Abstract
Objectives
In 2006, Medicare Part D transitioned prescription drug coverage for dual-eligible nursing home residents from Medicaid to Medicare and randomly assigned them to Part D prescription drug plans (PDPs). Because PDPs may differ in coverage, residents’ assigned plans may be relatively more or less restrictive for drugs they take. Taking advantage of the fact that randomization mitigates potential selection bias common in observational studies, this study seeks to assess the impact of PDP coverageon resident outcomes for three medication classes – antidepressants, antipsychotics, and cholinesterase inhibitors.
Design, Setting, Participants
Using Medicare claims, Minimum Data Set assessments, pharmacy claims, and PDP formulary information, we estimate the impact of coverage restrictions – including non-coverage and coverage with restrictions – on the following outcomes for dual-eligible nursing home residents randomized to PDPs in 2006–2008: depression; hallucinations/delusions; aggressive behaviors; cognitive performance; and activities of daily living. We further adjust for baseline health status to address any residual imbalances in the comparison groups.
Results
Across 5 outcomes in each of three medication classes of interest, PDP coverage restrictions impacted one resident health outcome: for cholinesterase inhibitor users, coverage restrictions were associated with a 0.04 point lower depression rating score relative to residents facing no restrictions. However, this result was not statistically significant after adjusting for multiple comparisons.
Conclusion
Our findings suggest that exogenous changes in coverage for three commonly-used medication classes had no detectable impact on nursing home resident health outcomes in 2006–2008. There are several possible explanations for this lack of association, including the role of policy protections for dual-eligible nursing home residents and the possibility that suitable clinical alternatives were identified or previously used medications offered little clinical benefit.
Keywords: Medicare Part D, coverage restrictions, nursing home
INTRODUCTION
In 2006, Medicare Part D transitioned prescription drug coverage for dual-eligible nursing home residents from Medicaid to Medicare and began randomly assigning these residents to Part D prescription drug plans (PDPs). Because PDPs differ in coverage restrictions, residents’ assigned plans may be relatively more or less generous for the drugs they are currently taking. Although previous studies have highlighted concerns related to the transition of nursing home residents to Part D, (1–4) none has yet analyzed the impact of PDP coverage restrictions on related resident health and functional outcomes.
Earlier studies examining the link between drug benefit coverage and medication use outside of the nursing home Part D setting typically measured the relative generosity of coverage in one of two ways: 1) the amount paid by the enrollee out of pocket;(5, 6) or 2) the share of total drug expenditures paid by the enrollee out of pocket.(7, 8) Because dual-eligible nursing home residents face no cost sharing under Part D, these approaches are inappropriate for this context. Instead, a PDP formulary coverage measure for this population must incorporate information on formulary coverage and utilization management, including prior authorization and step therapy requirements, for medications that residents take.
Among drugs used commonly by dual-eligible nursing home residents, who account for the majority of residents nationwide, studies have documented variation across PDPs with respect to benefit restrictions like formulary coverage, prior authorization, and step therapy requirements. (1, 9) Based on their early experience with Part D, nursing home and long-term care pharmacy (LTCP) stakeholders noted access challenges under Part D for a subset of drugs, including Alzheimer’s drugs, selected brand antidepressants and atypical antipsychotics, as well as alternate medication formulations (e.g., injectable, inhalation, topical, and infusion solutions).(1) Examination of one large LTCP’s rejected claims revealed a number of medications with a high volume of claims rejections, including the products Lexapro, Aricept, Fentanyl, Seroquel, Risperdal, Namenda, and Procrit.(10, 11) To date, the clinical impact of these coverage features has been unclear.
The randomization of dual-eligibles to PDPs offers an opportunity that potentially allows for causal estimation of the impact of medication coverage restrictions on outcomes. Combining multiple sources of data for approximately two-thirds of dual-eligible nursing home residents in the U.S., we build in this paper on previous work examining the impact of PDP coverage on the use of several medication classes commonly prescribed to nursing home residents.(4) Following randomization into Part D, only a small portion of residents faced non-coverage or utilization management requirements for drugs they were using at the time, when evaluated at the molecule level (i.e., brand and generic formulations of a molecule were treated as the same medication). Nonetheless, residents facing such restrictions were more likely to change medications within the class and have usage gaps. Although the study did not examine class- or condition-specific health outcomes, it did not find any statistically-significant differences in overall hospitalization or death rates between residents with less versus more restrictive medication coverage.
This paper analyzes the impact of PDP coverage on cognitive, behavioral, and functional outcomes in three therapeutic classes: antidepressants, used to treat clinical depression and other conditions; antipsychotics, used to treat schizophrenia and other psychotic disorders; and cholinesterase inhibitors, used to treat symptoms of Alzheimer’s disease and related neurological conditions. Each of these classes is commonly used by nursing home residents and shares a set of relevant health status outcomes. Two classes – antidepressants and antipsychotics – are currently “protected” under Part D, meaning PDPs must cover at least one formulation of every drug in the class. The third – cholinesterase inhibitors – is not a protected class; plans are required to cover only one drug in the class. For both protected and non-protected classes, plans are permitted to use utilization management tools like prior authorization and step therapy requirements to influence medication use. Our hypothesis was that residents who face relatively restrictive coverage of their medications after enrollment in their new plan would be more likely to experience worse health outcomes compared to residents who enroll in PDPs with relatively less restrictive coverage of their medications.
