Abstract
Withdrawal from chronic nicotine is associated with cognitive deficits. Therapies that ameliorate cognitive deficits during withdrawal aid in preventing relapse during quit attempts. Withdrawal-induced deficits in contextual learning are associated with nicotinic acetylcholine receptor (nAChR) upregulation. The aim of the present study was to determine if the acetylcholinesterase (AChE) inhibitor donepezil has the ability to reverse nicotine withdrawal-induced deficits in contextual learning. The results demonstrate that low doses of donepezil, which do not enhance contextual learning or alter locomotor activity/anxiety-related behavior, can reverse nicotine withdrawal-induced deficits in contextual learning. Thus, donepezil may have therapeutic value for ameliorating cognitive deficits associated with nicotine withdrawal and for preventing relapse.
Keywords: withdrawal, acetylcholinesterase, cognition, learning, addiction
Introduction
Since the first Surgeon General’s report in 1964, more than 20 million premature deaths have been attributed to smoking and this year alone more than 500,000 adults will die due to smoking-related illnesses. Cigarette smoking has been causally linked to diseases of all organs of the body, including common diseases such as diabetes mellitus, rheumatoid arthritis, and colorectal cancer (USDHHS, 2014). However, despite these drastic consequences, many individuals who wish to quit, simply cannot. As of 2008, over 70% of smokers wanted to quit completely, 40% attempted to quit, yet only 3–5% were able to do so for more than 6 months without assistance (Nides, 2008). The continuation of cigarette smoking despite known health hazards can be attributed to nicotine, the highly addictive psychoactive substance delivered by tobacco products (USDHHS, 1988).
In humans smoking cessation has been shown to result in cognitive deficits including impaired performance on tests of selective attention (Bell, Taylor, Singleton, Henningfield, & Heishman, 1999), sustained attention (Hughes & Keenan, 1989; Hughes, 2007), and working memory (Blake & Smith, 1997; Jacobsen et al., 2005). In addition, our lab has demonstrated that mice withdrawn from chronic nicotine show deficits in hippocampus-dependent learning, including contextual (Davis, James, Siegel, & Gould, 2005) and trace fear conditioning (Raybuck & Gould, 2009), as well as spatial object recognition (Kenney, Adoff, Wilkinson, & Gould, 2011). Importantly, impaired cognition during quit attempts predicts relapse (Patterson et al., 2010; Rukstalis, Jepson, Patterson, & Lerman, 2005), which has made ameliorating withdrawal associated cognitive deficits the target of pharmacotherapies for nicotine addiction (for review see Ashare, Falcone, & Lerman, 2014). Therefore, an important research goal is the evaluation of pharmacotherapies that may potentially ameliorate nicotine withdrawal-induced deficits in hippocampus-dependent learning (Davis & Gould, 2007; Portugal & Gould, 2007; Raybuck, Portugal, Lerman, & Gould, 2009; Wilkinson & Gould, 2011).
A neurophysiological characteristic of exposure to chronic nicotine is altered cholinergic signaling (Picciotto, Addy, Mineur, & Brunzell, 2008; Markou, 2008). Specifically, chronic nicotine leads to nicotinic acetylcholine receptor (nAChR) upregulation in rodents (Marks, Burch, & Collins, 1983; Schwartz & Kellar, 1985) and is associated with nAChR upregulation in humans (Cosgrove et al., 2009; Mamede et al., 2007). Furthermore, chronic nicotine results in a homeostatic change in the differential signaling contribution of nAChR subtypes (Besson et al., 2007). Thus, withdrawal may involve a homeostatic shift in cholinergic signaling once nicotine is removed from the system. Indeed, abstinent smokers show altered nAChR availability throughout various brain regions (Staley et al., 2006). In addition, the time-course of nicotine withdrawal-induced contextual learning deficits is associated with temporal changes in nAChR upregulation (Gould et al., 2012). Therefore, pharmacotherapies that modulate cholinergic signaling by increasing acetylcholine (ACh), the endogenous ligand for nAChRs, may provide valuable treatment options for nicotine addiction.
