Abstract
Recent evidence indicates that emotion enhances contrast thresholds in subsequent visual perception (Phelps, Ling, & Carrasco, 2006), and perceptual sensitivity for low-spatial-frequency but not high-spatial-frequency targets (Bocanegra & Zeelenberg, 2009b). However, these studies just report responses to various frequencies at a fixed contrast level or responses to various contrasts at a fixed frequency. In the current study, we measured the full contrast sensitivity function as a function of emotional arousal in order to investigate potential interactions between spatial frequency and contrast. We used a Bayesian adaptive inference with a trial-to-trial information gain strategy (Lesmes, Lu, Baek, & Albright, 2010) and a fear-conditioned stimulus to manipulate arousal level. The spatial frequency at which people showed peak contrast sensitivity shifted to lower spatial frequencies in the arousing condition compared with the non-arousing condition and people had greater contrast sensitivity function bandwidth in the arousing than in the non-arousing condition.
Introduction
Several pioneering psychophysical studies reveal that emotion alters early visual perception. For instance, presenting an arousing cue (e.g., a fearful face) can improve perception of subsequent neutral gratings (e.g., Gabor patches) (Phelps et al., 2006; see also A. J. Woods, Philbeck, & Wirtz, 2013). In these studies, increasing arousal lowered the contrast threshold, at least at the particular spatial frequency tested. Furthermore, emotionally arousing stimuli can increase activation in early visual cortex regions such as V1 (Padmala & Pessoa, 2008).
However, a recent study suggests that arousal does not globally enhance perception, but instead can either enhance or impair contrast sensitivity depending on the spatial frequency of the stimuli (Bocanegra & Zeelenberg, 2009b). In this study, spatial frequency was determined by the number of sinusoidal luminance cycles per degree (cpd) of visual angle in the Gabor patch; Figure 1A). Exposure to fearful faces decreased participants’ detection sensitivity of subsequent high-spatial-frequency gratings (e.g., 6 cpd) while increasing sensitivity of subsequent low-spatial-frequency gratings (e.g., 1.5 cpd). Similarly, in another study, exposure to fearful faces impaired subsequent judgments about high spatial frequency characteristics of words but enhanced judgments about low spatial frequency characteristics of words (Borst & Kosslyn, 2010). Emotions also seem to be more likely to be elicited by low-spatial-frequency stimuli than by high-spatial-frequency stimuli, although these emotion elicitation results are not entirely consistent across studies (for a review see De Cesarei & Codispoti, 2013).
Figure 1.
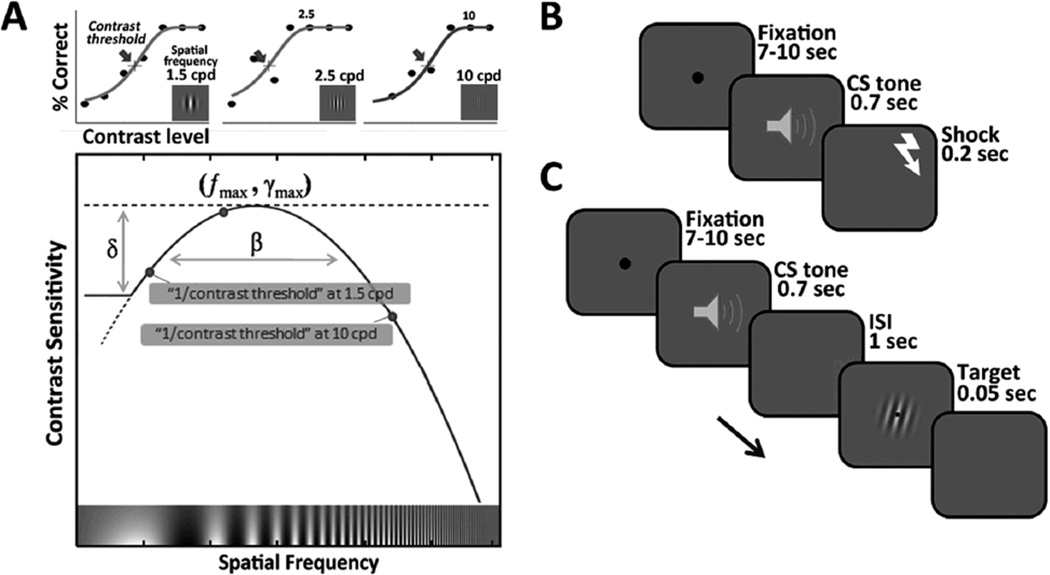
(A) Schematic representation of the contrast sensitivity function, which was constructed using the inverse of measured contrast thresholds, operationally defined as the point of inflection in the psychometric function, at each spatial frequency. The contrast sensitivity function has a maximum value at intermediate spatial frequencies, and decreases with both lower and higher spatial frequencies. In the quick contrast sensitivity function method, the spatial contrast-sensitivity-function parameters (the peak gain, γmax; the peak spatial frequency fmax; the bandwidth β, which describes the function's full width at half maximum; and the low-frequency truncation level δ) are directly estimated via Bayesian adaptive inference rather than measuring multiple contrast thresholds at each spatial frequency based on psychometric functions. The dashed line indicates predicted contrast sensitivities before truncation (B) Conditioning phase trial sequence. (C) Orientation identification task trial sequence. Note that stimuli are not drawn to scale here.