METHODS
Population
The study population includes dual-eligible nursing home residents aged 65 and older living in facilities that contracted with Omnicare as their pharmacy provider. Omnicare is the largest LTCP provider in the U.S, serving an estimated 50 percent of nursing home residents nationwide during the study period. For analytic purposes related to the control of unmeasured confounders, we focus on residents randomly assigned to their PDP. In 2006, the first year of Part D, all dual-eligible nursing home residents were randomly assigned to an initial PDP with a premium at or below the regional benchmark; in 2007 and 2008, residents whose PDP lost benchmark status (i.e., the plan’s premium bid for the coming year exceeded the benchmark) were randomly reassigned to another plan. For 2007 and 2008, we excluded individuals who elected to change PDPs in the previous year, since they would not have been re-randomized automatically. Importantly, if residents switched plans after randomization, their medication use and related outcomes were still assessed relative to the initial plan to which they were randomized (i.e., an “intent-to-treat” framework). Our focus is on residents enrolled in stand-alone PDPs.
We focus on residents taking medications on January 1st in each year in three classes: antipsychotics, antidepressants, and cholinesterase inhibitors. We define three separate cohorts of “current users” as those who filled at least two prescriptions in the class with 10 or more days of supply each or three prescriptions with any number of days supplied during the 100 days before PDP randomization on January 1st of the year in question. If residents use drugs in more than one class of interest, they could be included in more than one current user cohort within a particular year; similarly, if they were randomized to new PDPs in more than one year (e.g., 2006 and 2007), they may be included in more than one year. We exclude residents who used more than one drug in the class during the 100-day period, (7% of antipsychotic users; 17% of antidepressant users, and 1% of cholinesterase inhibitor users), as it introduced uncertainty in defining coverage restrictiveness and current users of particular medications.
Data
For the years 2005–2008, we link the following data for our study sample: pharmacy claims from Omnicare, which include all claims paid by Medicare (i.e., including those covered through Part D and those medications bundled in the Part A skilled nursing facility (SNF) payment), Medicaid, and other payers; resident demographic traits from Medicare Beneficiary Summary Files; assessments of residents’ health and functioning and date of death from the Minimum Data Set (MDS); dates of hospitalization from MedPAR files; and PDP formulary coverage and utilization requirements from the CMS Prescription Drug Plan Formulary, Pharmacy Network and Pricing Information files. We exclude individuals who died on January 1st of the year of randomized enrollment or who did not have at least one MDS assessment in the pre- and post-periods. All analyses present information on resident current user cohorts from all three study years combined.
Explanatory variable
The key explanatory variable of interest is PDP coverage restrictiveness, defined at the molecule – as opposed to product – level. For medications residents were taking when they were randomized to a new plan, we code coverage restrictiveness as a binary 0/1 variable, with 1 equaling either non-coverage or coverage with restrictions (i.e., prior authorization or step therapy) and 0 equaling coverage without restrictions.
Outcomes
In each class, we present information on a set of outcomes related to medication use and health and physical functioning. Following residents’ randomization to new plans, we track their subsequent medication use. Rather than present these usage patterns as terminal outcomes of interest, we present information about medication changes, gaps, stops, and continued use as mediators between PDP coverage limitations and the cognitive, behavioral, and functional outcomes we analyze. A medication change is indicated by a prescription claim for a drug in the same class other than the drug a resident was taking during the 100 days before randomized enrollment. Given our focus at the molecule level, changes between brand and generic formulations of the same drug (e.g., brand Paxil tablet to generic paroxetine tablet) were not considered as a “change.” A medication gap is identified as a period of 31 or more days with no prescriptions for any drug in the class followed by a prescription in the class within the observation window. Medication stops are identified as no prescriptions for drugs in the class during the observation window. Residents “continued use” if they experienced no gaps, changes, or stops. If residents experienced more than one outcome during the observation window (e.g., a medication change followed by a gap), we count only the event that occurred first. Following previous work,(4) we define the observation window as up to 30 days following the Part D “transition period” over which CMS issued PDPs guidance to fill prescriptions for non-covered drugs for nursing home residents while alternate therapies were considered (up to 210 days in 2006 and 120 days in 2007 and 2008). For individuals who were hospitalized or who died, we truncated the windows at the date of hospitalization or death.
Our primary analyses focus on the following MDS health and functional measures: depression rating scale (0–14 scale, where 0–2 indicates no depression, 3–5 diagnosable depression, and 6+ more severe depression)(12); presence of hallucinations or delusions in the last seven days (yes or no); aggressive behavior scale (0–12 scale, where 1–4 indicates mild to moderate aggressive behavior and 5+ more severe aggression)(13); cognitive performance scale (a 0–6 scale, where 0–1 indicates no or minimal impairment, 2–4 moderate impairment, and 5–6 severe impairment)(14); and activities of daily living score (0–28 scale, with higher scores indicating worse function).(15)
Analyses
We first present demographic and baseline health status information for the current users of each medication class (Table 1). We present information on health and functional measures at baseline, using the last MDS assessment prior to January 1st of the year of randomized enrollment.
TABLE 1.