In the present study the acetylcholinesterase (AChE) inhibitor donepezil was tested for its efficacy in ameliorating nicotine withdrawal-induced deficits in contextual conditioning. Donepezil has shown promise in treating nicotine addiction as it attenuates nicotine self-administration and reinstatement of nicotine seeking in rats (Kimmey, Rupprecht, Hayes, & Schmidt, 2012) and improves working memory in smokers (Ashare, Ray, Lerman, & Strasser, 2012). Donepezil has also been shown to ameliorate learning deficits in a number of models of disrupted cognition (Csernansky et al., 2006; Easton et al., 2013; Zurkovsky et al, 2013; Tota et al., 2012) and has been approved by the FDA to treat mild cognitive impairment associated with Alzheimer’s disease (Shigeta & Homma, 2001). Finally, donepezil has fewer side effects (e.g. gastrointestinal disturbances) compared to other AChE inhibitors (Lanctôt et al., 2003).
Methods
Subjects
Subjects were male C57BL/6J mice aged 10–12 weeks at training (Jackson Laboratories, Bar Harbor, ME). Mice were housed 2–4 per cage and provided ad libitum access to food and water. A 12 h light-dark cycle was maintained throughout the study and all experimental procedures were conducted between the hours of 9:00 a.m. and 5:00 p.m. The Temple University Animal Care and Use Committee approved all procedures.
Surgeries
Mice were anesthetized with isoflurane (2.5% induction and maintenance) and surgically implanted with subcutaneous osmotic mini-pumps (Alzet, Model 1002) slightly posterior to the scapulae. The pumps delivered saline or 12.6 mg/kg/day nicotine for 12 days. The pumps were removed 12 days after implantation.
Drugs and administration
Nicotine hydrogen tartrate salt (Sigma, N5260) and donepezil hydrochloride monohydrate (Sigma, D6821) were dissolved in 0.9% sterile saline. For the dose response experiment, donepezil (0.015, 0.05, 0.15, 0.5, 1.5 mg/kg, doses based on previous research (Geerts et al., 2005)) or saline was administered s.c. 30 mins prior to fear conditioning training and testing. For the withdrawal study, chronic nicotine (12.6 mg/kg/day, dose based on previous research (André, Gulick, Portugal, & Gould, 2008)) or saline was administered s.c. via osmotic-mini pumps for 12 days; mice were then trained 24 hours after cessation of nicotine administration.
Apparatus
Mice were trained and tested for contextual conditioning in four identical clear Plexiglas chambers (17.78 x 19.05 x 38.10 cm) housed in sound attenuating boxes (Med-Associates, St. Albans, VT) (Davis, James, Siegel, & Gould, 2005). The floor of each chamber was made of 18 metal rods connected to a shock generator and scrambler (Med Associates, Model ENV-414). Ventilation fans mounted on the sides of each box provided background noise. A 4 W light was mounted above each box for illumination. Testing for cued conditioning occurred in an altered context consisting of four chambers (20.3 x 22.9 x 17.8 cm) that differed in visual, spatial, tactile, and olfactory cues. All chambers were cleaned with 70% ethanol before and after all behavioral procedures.
Behavioral procedures
Contextual fear conditioning
Mice were placed into one of the four conditioning chambers. Baseline freezing was scored for 120 s. Freezing, defined as the absence of movement besides respiration (Blanchard & Blanchard, 1969), was sampled 1 s every 10 s as a measure of learning as described previously (Davis, James, Siegel, & Gould, 2005). The baseline period was followed by two CS (30 s 85 dB white noise) – US (2 s 0.57 mA foot-shock) pairings separated by 120 s inter-trial interval. Immediate freezing was scored between the first and second CS-US pairing. After the last CS-US pairing, mice remained in the chambers for an additional 30 s before being returned to their home cages. Twenty-four hours later, mice were placed back into the original training context without the CS and freezing to the context was scored for 5 mins. One hour after contextual testing, mice were placed in the altered context for 6 mins. Freezing was scored for 3 mins in the absence of the auditory CS (pre-CS period), followed by scoring for 3 mins in the presence of the auditory CS. The experimenter was blind to all experimental conditions.