Researchers have accounted for findings of different effects of arousal on low-versus high-spatial-frequency stimuli by arguing that the amygdala responds to emotional arousal by potentiating magnocellular-type channels in the visual system while suppressing parvocellular-type channels (Bocanegra & Zeelenberg, 2009b, 2011a; Borst & Kosslyn, 2010). This bias favoring magnocellular-type channels should preferentially enhance processing of low-spatial-frequency information because magnocellular cells within the lateral geniculate nucleus of the thalamus have a lower spatial resolution than parvocellular cells (Sincich & Horton, 2005).
Although the evidence to date indicates that emotion has a strong influence in early visual perception, the results from the previous studies described above each gave information about the influence of only one dimension of early visual perception as a function of emotional arousal. Phelps et al. (2006) investigated the contrast psychometric function, i.e., performance as a function of stimulus contrast, at a fixed spatial frequency, whereas Bocanegra and Zeelenberg (2009b) investigated the orientation discrimination sensitivity for various spatial frequencies at a fixed contrast level. Yet both spatial frequency and contrast are critical for visual processing and they interact. For example, visual perception at a particular contrast level depends on the spatial frequency of the target object (R.L. De Valois, Morgan, & Snodderly, 1974; Georgeson & Sullivan, 1975; Keller, Strasburger, Cerutti, & Sabel, 2000; Owsley, 2003; Pasternak & Merigan, 1981). Therefore, to globally assess the effects of emotion on perception, investigations should probe a range of both spatial frequencies and contrasts. Indeed, one recent neurophysiological study considered these two factors in how emotion influences perception (Song & Keil, 2013). In this study, the authors probed early visual modulation, as indexed by steady-state visual potential (ssVEPs), by presenting an emotionally arousing picture followed by a contrast-varying Gabor patch. The Gabor patches were presented in one of two different spatial frequencies (2 vs. 6 cpd). For low-spatial-frequency targets, viewing emotionally arousing pictures just beforehand increased the accuracy of judging the orientation of subsequent low spatial frequency targets and increased the ssVEP amplitude. In contrast, for high-spatial-frequency targets, preceding emotionally arousing pictures decreased detection accuracy and ssVEP amplitude.