Sample Characteristics of Dual-Eligible Nursing Home Residents Using Three Medication Classes, 2006–2008
| MEDICATION CLASS (current users, 2006–2008) | |||
|---|---|---|---|
|
| |||
| Antidepressants (N=70,662) | Antipsychotics (N=43,578) | Cholinesterase Inhibitors (N=38,199) | |
|
| |||
| Sex: | |||
| Female | 76.8% | 73.2% | 76.0% |
| Male | 23.2% | 26.8% | 24.0% |
|
| |||
| Age: | |||
| 65–74 | 18.6% | 13.8% | 22.7% |
| 75–84 | 40.4% | 43.8% | 42.3% |
| 85–94 | 36.2% | 38.8% | 31.3% |
| 95+ | 4.8% | 3.7% | 3.7% |
|
| |||
| Race: | |||
| White | 86.5% | 82.9% | 83.6% |
| Black | 9.8% | 13.6% | 12.3% |
| Other | 3.7% | 3.5% | 4.1% |
|
| |||
| Outcome Measures (baseline): | |||
| Mean (SD) | 1.2 (1.7) | 1.4 (1.9) | 1.1 (1.7) |
| Depression Rating Scale (0–14 score) | 83.5% | 80.3% | 84.6% |
| No depression % | 13.2% | 15.3% | 12.4% |
| Diagnosable Depression % | 4.4% | 3.3% | 3.1% |
| Severe Depression % | 5.4% | 4.8% | 9.2% |
| Hallucinations or delusions during last 7 days (0/1) % (Total) | 0.7 (1.5) | 1.1 (1.9) | 0.8 (1.6) |
| Aggressive Behavior (0–12 score) | |||
| None % | 71.1% | 58.3% | 68.3% |
| Mild to Moderate % | 25.6% | 35.6% | 27.9% |
| Severe % | 3.3% | 6.2% | 3.8% |
| Cognitive Performance Scale (0–6 score) | 2.8 (1.6) | 3.2 (1.4) | 3.2 (1.3) |
| No or minimal impairment % | 20.5% | 12.2% | 8.8% |
| Moderate % | 65.5% | 69.2% | 74.9% |
| Severe % | 14.0% | 18.6% | 16.3% |
| Activities of Daily Living Score (0–28) | 15.3 (8.1) | 15.2 (8.2) | 14.6 (8.0) |
Notes: Depression Rating Scale (0–2=no depression; 3–5=diagnosable depression; 6+=more severe depression, 6+); Aggressive Behavior (1–4 score indicates mild to moderate aggressive behavior; 5+ = more severe aggression); Cognitive Performance Scale (0–1= no or minimal impairment; 2–4=moderate impairment; 5–6=severe impairment).
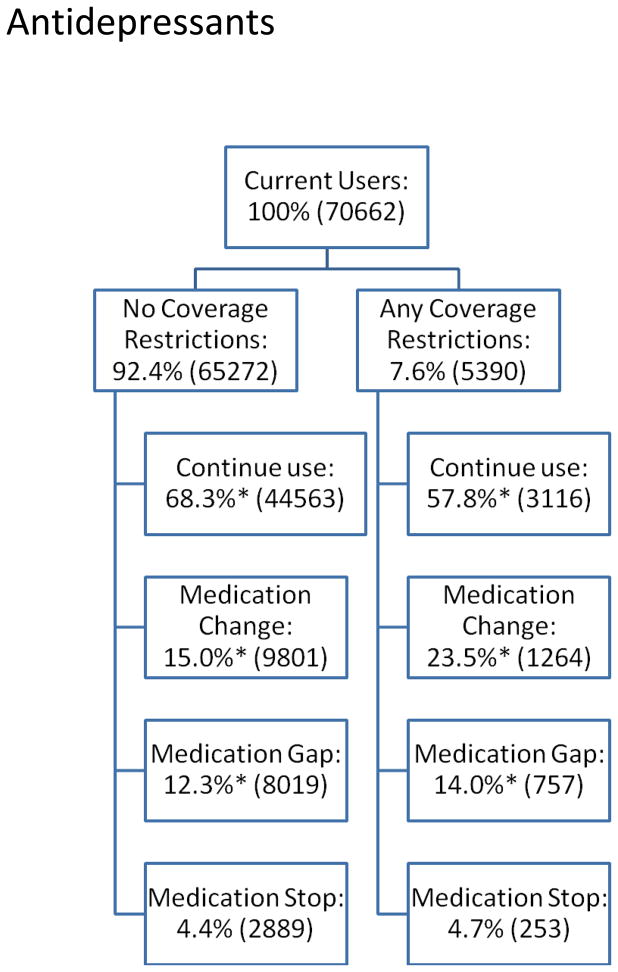
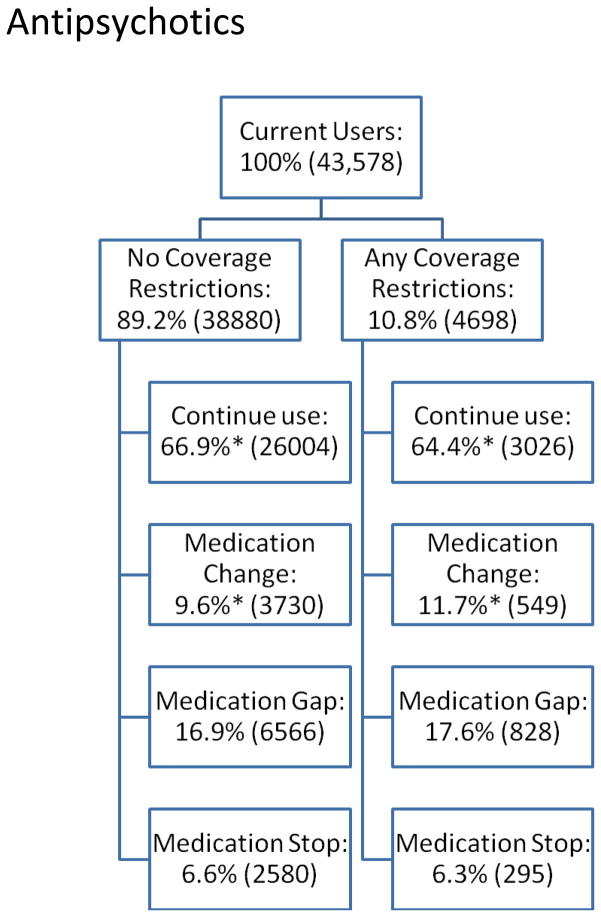
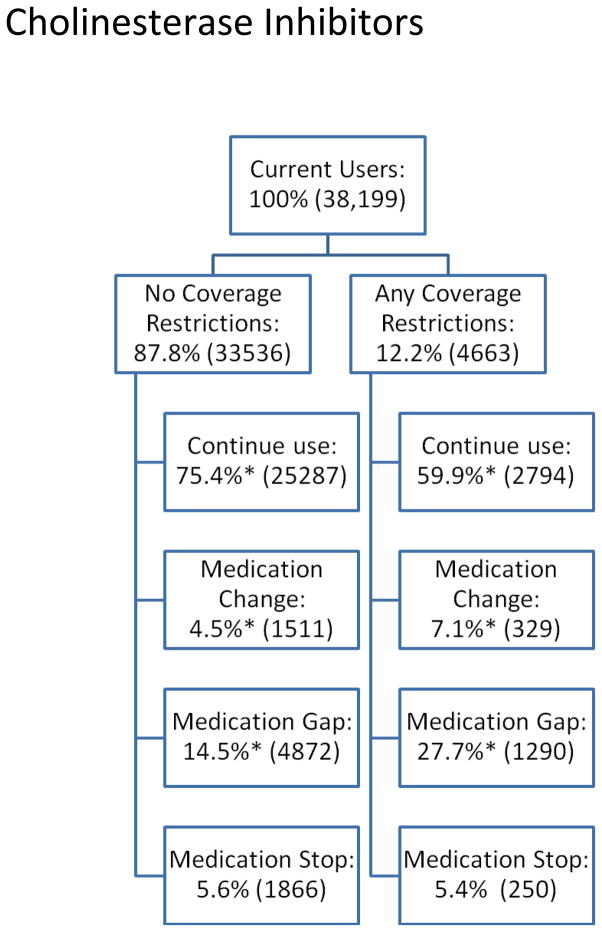
We next present descriptive data about PDP coverage restrictions (“any” versus “none”) and subsequent medication use outcomes (i.e., change, gap, stop, and continued use) for residents randomized into new PDPs (Figures 1a–1c).