Open-field
In order to assess whether acute donepezil administered prior to training and testing affected basal levels of activity, locomotion was assessed in a square open-field (59.69 x 49.53 cm) in three sessions over three consecutive days. The open-field procedure was modeled after Czerniawski and colleagues (2012). On day 1, mice were placed in the center of the open chamber and allowed to explore freely for 10 mins. On days 2 and 3, mice received s.c. injections of either saline or donepezil (0.05 or 0.15 mg/kg) 30 mins prior to being placed in the center of the open-field. Each session was tracked using Panlab Smart software to determine the total distance traveled compared to the distance traveled in three separate zones (i.e. the wall, corners, and center of the open-field). Anxiety-like behavior was assessed by calculating the thigmotactic ratio (i.e. the distance traveled in the center divided by the total distance) (Lipkind et al., 2004; McIlwain, Merriweather, Yuva-Paylor, & Paylor, 2001). Experimenters were blind to all experimental conditions and mice were tested between 10:00 a.m. and 2:00 p.m. during the same hour on each day.
Statistical analyses
Data were analyzed using one-way ANOVA’s, followed by Dunnett’s Multiple Comparison tests. Statistical significance was set at the p < 0.05 level.
Results
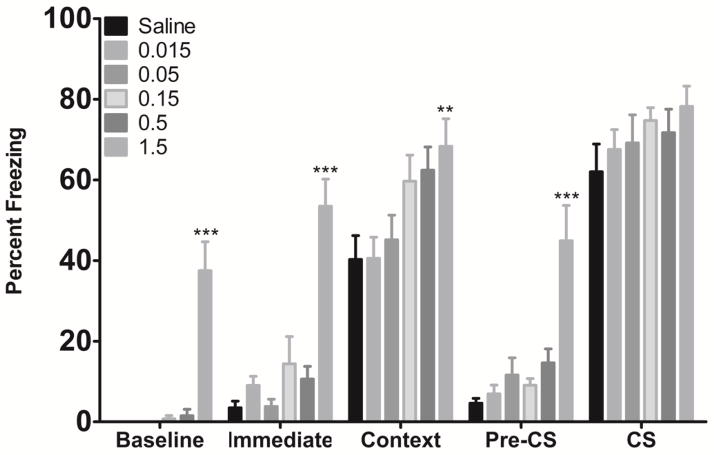
To determine if acute donepezil (0.015, 0.05, 0.15, 0.5, or 1.5 mg/kg) had an effect on hippocampus-dependent (contextual) or hippocampus-independent (cued) learning (Phillips & LeDoux, 1992), mice were trained and tested in contextual and cued fear conditioning after acute injections of donepezil (Figure 1). A significant effect of treatment on baseline [F(5, 68) = 24.24, p < 0.0001], immediate [F(5, 68) = 19.85, p < 0.0001], context [F(5, 68) = 4.13, p < 0.01], and pre-CS freezing [F(5, 68) = 11.53, p < 0.0001] was found. Freezing to the cue was not affected by donepezil treatment (all p’s > 0.05). Post-hoc tests revealed that mice administered 1.5 mg/kg donepezil froze significantly more than the saline group during baseline, immediate, context, and pre-CS periods (all p’s < 0.05). The high levels of freezing after acute exposure to 1.5 mg/kg donepezil indicated the drug may have non-specific effects on locomotion and/or anxiety.
Figure 1.
The effects of acute donepezil on fear conditioning (n=11–12 for each dose group). Doses of donepezil between 0.015 and 0.5 mg/kg had no effect on fear conditioning. The highest dose of donepezil tested (1.5 mg/kg) increased baseline, immediate, context, and pre-CS freezing. *Significantly different from Saline. Error bars represent ±SEM.