The current study was designed to provide a more complete portrayal of the modulatory role of emotional arousal over both contrast and spatial frequency in the early visual system. To accomplish the current goal, we measured the contrast sensitivity function (Cornsweet, 1970) as a function of emotional arousal (Figure 1A). In visual psychophysics, threshold refers to the minimum physical strength of a stimulus that can be detected or discriminated at some target accuracy level, such as 82% correct. Sensitivity is defined as the reciprocal of threshold (1/threshold). The contrast sensitivity function (CSF) characterizes sensitivity as a function of the spatial frequency (the coarseness or fineness) of visual stimuli. The fundamental advantage of measuring the contrast sensitivity function is that it reflects various contrast sensitivity at different spatial frequency channels, each tuned to a preferred spatial scale, and consequently it provides information about the global tuning of the visual system in response to various combination of contrast and spatial frequency of visual environment (Billock & Harding, 1996; Blakemore & Campbell, 1969; F. W. Campbell & J. Robson, 1968; Russell L De Valois, Albrecht, & Thorell, 1982; Hubel & Wiesel, 1968; Mallat, 1989; Pantle & Sekuler, 1968; Robert Sekuler, Wilson, & Owsley, 1984). Indeed, the contrast sensitivity function has been extensively used as a model to investigate the early visual system with various visual tasks (F. W. Campbell & J. G. Robson, 1968; Chung, Legge, & Tjan, 2002; Enroth-Cugell & Robson, 1966; Kwon & Legge, 2011; Legge & Foley, 1980; Movshon, Thompson, & Tolhurst, 1978; Watson & Ahumada, 2005; Watson & Solomon, 1997).
The conventional data collection method, the method of constant stimuli, usually requires several hundred trials to estimate a threshold at a single spatial frequency. To obtain a contrast sensitivity function, researchers usually measure contrast thresholds at several different spatial frequencies. The burden of data collection is multiplied by the number of spatial frequencies tested. Even with the most advanced adaptive procedures for estimating a single contrast threshold (e.g. QUEST or Psi method), measurement of a contrast sensitivity function typically takes 500–1000 trials. During such long sessions, visual performance is likely to change and emotional responses are likely to habituate. Thus, measuring the full contrast sensitivity function using the conventional method faces obstacles in terms of testing time and data precision. To overcome the disadvantage of the conventional method, we adopted the quick CSF (qCSF) method (Lesmes et al., 2010), a Bayesian adaptive procedure for efficient estimation of contrast sensitivity functions. Instead of measuring contrast thresholds at individual spatial frequencies, the qCSF directly estimates the parameters of the contrast sensitivity function and computes contrast sensitivities over the full spatial-frequency range based on the parameters values. By directly estimating the model parameters, the qCSF method improves contrast sensitivity function estimation in both testing time and precision with a relatively small number of trials (e.g., less than 100 trials; Hou et al., 2010).
Another important aspect of the current study is that we excluded confounds found in previous studies evaluating emotion’s role in visual perception. Although one role of emotion is multiplicative amplification of transient covert attention’s influence on the contrast threshold (Phelps et al., 2006) or spatio-temporal resolution (Bocanegra & Zeelenberg, 2011b), other evidence implies that emotional arousal can also influence perception irrespective of attention (Bocanegra, Huijding, & Zeelenberg, 2012; Bocanegra & Zeelenberg, 2009a, 2009b; Phelps et al., 2006; Zeelenberg & Bocanegra, 2010). In the current study, we avoided confounding attentional effects induced by transient covert attention by presenting just one target Gabor on the center of the screen. By using a fixed location for target presentation, we also excluded the effect of spatial uncertainty for the possible peripheral target’s location; uncertainty influences overall visual perception as it changes visual system resource allocation (Cameron, Tai, & Carrasco, 2002; Carrasco, Penpeci-Talgar, & Eckstein, 2000; Foley & Schwarz, 1998; Pelli, 1985; Solomon, Lavie, & Morgan, 1997). Although there are several accounts of how attention benefits early visual perception, such as contrast gain (e.g., Sclar, Lennie, & DePriest, 1989) or response gain (e.g., McAdams & Maunsell, 1999), the key point is that transient covert attention, induced by the sudden appearance of a target stimulus at a peripheral location, enhances visual perception. We wanted to avoid having this as a potential mediator of our emotion effects (for more reviews, see, Carrasco, 2011; Carrasco, Ling, & Read, 2004; Carrasco et al., 2000).