Figure 1.
Figure 1a: Antidepressant Medication Use Behavior by Presence of Coverage Restrictions among Dual-Eligible Nursing Home Residents, 2006–2008.
Figure 1b: Antipsychotic Medication Use Behavior by Presence of Coverage Restrictions among Dual-Eligible Nursing Home Residents, 2006–2008.
Figure 1c: Cholinesterase Inhibitor Medication Use Behavior by Presence of Coverage Restrictions among Dual-Eligible Nursing Home Residents, 2006–2008.
Finally, we present regression results on the impact of coverage restrictions on resident outcomes (Table 2). Using regression models, we compare outcomes measured over the entire calendar year following randomized enrollment for dual-eligible residents to PDPs with no coverage restrictions versus residents randomized to PDPs with some restriction. We use linear regression for the numerical scales (depression, aggressive behavior, cognitive performance, and activities of daily living) and present results based on the mean (across all MDS assessments conducted during the year) scores for residents during the calendar year, weighted by the number of assessments for an individual. We use logistic regression for the dichotomous outcome (presence of hallucinations or delusions) and look for the presence of this outcome at any point over the same time period. Regression models were adjusted for the following: age (65–74, 75–84, 85–94, 95+); gender (male, female); race (black, white, other); region (midwest, northeast, south, west); dummy variables for the drug the resident was taking before randomization; year of randomized enrollment (2006, 2007, 2008); and the baseline measure of the outcome of interest. We present regression coefficients to denote the impact of PDP coverage restrictions for the MDS scales of interest (for depression, aggressive behavior, cognitive performance, and ADLs). For hallucinations/delusions, we present odds ratios comparing the likelihood of event occurrence. We also provide unadjusted results in Appendix Table 2.
Table 2.
Impact of Part D Coverage Restrictions for Antidepressants, Antipsychotics, and Cholinesterase Inhibitors on Nursing Home Resident Outcomes, 2006–2008
| OUTCOME MEASURES | |||||
|---|---|---|---|---|---|
| CLASS | Depression Rating Scale (p-value) [CI] | Hallucinations or Delusions Odds Ratio (p-value) [CI] | Aggressive Behavior Scale (p-value) [CI] | Cognitive Performance Scale (p-value) [CI] | Activities of Daily Living Limitations (p-value) [CI] |
| Antidepressants (n= 70,662) | 0.02 (0.24) [−0.01–0.04] | 1.08 (0.21) [0.96–1.22] | 0.00 (0.90) [−0.02–0.03] | −0.00 (0.76) [−0.02–0.02] | 0.01 (0.81) [−0.09–0.11] |
| Antipsychotics (n= 43,578) | −0.02 (0.19) [−0.05–0.01] | 1.00 (0.96) [0.90–1.12] | 0.00 (0.94) [−0.03–0.03] | 0.00 (0.88) [−0.02–0.02] | −0.02 (0.60) [−0.13–0.10] |
| Cholinesterase Inhibitors (n= 38,199) | −0.04 (0.02) [−0.07–0.00] | 0.94 (0.36) [0.82–1.07] | −0.01 (0.70) [−0.04–0.03] | 0.01 (0.25) [−0.01–0.03] | 0.10 (0.12) [−0.02–0.22] |
Note: Regression coefficients denote the impact of PDP coverage restrictions on the measures of interest, comparing residents randomized to PDPs with coverage restrictions to those who were randomized to plans with no such restrictions. We use linear regression for the MDS scales of interest (for depression, aggressive behavior, cognitive performance, and ADLs) and present results based on mean resident scores during the calendar year. We use logistic regression for the presence of hallucinations or delusions at any point over the same time period and present odds ratios of the likelihood of event occurrence. All models adjust for age, gender, race, geographic region, the drug a resident was taking before randomization, year, and a baseline measure of the outcome of interest. After correcting for multiple comparisons using the Bonferroni correction, the one statistically significant result was no longer significant, as the threshold for significance at the 0.05 level was 0.0033.
Appendix Table 2.
