the literature documenting changes in cognitive function in older Mexican Americans associated with levels of physical activity. A large sample was examined for cognitive decline over 14-years. Analyses of the memory and non-memory components of cognition as represented in the MMSE were also conducted. An additional contribution of our study is the focus on an underserved population. Latinos are the fastest growing segment of the older population and a group where relatively little is known about patterns of physical activity related to cognitive function, particularly in persons over 75 years of age.
Historically, Hispanics (including Mexican Americans) report lower levels of physical activity compared to non-Hispanic whites.24 The mean PASE score in our sample was 97.8 (SD=63.8), which is lower than the average score of 118.9 among adults ≥65 years in a validity study for the PASE,14 and lower than other previous reports involving non-Hispanic community-dwelling samples.25,26
An important finding in our investigation is the lack of a positive association between lower quartiles of physical activity and a reduced decline in cognitive function (Figure 1). A previous study involving the Hispanic EPESE participants found a positive association between low levels of physical activity and reduced mortality.27 The lack of a positive effect for low levels of physical activity on changes in cognitive status has implications for the development and intensity of activity intervention programs for this population and represents an area for future research.
Our results suggest that engagement in a moderate amount of routine daily activities, such as walking, gardening, mowing the lawn and housework may have beneficial effects in maintaining cognitive function in older Mexican Americans, a population that currently lacks targeted, culturally relevant, evidence-based recommendations to maintain or enhance physical activity. Consistent with previous intervention studies, our results suggest that the protective effect of physical activity on cognitive decline is associated with memory aspects of cognition.20,21 Chang and colleagues reported that physical activity facilitated working memory, while Ruscheweyh et al. showed a positive association between activity and memory, regardless of intensity.20,21 Physical activity and memory were positively associated in a study among Japanese adults with mild cognitive impairment.28 However, our findings are in contrast to a previous study in the Netherlands among 45–75 year olds that observed an association between changes in physical activity intensity and cognitive processing speed, but not in memory.8 Additional research is needed to develop a better understanding of the potential differential impact of physical activity on the memory and non-memory components of cognitive function.
Limitations
Our study has limitations. Measures of physical activity were based on self-report and available only at baseline. The PASE instrument incorporates frequency, duration, and intensity to calculate a score. It is not possible to separate out the influence of these individual components of physical activity. The MMSE is used primarily as a screening assessment for cognitive function and has limited sensitivity to detect changes over time, particularly in specialized areas of cognition. The study team did not have access to medical records, diagnostic images or serum markers to confirm participant self-reports of medical conditions.29 It is also important to note that our findings apply to Mexican American older adults and cannot be generalized to the larger Hispanic population.
This study has several strengths, including the large community-based sample, the prospective design, and our exploration of the potential role of physical activity on the memory and non-memory components of cognitive function in older Mexican Americans.
CONCLUSION
A positive association was observed between levels of physical activity and changes in cognitive function over 14-years in a well-defined cohort of Mexican American older adults living in the community. Our findings suggest that greater physical activity is associated with reduced cognitive decline in this population; and that the protective effect of physical activity may be more evident in memory than non-memory aspects of cognition. Additional investigation is necessary to confirm and refine our findings and to help translate research on physical activity into intervention programs that can prevent or reduce cognitive decline and improve health in older adults.
Acknowledgments
Funding: This work was supported by the National Institute of Aging (R01 AG10939, R01 AG17638, P30 AG024832), the National Institute of Child Health and Human Development (R24 HD065702) at the National Institutes of Health, and the National Institute on Disability and Rehabilitation Research (H133P110012) Department of Education.
Drs. Al Snih, Markides, Graham, and Ottenbacher are all supported by federal research grants from the National Institutes of Health (NIH). Dr. Samper-Ternent was supported by a grant from the Department of Education during the period of the study. As noted in the acknowledgements, the NIH and Dept. of Education had no influence on the study’s design or findings.
Sponsor’s Role: The funding agencies (NIH and Department of Education) had no input on the study design, results, or interpretation of findings. Thus, the opinions and conclusions included in this paper are the authors’ and have not been reviewed or endorsed by the NIH or Department of Education.
Footnotes
Conflict of Interest: The authors report no conflicts of interest related to this study.
Author Contributions: Study design – Allison J. Ottenbacher, Soham Al Snih, Kenneth J. Ottenbacher O. Initial draft – Allison J. Ottenbacher, Soham Al Sni. Data analysis – Allison J. Ottenbacher, Soham Al Sni. Interpretation of results – Allison J. Ottenbacher, Soham Al Sni, James E. Graham. Clinical implications – Saad M. Bindawas, Rafael Samper-Ternent, Mukaila Raji. Critical review – Saad M. Bindawas, Kyriakos S. Markides, James E. Graham, Rafael Samper-Ternent, Mukaila Raji, Kenneth J. Ottenbacher O. Funding – Kyriakos S. Markides, Kenneth J. Ottenbacher.