To rule out non-specific effects of donepezil on locomotor activity and/or anxiety, a three day open-field assay was run where two sub-threshold doses of donepezil (0.05 or 0.15 mg/kg) or saline were administered 30 mins prior to testing on days 2 and 3. Total distance traveled and thigmotaxis were analyzed. Results indicated that the doses of donepezil tested did not alter locomotor activity or anxiety as measured by thigmotaxis (all p’s > 0.05, data not shown).
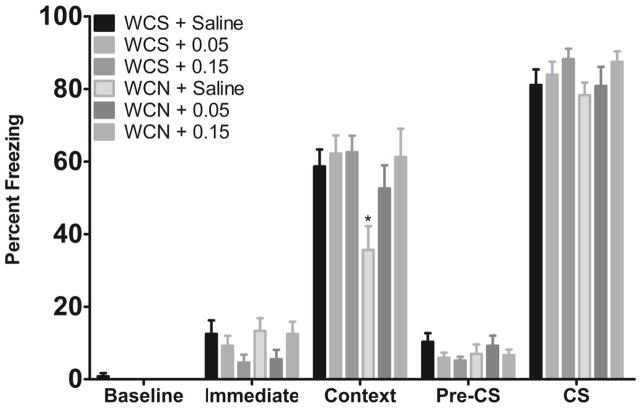
The effects of acute donepezil (0.05 or 0.15 mg/kg) on nicotine withdrawal-induced deficits in contextual conditioning were assessed in mice undergoing withdrawal from chronic saline (WCS) or withdrawal from chronic nicotine (WCN) (Figure 2). A significant effect of treatment on contextual freezing [F(5, 54) = 3.32, p < 0.05] was found. Post-hoc tests revealed that mice withdrawn from chronic nicotine and treated with saline prior to training and testing (WCN + Saline) showed deficits in contextual learning (p < 0.05). However, mice withdrawn from chronic nicotine and treated with acute donepezil prior to training and testing (WCN + 0.05 mg/kg and WCN + 0.15 mg/kg) showed contextual learning that was not significantly different from saline controls (p > 0.05). No significant changes were seen in any other fear conditioning measure (all p’s > 0.05). Data suggest that donepezil reverses deficits in contextual conditioning produced by nicotine withdrawal.
Figure 2.
The effects of acute donepezil on nicotine withdrawal-induced deficits in contextual conditioning (n=8–10 for each dose group). Donepezil reversed deficits in contextual learning induced by withdrawal from chronic nicotine. WCS = withdrawal from chronic saline. WCN = withdrawal from chronic nicotine. *Significantly different from WCS + Saline. Error bars represent ±SEM.
Discussion
The purpose of the present study was to investigate the potential efficacy of the acetylcholinesterase (AChE) inhibitor donepezil in ameliorating cognitive deficits induced by withdrawal from chronic nicotine. To this end, contextual fear conditioning was used to examine the effects of donepezil on hippocampus-dependent (contextual) and hippocampus-independent (cued) learning (Phillips & LeDoux, 1992) in male C57BL/6J mice withdrawn from chronic nicotine. First, a dose response was conducted to determine the effects of donepezil on fear conditioning; donepezil dose-dependently increased conditioned freezing. Next, two doses of donepezil that did not enhance conditioned freezing or alter locomotion/anxiety-like behavior in the open-field were tested for their effects on contextual learning during withdrawal from 12 days of chronic nicotine or saline. We found that mice withdrawn from chronic nicotine and treated with saline displayed significant deficits in contextual learning. However, mice withdrawn from chronic nicotine and treated with donepezil showed contextual learning that was not significantly different from control mice. Thus, donepezil effectively reversed nicotine withdrawal-induced deficits in contextual learning.