Impact of Part D Coverage Restrictions for Antidepressants, Antipsychotics, and Cholinesterase Inhibitors on Nursing Home Resident Outcomes, 2006–2008 (UNADJUSTED RESULTS)
| OUTCOME MEASURES | |||||
|---|---|---|---|---|---|
| CLASS | Depression Rating Scale (p-value) [CI] | Hallucinations or Delusions Odds Ratio (p-value) [CI] | Aggressive Behavior Scale (p-value) [CI] | Cognitive Performance Scale (p-value) [CI] | Activities of Daily Living Limitations (p-value) [CI] |
| Antidepressants (n= 70,662) | 0.06 (0.004) [0.02 to 0.11] | 1.00 (0.98) [0.90 to 1.10] | −0.03 (0.16) [−0.06 to 0.01] | −0.05 (0.02) [−0.09 to −0.01] | 0.18 (0.10) [−0.03 to 0.38] |
| Antipsychotics (n= 43,578) | 0.00 (0.86) [−0.05 to 0.06] | 0.99 (0.86) [0.90 to 1.08] | −0.04 (0.17) [−0.08 to 0.01] | −0.04 (0.07) [−0.08 to 0.00] | −0.22 (0.06) [−0.45 to −0.01] |
| Cholinesterase Inhibitors (n= 38,199) | 0.05 (0.04) [0.00 to 0.10] | 1.10 (0.05) [1.00 to 1.21] | 0.05 (0.04) [0.00 to 0.09] | 0.04 (0.06) [−0.00 to 0.08] | −0.23 (0.06) [−0.46 to 0.01] |
Note: Regression coefficients denote the impact of PDP coverage restrictions on the measures of interest, comparing residents randomized to PDPs with coverage restrictions to those who were randomized to plans with no such restrictions. We use linear regression for the MDS scales of interest (for depression, aggressive behavior, cognitive performance, and ADLs) and present results based on mean resident scores during the calendar year. We use logistic regression for the presence of hallucinations or delusions at any point over the same time period and present odds ratios of the likelihood of event occurrence. After correcting for multiple comparisons using the Bonferroni correction, none of the statistically significant results remained significant, as the threshold for significance at the 0.05 level was 0.0033.
A concern in analyzing five outcomes across each of three medication classes is that the chance of finding a statistically significant result when none exists is higher than the 0.05 level of a single test. We used the Bonferroni correction to account for this.(16) Using this conservative approach to correct for our multiple tests, a p-value in any test must be less than 0.0033 in order to report a significant finding.
Given the possibility that our results might be sensitive to modeling choices, we performed several sensitivity analyses. In addition to combining current user groups across study years, we examined 2006 – the transition year to Part D – separately from 2007–2008 combined. In addition to examining the effect on health outcomes of any coverage restrictions relative to none, we looked separately at non-coverage and utilization management requirements relative to no coverage restrictions. In addition to using the mean score of a particular outcome over the calendar year post randomization, we used the highest/worst assessment observed over the same time period. We also examined the percent of residents whose status worsened for a given outcome from baseline to the last assessment and whose assessments placed them into the most severe categories for our outcome scales of interest (e.g., severe depression, aggression, and cognitive impairment).
RESULTS
Population
In the three medication classes studied, study cohorts of dual-eligible nursing home residents randomized to PDPs in 2006–2008 are comprised of 70,662 (antidepressants), 43,578 (antipsychotics), and 38,199 (cholinesterase inhibitors) residents (Table 1). Across these classes, around three-quarters of current users are female, a similar percent are aged 75 and above, and more than 80% are white. Baseline health status is similar for current user groups, with some exceptions. For example, cholinesterase inhibitor users have higher levels of hallucinations/delusions (9%) compared to antidepressant and antipsychotic users (both around 5%). Similarly, a higher proportion of antipsychotic users have mild-moderate and severe aggressive behaviors (36% and 6%, respectively) compared to antidepressant and cholinesterase inhibitor users (26–28% and 3–4%, respectively).
Coverage restrictions and medication use
Figures 1a–1c show the proportion of current users facing no coverage restrictions (versus any restrictions) in the year of randomized enrollment and medication use behaviors that occur subsequently. For antidepressants, almost 8% of current users faced coverage restrictions subsequent to randomization. Compared to residents who did not face such restrictions, antidepressant users facing coverage restrictions had lower continuation rates (58% vs. 68%, p<0.05) and significantly higher rates of changes (23% vs. 15%, p<0.05) and gaps in use (14% vs. 12%, p<0.05) after randomized enrollment. Compared with antidepressant users, a slightly higher proportion of antipsychotic users faced coverage restrictions following randomization (11%), although the differences in subsequent medication use are smaller: 64% vs. 67% (residents facing any vs. no restrictions, respectively (p<0.05)) continued their antipsychotic; 12% vs. 10% changed antipsychotics (p<0.05); and similar proportions had gaps in use (18% vs. 17%, not significantly different). For cholinesterase inhibitor users, 12% faced coverage restrictions after randomization. Of these, 60% continued to use the same cholinesterase inhibitor compared to 75% of those who do not face restrictions (p<0.05); 7% vs. 5% change cholinesterase inhibitors (p<0.05); and 28% vs. 15% have gaps in use (p<0.05). In all three classes, similar proportions stopped using any drug within the class altogether, regardless of coverage restrictions.
Coverage restrictions and health outcomes
Table 2 shows the impact of Part D coverage restrictions on the clinical outcomes of nursing home residents using antidepressants, antipsychotics, and cholinesterase inhibitors, comparing residentsc who were randomized to PDPs with coverage restrictions for these drugs to those who were randomized to plans with no restrictions. Across the five outcomes examined for users in the three classes of interest, only one outcome in one drug class is statistically significantly affected by coverage restrictions, prior to adjustment for multiple comparisons. Cholinesterase inhibitor users randomized to a plan with any coverage restrictions for these medications have a subsequent depression rating scale score that is 0.04 points worse (p=0.0185) compared to cholinesterase inhibitor users randomized to a plan without any coverage restrictions (the mean baseline depression rating for these individuals was 1.10). After correcting for multiple comparisons, this result was not statistically significant. All sensitivity analyses produced results consistent with these findings, i.e., no association between coverage restrictions and outcomes.