References
- 1.Deary IJ, Corley J, Gow AJ, et al. Age-associated cognitive decline. Br Med Bull. 2009;92:135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- 2.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 3.Middleton LE, Mitnitski A, Fallah N, et al. Changes in cognition and mortality in relation to exercise in late life: A population based study. PLoS One. 2008;3:e3124. doi: 10.1371/journal.pone.0003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: A cross-sectional and longitudinal examination. J Gerontol B Psychol Sci Soc Sci. 2005;60:113–120. doi: 10.1093/geronb/60.3.p113. [DOI] [PubMed] [Google Scholar]
- 5.Kerr J, Marshall SJ, Patterson RE, et al. Objectively measured physical activity is related to cognitive function in older adults. J Am Geriatr Soc. 2013;61:1927–1931. doi: 10.1111/jgs.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med. 2011;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 8.Angevaren M, Vanhees L, Nooyens ACJ, et al. Physical activity and 5-year cognitive decline in the Doetinchem cohort study. Ann Epidemiol. 2010;20:473–479. doi: 10.1016/j.annepidem.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Lindwall M, Cimino CR, Gibbons LE, et al. Dynamic associations of change in physical activity and change in cognitive function: Coordinated analyses of four longitudinal studies. J Aging Res. 2012:493598. doi: 10.1155/2012/493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 11.National Archive of Computerized Data on Aging, Inter-university Consortium for Political and Social Research. [Accessed April 2, 2014]; Available at: http://www.icpsr.umich.edu/icpsrweb/NACDA/
- 12.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 13.Uhlmann RF, Larson EB. Effect of education on the mini-mental state examination as a screening test for dementia. J Am Geriatr Soc. 1991;39:876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 14.Washburn RA, McAuley E, Katula J, et al. The Physical Activity Scale for the elderly (PASE): Evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 15.Krarup LH, Truelsen T, Gluud C, et al. Pre-stroke physical activity is associated with severity and long-term outcome from first-ever stroke. Neurology. 2008;71:1313–1318. doi: 10.1212/01.wnl.0000327667.48013.9f. [DOI] [PubMed] [Google Scholar]
- 16.Cousins JM, Petit MA, Paudel ML, et al. Muscle power and physical activity are associated with bone strength in older men: The osteoporotic fractures in men study. Bone. 2010;47:205–211. doi: 10.1016/j.bone.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64:862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 20.Ruscheweyh R, Willemer C, Krüger K, et al. Physical activity and memory functions: An interventional study. Neurobiol Aging. 2011;32:1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Chang YK, Huang CJ, Chen KF, et al. Physical activity and working memory in healthy older adults: An ERP study. Psychophysiology. 2013;50:1174–1182. doi: 10.1111/psyp.12089. [DOI] [PubMed] [Google Scholar]
- 22.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 23.Eggermont LH, Milberg WP, Lipsitz LA, et al. Physical activity and executive function in aging: The MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57:1750–1756. doi: 10.1111/j.1532-5415.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neighbors CJ, Marquez DX, Marcus BH. Leisure-time physical activity disparities among Hispanic subgroups in the United States. Am J Public Health. 2008;98:1460–1464. doi: 10.2105/AJPH.2006.096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chad KE, Reeder BA, Harrison EL, et al. Profile of physical activity levels in community-dwelling older adults. Med Sci Sports Exerc. 2005;37:1774–1784. doi: 10.1249/01.mss.0000181303.51937.9c. [DOI] [PubMed] [Google Scholar]
- 26.Janney CA, Cauley JA, Cawthon PM, et al. Longitudinal physical activity changes in older men in the osteoporotic fractures in men study. J Am Geriatr Soc. 2010;58:1128–1133. doi: 10.1111/j.1532-5415.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottenbacher AJ, Snih SA, Karmarkar A, et al. Routine physical activity and mortality in Mexican Americans aged 75 and older. J Am Geriatr Soc. 2012;60:1085–1091. doi: 10.1111/j.1532-5415.2012.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanigawa T, Takechi H, Arai H, et al. Effect of physical activity on memory function in older adults with mild Alzheimer’s disease and mild cognitive impairment. Geriatr Gerontol Int. 2014 doi: 10.1111/ggi.12159. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]