Changes in the cholinergic system are associated with nicotine withdrawal-induced contextual learning deficits (Gould et al., 2012). Specifically, nAChR upregulation in the hippocampus parallels the duration of withdrawal-induced deficits in contextual learning (Gould et al., 2012). Thus, the present finding that donepezil reverses deficits in contextual learning may suggest that donepezil acts to ameliorate dysfunctional cholinergic signaling during withdrawal. Importantly, it has been proposed that attempts to alleviate these withdrawal associated cognitive deficits during quit attempts may precipitate relapse (Gould, 2006). Consistent with this view, poorer cognitive performance during abstinence predicts smoking resumption (Patterson et al., 2010). Therefore, pharmacotherapies such as donepezil, that modulate the cholinergic system and ameliorate nicotine withdrawal-induced cognitive deficits, may prolong abstinence from nicotine and decrease the risk of relapse.
Our lab has previously demonstrated that withdrawal from chronic nicotine produces deficits in contextual learning (André et al., 2008; Davis, James, Siegel, & Gould, 2005; Gould et al., 2012; Portugal & Gould, 2009; Wilkinson & Gould, 2013; Wilkinson, Turner, Blendy, & Gould, 2013). In addition, we have found that galantamine (an AChE inhibitor and positive allosteric modulator of α4β2 nAChRs) can reverse deficits in contextual learning produced by withdrawal from chronic nicotine (Wilkinson & Gould, 2011). Together, these results suggest that withdrawal from chronic nicotine impairs hippocampus-dependent learning, and that AChE inhibition may have beneficial effects on hippocampus-dependent learning during withdrawal. However, until now it was unknown if withdrawal-induced contextual learning deficits could be ameliorated by inhibiting AChE alone – without concurrent direct modulation of nAChR activity.
The mechanisms underlying the amelioration of nicotine withdrawal-induced deficits by donepezil have yet to be determined; however, it is known that donepezil increases ACh levels in the hippocampus (Hatip-Al-Khatib, Takashi, Egashira, Iwasaki, & Fujiwara, 2004; Ihalainen et al., 2011). Therefore, the pro-cognitive effects of donepezil during nicotine withdrawal may be the result of increased acetylcholine levels in the hippocampus. However, future work will be necessary to determine the exact mechanism by which donepezil ameliorates nicotine withdrawal-induced deficits.
Although the mechanisms underlying the effects of donepezil on nicotine withdrawal-induced deficits in contextual conditioning are not known, we believe donepezil reversed nicotine withdrawal-induced deficits in acquisition rather than deficits in retrieval. In support, previous work demonstrated that nicotine withdrawal disrupts new contextual learning and not recall (Portugal & Gould, 2009). Thus, while not directly tested, our findings suggest that donepezil may be reversing acquisition deficits.
To date no clinical trials have examined the effects of donepezil on nicotine withdrawal-induced cognitive deficits. However, donepezil has been approved by the FDA to treat mild to moderate dementia related to Alzheimer’s disease (Herrmann, Chau, Kircanski, & Lanctôt, 2011), and is a safe and highly tolerable drug (Lanctôt et al., 2003). In addition, donepezil improves working memory in non-treatment seeking smokers (Ashare, Ray, Lerman, & Strasser, 2012) and reduces nicotine self-administrating and reinstatement to nicotine seeking in rats (Kimmey, Rupprecht, Hayes, & Schmidt, 2012). Therefore, donepezil may have multiple beneficial effects related to treating nicotine addiction that are only beginning to be recognized.
The results of the present study are promising as pharmacotherapies that reverse nicotine withdrawal-associated deficits in contextual learning such as varenicline (Raybuck, Portugal, Lerman, & Gould, 2009) and bupropion (Portugal & Gould, 2007) have been successful as treatments for nicotine addiction (Turner, Gold, Schnoll, & Blendy, 2013). However, many treatment seeking smokers are not able to quit using these drugs (Nides, 2008), or they experience adverse effects (Cahill, Stead, & Lancaster, 2012). Interestingly, both varenicline and bupropion act directly at nAChRs while donepezil does not. Therefore, donepezil might be efficacious in individuals who do not benefit from drugs that act directly on nAChRs. Overall, there is a need for new treatment options for smoking cessation, and donepezil may be such an option.