DISCUSSION
Our findings suggest that changes to Part D plan coverage for three commonly-used medication classes resulted in treatment disruptions but did not have a detectable impact on outcomes for dual-eligible nursing home residents over the period 2006–2008. While we observed a modest negative effect on depression scores for cholinesterase users, this finding was not statistically significant after correcting for multiple comparisons. Our results are consistent for users of antidepressants, antipsychotics, and cholinesterase inhibitors across the common set of outcomes we examine and are robust to different modeling specifications.
Although it is unclear why coverage restrictions had no detectable impact on resident health outcomes, two factors stand out as potential explanations: policy protections for dual-eligible nursing home residents generally have been effective in ensuring access to necessary and clinically appropriate medications; or PDP coverage restrictions could have little impact on residents’ health if suitable alternatives exist or if previously used medications offered little clinical benefit.
Policy protections
Since Medicare Part D moved most nursing home residents from a Medicaid-financed, state-administered drug benefit to one administered by private plans in 2006, concerns have been raised about the potential negative impact on residents.(2) Beyond the transition’s administrative and logistical challenges, a key focal point of concern has been that these beneficiaries are assigned randomly to plans without regard for their medication needs. To mitigate the potential for harmful disruptions while remaining consistent with the program’s overall orientation toward private plans and consumer choice, a number of safeguards were instituted, some of which are specific to the nursing home population or the dually eligible. These include the designation of six protected classes, including two analyzed here (antidepressants and antipsychotics).
Beyond these class-specific protections, CMS directs PDPs to cover up to a 90-day supply of coverage limited drugs for new PDP enrollees in a nursing home, including enrollees reassigned when their plan loses benchmark status (in 2006, the first year of our analysis, this protection was up to 180 days). CMS also directs PDPs to cover a one-time temporary or emergency supply (one fill or up to a 31-day supply) of non-formulary medications for long-term care residents to lessen coverage gaps while exceptions or appeals are adjudicated. Dual-eligible nursing home residents pay no copayments and are permitted to change PDPs monthly, although there are concerns that relatively high levels of cognitive impairment may affect residents’ ability to weigh plan options and complete the administrative process to switch plans (in reality, few dual eligible beneficiaries switch plans during the year). A final, but important stop-gap protection beyond Part D is that nursing homes are required by statute to deliver care in accordance with residents’ clinical care plans, even in the absence of payment from a PDP. One implication of this policy is that residents may be switched back to their previous drugs if problems arise in taking a new medication.
In sum, the safeguards for nursing home residents enrolled in Part D are substantial and, ideally, further bolstered by responsibilities of nursing home and LTCP clinical staff. Our results provide some reassurance that these protections, individually and in combination, were effective in minimizing the potential negative effects of coverage gaps on nursing home residents in the initial years of Part D, at least in the classes we analyze. For example, even though nearly 71,000 antidepressant users were randomized to a new PDP during our study period, fewer than 10% of these residents faced coverage restrictions for these medications, and more than half of those who did continued their medications subsequently. Importantly, these protections can change, with CMS recently proposing a rule that would end designation of antidepressants and antipsychotics as protected classes.(17)
Medication efficacy
The clinical impact of coverage restrictions depends on a number of factors, including prevalence of use, available alternatives, and the efficacy of specific medications. For instance, if a drug is seldom used because there are clinically superior alternatives, low coverage levels may be appropriate and cause minimal burden clinically. It is possible that we did not see an association between plan coverage and outcomes because residents did not derive a clinical benefit from the drugs. Similarly, high levels of prior authorization might add valuable safeguards in cases where prescribing could be questionable or potentially harmful, either due to controversy about efficacy or concerns about risks or side effects. Among the medication classes we analyze, antipsychotics are perhaps the best example of this, as they represent an area where overuse has been problematic among nursing home residents, with policymakers and clinicians working together to reduce their utilization.(18) Moreover, studies are unclear as to whether or not cholinesterase inhibitors improve or maintain cognitive function among nursing home residents who have moderate to advanced cognitive impairment.(19) In contrast to clear, high-quality studies of depression in cognitively intact elders that demonstrate efficacy of antidepressant therapy for depression, the small number of randomized controlled studies published to date do not demonstrate efficacy of antidepressive therapy in older adults with both depression and dementia, potentially lessening the impact of interruptions or discontinuation of therapy.(20)
Limitations
Our study has several limitations. First, the experience of dual-eligible nursing homes residents may not generalize to other populations in Part D (e.g., who do not have similar policy protections) or to other medication classes. Second, we tried to select a range of relevant health and functional outcomes that have been validated and used in the literature, but it is possible that the outcomes were not sensitive enough to capture fully the impact of coverage restrictions on residents’ health. Third, although MDS data have moderate to high validity and reliability, potential inaccuracies in coding can arise due to poor medical historical data and inter-rater variability.(21) Fourth, our analyses excluded residents using more than one drug within a particular class; although these subgroups were small for antipsychotic and cholinesterase inhibitor users, they accounted for 17% of antidepressant users. Fifth, although our data captured the impact of coverage restrictions on health in the initial years of the Part D program, it is possible that plan utilization strategies have changed sufficiently that their impact has become more pronounced, thus warranting further study. Finally, given the limited number of residents who experienced medication use disruptions due to coverage restrictions, we may have lacked adequate power to detect changes in resident outcomes, especially in light of adjustment for multiple testing.
Conclusion
Medicare Part D produced a sea-change in the financing and administration of prescription drugs for our nation’s elderly dual-eligible nursing home residents. Despite some residents being randomized to plans with more restrictive coverage of their medications, we find little detrimental impact of these changes on residents’ health and functional status in three classes. Importantly, our findings should be interpreted in the context of the numerous Part D safeguards for selected medication classes and for nursing home residents generally. As the PDP marketplace continues to evolve, policymakers should remain vigilant that Part D and its related protections continue to work well for nursing home residents and other vulnerable Medicare beneficiaries.