Acknowledgments
We would like to thank David Braak for his assistance with data analysis. This work was supported by grants from the National Institute on Drug Abuse (NIDA, DA017949 and CA143187).
Footnotes
Disclosure
All authors report no potential conflicts of interest.
References
- André JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behavioural Brain Research. 2008;190(2):174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014:581–91. doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Ray R, Lerman C, Strasser AA. Cognitive effects of the acetylcholinesterase inhibitor, donepezil, in healthy, non-treatment seeking smokers: a pilot feasibility study. Drug and Alcohol Dependence. 2012;126(1–2):263–7. doi: 10.1016/j.drugalcdep.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine & Tobacco Research. 1999;1(1):45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Besson M, Granon S, Mameli-Engvall M, Cloez-Tayarani I, Maubourguet N, Cormier A, Cazala P, David V, Changeux JP, Faure P. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proceedings of the National Academy of Sciences of the United States of America. 104(19):8155–8160. doi: 10.1073/pnas.0702698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J, Smith A. Effects of smoking and smoking deprivation on the articulatory loop of working memory. Human Psychopharmacology Clinical and Experimental. 1997;12:259–264. doi: 10.1002/(SICI)1099-1077(199705/06)12:3<259::AID-HUP866>3.0.CO;2-F. [DOI] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. Journal of Comparative and Physiological Psychology. 1969;68(1):129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(4):1–119. doi: 10.1002/14651858.CD006103.pub6. [DOI] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O’Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK. β2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives of General Psychiatry. 2009;66(6):666–76. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H. Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology. 2006;30(12):2135–2143. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Otto T. Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: differential effects of regionally selective NMDA receptor antagonism on acquisition and expression. Hippocampus. 2012;22(7):1528–39. doi: 10.1002/hipo.20992. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology. 2007;32(9):2011–19. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of Neuroscience. 2005;25(38):8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Sankaranarayanan S, Tanghe A, Terwel D, Lin AX, Hoque N, Bourin C, Gu H, Ahlijanian M, Bristow L. Effects of sub-chronic donepezil on brain Abeta and cognition in a mouse model of Alzheimer’s disease. Psychopharmacology. 2013;230(2):279–89. doi: 10.1007/s00213-013-3152-3. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Research. 2005;1033(2):186–93. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning. Molecular Neurobiology. 2006;34(2):93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62(5–6):2118–25. doi: 10.1016/j.neuropharm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatip-Al-Khatib I, Takashi A, Egashira N, Iwasaki K, Fujiwara M. Comparison of the effect of TAK-147 (zanapezil) and E-2020 (donepezil) on extracellular acetylcholine level and blood flow in the ventral hippocampus of freely moving rats. Brain Research. 2004;1012(1–2):169–76. doi: 10.1016/j.brainres.2004.03.067. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Chau SA, Kircanski I, Lanctôt KL. Current and emerging drug treatment options for Alzheimer’s disease. Drugs. 2011;71(15):2031–65. doi: 10.2165/11595870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine & Tobacco Research. 2007;9(3):315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keenan RM. Effect of tobacco withdrawal on sustained attention. Addictive Behaviors. 1989;14:577–580. doi: 10.1016/0306-4603(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Ihalainen J, Sarajärvi T, Rasmusson D, Kemppainen S, Keski-Rahkonen P, Lehtonen M, Banerjee PK, Semba K, Tanila H. Effects of memantine and donepezil on cortical and hippocampal acetylcholine levels and object recognition memory in rats. Neuropharmacology. 2011;61(5–6):891–9. doi: 10.1016/j.neuropharm.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology. 2011;217(3):353–65. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmey BA, Rupprecht LE, Hayes MR, Schmidt HD. Donepezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats. Addiction Biology. 2012 doi: 10.1111/adb.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. Canadian Medical Association. 2003;169(6):557–64. [PMC free article] [PubMed] [Google Scholar]