Appendix Table 1.
Antidepressant, Antipsychotic, and Cholinesterase Inhibitor Medications Currently Used at the Time of Randomized Enrollment among Dual-eligible Nursing Home Residents, 2006–2008
| MEDICATION CLASS | DRUG | CURRENT USERS (#) | CURRENT USERS (%) |
|---|---|---|---|
| Antidepressants | |||
| Amitriptyline HCl Tablet | 680 | 0.96% | |
| Amoxapine Tablet | 7 | 0.01% | |
| Bupropion HCl Sustained Release Tablet | 520 | 0.74% | |
| Bupropion HCl Sustained Release Tablet Xl | 245 | 0.35% | |
| Bupropion HCl Tablet | 353 | 0.50% | |
| Citalopram Hydrobromide Reformulation Solution | 89 | 0.13% | |
| Citalopram Hydrobromide Reformulation Tablet | 17193 | 24.33% | |
| Citalopram Hydrobromide Solution | 53 | 0.08% | |
| Citalopram Hydrobromide Tablet | 5252 | 7.43% | |
| Clomipramine HCl Capsule | 51 | 0.07% | |
| Desipramine HCl Tablet | 42 | 0.06% | |
| Duloxetine HCl Delayed Release Capsule | 1740 | 2.46% | |
| Fluoxetine HCl Delayed Release Capsule | 76 | 0.11% | |
| Fluoxetine HCl Solution | 99 | 0.14% | |
| Fluoxetine HCl Tablet | 3211 | 4.54% | |
| Fluvoxamine Maleate Tablet | 230 | 0.33% | |
| Imipramine HCl Tablet | 110 | 0.16% | |
| Imipramine Pamoate Capsule | 1 | 0.00% | |
| Maprotiline HCl Tablet | 5 | 0.01% | |
| Mirtazapine Dissolvable Tablet | 4350 | 6.16% | |
| Mirtazapine Tablet | 9279 | 13.13% | |
| Nefazodone HCl Tablet | 26 | 0.04% | |
| Nortriptyline HCl Capsule | 564 | 0.80% | |
| Nortriptyline HCl Solution | 6 | 0.01% | |
| Paroxetine HCl Reformulation Tablet | 34 | 0.05% | |
| Paroxetine HCl Suspension | 33 | 0.05% | |
| Paroxetine HCl Sustained Release Tablet | 282 | 0.40% | |
| Paroxetine HCl Tablet | 4783 | 6.77% | |
| Phenelzine Sulfate Tablet | 7 | 0.01% | |
| Protriptyline HCl Tablet | 4 | 0.01% | |
| Selegiline Patch/Disc | 5 | 0.01% | |
| Sertraline HCl Suspension | 61 | 0.09% | |
| Sertraline HCl Tablet | 13177 | 18.65% | |
| Tranylcypromine Sulfate Tablet | 1 | 0.00% | |
| Trazodone HCl Tablet | 4312 | 6.10% | |
| Trimipramine Maleate Capsule | 2 | 0.00% | |
| Venlafaxine HCl Continuous Delivery Caps | 2792 | 3.95% | |
| Venlafaxine HCl Tablet | 987 | 1.40% | |
| Atypical Antipsychotics | |||
| Aripiprazole Dissolvable Tablet | 1 | 0.00% | |
| Aripiprazole Solution | 16 | 0.04% | |
| Aripiprazole Tablet | 1756 | 4.03% | |
| Clozapine Dissolvable Tablet | 18 | 0.04% | |
| Clozapine Tablet | 322 | 0.74% | |
| Haloperidol Suspension | 52 | 0.12% | |
| Haloperidol Tablet | 1252 | 2.87% | |
| Loxapine Succinate Capsule | 56 | 0.13% | |
| Molindone HCl Tablet | 22 | 0.05% | |
| Olanzapine Dissolvable Tablet | 998 | 2.29% | |
| Olanzapine Tablet | 10183 | 23.37% | |
| Quetiapine Fumarate Sustained Release Tablet | 1 | 0.00% | |
| Quetiapine Fumarate Tablet | 14041 | 32.22% | |
| Risperidone Dissolvable Tablet | 180 | 0.41% | |
| Risperidone Solution | 497 | 1.14% | |
| Risperidone Sustained Release Tablet | 7 | 0.02% | |
| Risperidone Tablet | 13391 | 30.73% | |
| Thiothixene Capsule | 100 | 0.23% | |
| Ziprasidone HCl Capsule | 685 | 1.57% | |
| Cholinesterase Inhibitors* | |||
| Donepezil HCl Dissolvable Tablet | 112 | 0.29% | |
| Donepezil HCl Tablet | 29196 | 76.43% | |
| Galantamine Hydrobromide Solution | 20 | 0.05% | |
| Galantamine Hydrobromide Sustained Release | 1408 | 3.69% | |
| Capsule | |||
| Galantamine Hydrobromide Tablet | 3326 | 8.71% | |
| Rivastigmine Patch/Disc | 27 | 0.07% | |
| Rivastigmine Tartrate Capsule | 4082 | 10.69% | |
| Rivastigmine Tartrate Solution | 28 | 0.07% |
Namenda is a NMDA agonist and not a cholinesterase inhibitor; thus, it is not included in our analyses.
Acknowledgments
FUNDING: The National Institute on Aging (R01 AG034085) provided financial support for the work. Dr. Stevenson was supported by NIH-NIA K01 AG038481. Dr. Mitchell was supported by NIH-NIA K24AG033640. Dr. Dusetzina is supported by the NIH Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12 Program (5K12HD001441-12) and the North Carolina Translational and Clinical Sciences Institute (UL1TR001111).
Footnotes
Sponsor’s Role: NIA played no substantive role in the design and execution of the study.