- Lipkind D, Sakov A, Kafkafi N, Elmer GI, Benjamini Y, Golani I. New replicable anxiety-related measures of wall vs. center behavior of mice in the open field. Journal of Applied Physiology. 2004;97(1):347–59. doi: 10.1152/japplphysiol.00148.2004. [DOI] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. Journal of Nuclear Medicine. 2007;48(11):1829–35. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Philosophical Transactions of the Royal Society of London. 2008;363(1507):3159–68. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance and nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1983;226(3):817–25. [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: Effects of training history. Physiology & Behavior. 2001;73(5):705–17. doi: 10.1016/S0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Nides M. Update on pharmacologic options for smoking cessation treatment. The American Journal of Medicine. 2008;121(4 Suppl 1):S20–31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106(1):1–8. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in Neurobiology. 2008;84(4):329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacology Biochemistry and Behavior. 2007;88(2):179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Nicotine withdrawal disrupts new contextual learning. Pharmacology, Biochemistry, and Behavior. 2009;92(1):117–23. doi: 10.1016/j.pbb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice--a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. The European Journal of Neuroscience. 2009;29(2):377–87. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behavioral Neuroscience. 2009;122(5):1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulse symptoms predict relapse among smokers in nicotine replacement therapy. Journal of Substance Abuse. 2005;28(4):297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In Vivo Regulation of [ 3H ] Acetylcholine Recognition Sites in Brain by Nicotinic Cholinergic Drugs. Journal of Neurochemistry. 1985;45(2):427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x/full. [DOI] [PubMed] [Google Scholar]
- Shigeta M, Homma A. Donepezil for Alzheimer’s disease: Pharmacodynamic, pharmacokinetic, and clinical profiles. CNS Drug Reviews. 2001;7(4):353–68. doi: 10.1111/j.1527-3458.2001.tb00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. The Journal of Neuroscience. 2006;26(34):8707–14. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tota S, Goel R, Pachauri SD, Rajasekar N, Najmi AK, Hanif K, Nath C. Effect of angiotensin II on spatial memory, cerebral blood flow, cholinergic neurotransmission, and brain derived neurotrophic factor in rats. Psychopharmacology. 2012;226(2):357–69. doi: 10.1007/s00213-012-2913-8. [DOI] [PubMed] [Google Scholar]
- Turner JR, Gold A, Schnoll R, Blendy JA. Translational research in nicotine dependence. Cold Spring Harbor perspectives in Medicine. 2013;3(3):a012153. doi: 10.1101/cshperspect.a012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. A report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Disease Prevention and Health Promotion, Office on Smoking and Health; Washington, DC: 1988. The health consequences of smoking: Nicotine addiction. [Google Scholar]
- USDHHS. The health consequences of smoking - 50 years of progress: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; Atlanta, GA: 2014. [Google Scholar]
- Wilkinson DS, Gould TJ. The effects of galantamine on nicotine withdrawal-induced deficits in contextual fear conditioning in C57BL/6 mice. Behavioural Brain Research. 2011;223(1):53–7. doi: 10.1016/j.bbr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Gould TJ. Withdrawal from chronic nicotine and subsequent sensitivity to nicotine challenge on contextual learning. Behavioural Brain Research. 2013;250:58–61. doi: 10.1016/j.bbr.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology. 2013;225(1):201–8. doi: 10.1007/s00213-012-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Bychkov E, Tsakem EL, Siedlecki C, Blakely RD, Gurevich EV. Cognitive effects of dopamine depletion in the context of diminished acetylcholine signaling capacity in mice. Disease Models & Mechanisms. 2013;6(1):171–83. doi: 10.1242/dmm.010363. [DOI] [PMC free article] [PubMed] [Google Scholar]