Author Contributions:
Study concept and design: DGS, AJO, SBD, SLM, BJZ, MEC, JPN, HAH
Acquisition of subjects and/or data: DGS, HAH
Analysis and interpretation of data: DGS, AJO, SBD, SLM, BJZ, MEC, JPN, HAH
Preparation of manuscript: DGS, AJO, SBD, SLM, BJZ, MEC, JPN, HAH
Conflict of Interest
J.P.N. is the director of and holds equity in Aetna, which sells Part D plans. B.J.Z. is an employee of Omnicare Inc., and holds Omnicare Inc., stock. She participates in research projects funded by grants from Amgen, Sanofi-Aventis, Mylan, AbbVie, Astellas, and Optimer. M.E.C. reports the following potential conflicts: Research support: CareFirst, NIA, The Commonwealth Fund, Charles H. Hood Foundation, InHealth, Institute of Medicine, Pfizer Inc., Robert Wood Johnson Foundation; Board memberships: Commonwealth Fund Commission of High Performance, Congressional Budget Office, Medicare Payment Advisory Commission, Abbott, FairHealth, Health Research & Educational Trust, National Institute of Health Care Management, CMS Technical Advisory Panel, NC Prevention Partner Advisory Council on Evidence-based Incentives and Benefits, and Massachusetts Medical Society; Consultancy: Massachusetts Medical Society, America’s Health Insurance Plans, Abbott, Humana, SEIU, Precision Health Economics, US Chamber of Commerce, and Milliman; LLC Equity: Benefit Based Designs, Value Based Insurance Design Institute, and Value Based Insurance Design Health; Payment for Manuscript Prep/development: Truven Healthcare Analytics, Precision Health Economics, New America Foundation, and London School of Economics; Role as Journal Editor: Health Services Research and American Journal of Managed Care. The remaining authors declare no conflict of interest.
References
- 1.Stevenson DG, Huskamp HA, Keating NL, et al. Medicare Part D and nursing home residents. Journal of the American Geriatrics Society. 2007;55:1115–1125. doi: 10.1111/j.1532-5415.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson DG, Huskamp HA, Newhouse JP. Medicare part D and the nursing home setting. Gerontologist. 2008;48:432–441. doi: 10.1093/geront/48.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson DG, Newhouse JP, Huskamp HA. Medicare Part D, Nursing Homes, and Long-Term Care Pharmacies. Washington, DC: Medicare Payment Advisory Commission; 2007. ( http://www.medpac.gov/documents/Jun07_Part_D_contractor.pdf) [Google Scholar]
- 4.Huskamp HA, Stevenson DG, O’Malley AJ, et al. Medicare Part D plan generosity and medication use among dual-eligible nursing home residents. Med Care. 2013;51:894–900. doi: 10.1097/MLR.0b013e31829fafdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman DP, Joyce GF, Escarce JJ, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291:2344–2350. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- 6.Joyce GF, Escarce JJ, Solomon MD, et al. Employer drug benefit plans and spending on prescription drugs. JAMA. 2002;288:1733–1739. doi: 10.1001/jama.288.14.1733. [DOI] [PubMed] [Google Scholar]
- 7.Doshi JA, Polsky D. Drug benefit generosity and essential medication use among Medicare-eligible retirees. Am J Manag Care. 2007;13:425–431. [PubMed] [Google Scholar]
- 8.Artz MB, Hadsall RS, Schondelmeyer SW. Impact of generosity level of outpatient prescription drug coverage on prescription drug events and expenditure among older persons. Am J Public Health. 2002;92:1257–1263. doi: 10.2105/ajph.92.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huskamp HA, Stevenson DG, Donohue JM, et al. Coverage and prior authorization of psychotropic drugs under Medicare Part D. Psychiatr Serv. 2007;58:308–310. doi: 10.1176/ps.2007.58.3.308. [DOI] [PubMed] [Google Scholar]
- 10.Huskamp HA, Stevenson DG, Keating NL, et al. Rejections of drug claims for nursing home residents under Medicare Part D. Health Aff (Millwood) 2008;27:560–567. doi: 10.1377/hlthaff.27.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson DG, Keohane LM, Mitchell SL, et al. Medicare part D claims rejections for nursing home residents, 2006 to 2010. Am J Manag Care. 2012;18:647–654. [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows AB, Morris JN, Simon SE, et al. Development of a minimum data set-based depression rating scale for use in nursing homes. Age Ageing. 2000;29:165–172. doi: 10.1093/ageing/29.2.165. [DOI] [PubMed] [Google Scholar]
- 13.Perlman CM, Hirdes JP. The aggressive behavior scale: A new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56:2298–2303. doi: 10.1111/j.1532-5415.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 14.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 15.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54A:M546–553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 16.Christenson RP. Answers to Complex Questions: The Theory of Linear Models. New York: Springer-Verlag; 1996. [Google Scholar]
- 17.Thomas K, Pear R. New York Times. 2014. Feb 21, Plan to Limit Some Drugs in Medicare is Criticized. Sect. B1. [Google Scholar]
- 18.Mitka M. CMS seeks to reduce antipsychotic use in nursing home residents with dementia. JAMA. 2012;308:119, 121. doi: 10.1001/jama.2012.7422. [DOI] [PubMed] [Google Scholar]
- 19.Qaseem A, Snow V, Cross JT, Jr, et al. Current pharmacologic treatment of dementia: A clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148:370–378. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee S, Hellier J, Dewey M, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): A randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 2011;378:403–411. doi: 10.1016/S0140-6736(11)60830-1. [DOI] [PubMed] [Google Scholar]
- 21.Shin JH, Scherer Y. Advantages and disadvantages of using MDS data in nursing research. J Gerontol Nurs. 2009;35:7–17. doi: 10.3928/00989134-20090101-09. [DOI] [PubMed] [Google Scholar]