We also avoided adaptation effects potentially induced by using the same sensory modality to evoke emotional arousal and to measure perception (e.g., inducing arousal via a visual cue and testing perception via a visual target). Arousing and non-arousing visual stimuli can differ in low-level visual features such as the energy distribution of frequencies (e.g., Delplanque, N’diaye, Scherer, & Grandjean, 2007); this is important as spatial frequency adaptations can change subsequent perception and global tuning of the visual system (see K. K. De Valois, 1977; Maffei, Fiorentini, & Bisti, 1973; Wilson & Humanski, 1993). To eliminate these concerns, we used fear-conditioned auditory tones as the arousing and non-arousing cues. Classical fear conditioning is a process in which affective significance is endowed to a previously neutral stimulus (conditioned stimulus; CS) by pairing it with an aversive stimulus such as an electric shock (unconditioned stimulus; US). After repeated pairings with US, the CS becomes capable of increasing emotional arousal (CS+) and triggers a series of autonomic response such as increased skin conductance response (conditioned response; CR). A previous study showed that fear conditioned auditory cues modulated activity in early visual cortex regions such as V1 (see the conditioning 2 section of Padmala & Pessoa, 2008), thus arousal induced by auditory stimuli can modulate basic visual processes. In the current study, we used two different tones (500 vs. 1500Hz) as the CS’s. The pitch of the CS+ tone was counterbalanced across observers; for example, some observers received the electric shock after hearing the high-pitched tone, and some after hearing the low-pitched tone (see method for more details). By adopting classical fear conditioning, we avoided all possible confounding sources in terms of low-level visual features of experimental stimuli.
Materials and Methods
Observers
Twenty-eight observers (16 male)1 with corrected-to-normal vision volunteered for this study and gave informed consent. Observers were naïve to the purpose of the experiment. During the experiment, observers performed a fear-conditioning session followed by a perceptual detection session using the conditioned tones as modulators of arousal in each trial.
Stimuli and Apparatus
Target displays contained single Gabor patches (5.2° × 5.2° of visual angle; 1.9° sinusoidal gratings enveloped by a Gaussian). For the current study, the 16 possible grating spatial frequencies were spaced log linearly from 0.25 to 36 cpd; the 60 possible grating contrasts were spaced log linearly from 0.1% to 100%. All the visual stimuli were generated in real time using the quick-contrast-sensitivity-function (qCSF) toolbox (http://lobes.osu.edu/qMethods.php) and the PsychToolbox extensions (Brainard, 1997; Pelli, 1997) based on Matlab 2010b (The MathWorks Corp. Natrick, MA). The visual stimuli were presented at 85 Hz on a 19-in. CRT monitor (NEC AccuSync 90; NEC Corp. Tokyo, Japan) with a resolution of 1280 × 960 pixels. Using a display attenuator that combines two 8-bit output channels of the graphics card, the display system produced 14-bit gray-level resolution (Li, Lu, Xu, Jin, & Zhou, 2003), and then was gamma-corrected. Observers viewed the stimuli in a soundproof and dimly lit room at a viewing distance of 2.5 m with their head position stabilized by a chin rest. The mild electric shock used as an unconditioned stimulus (US) was delivered to the third and fourth fingers of the left hand via a shock stimulator for humans (E13–22; Coulbourn Instruments, Allentown, PA). Two tones (500 Hz and 1500 Hz) were adopted as conditioned stimuli (i.e., CSs) to avoid possible confounding effects of using stimuli in the same sensory modality to induce emotional arousal and to measure perceptual processing. To confirm the success of the emotional arousal manipulation, skin conductance responses as a function of CSs (i.e., fear conditioned-arousing tone vs. non-arousing tone) were measured using a BIOPAC MP-150 system (250 Hz sampling rate; BIOPAC Systems, Goleta, CA) during the experiment.
Procedure
An initial fear-conditioning session established the emotionally arousing nature of the CS+ tone. During the session, one of the tones was paired with electric shock (CS+) while the other tone was not paired with shock (CS−). Fourteen observers were conditioned with the high-pitched tone and 13 with the low-pitched tone as the CS+. Each trial began with a fixation cross jittered to appear for 7 to 10 s. Then one of the CS tones played for 0.7 s. If the tone was designated as the CS+, a shock was delivered right after the offset of the CS+ tone, and terminated after 0.2 s (Figure 1B). On each trial, observers were asked to press a button to indicate whether the tone pitch was low or high. A total of 30 trials were presented in a random order: 10 CS+ with shock; 10 CS+ without shock; 10 CS− tones. Therefore, CS+ tones were followed by a shock with a 50% partial reinforcement schedule. Prior to the experiment, observers were informed which tone predicts the US, but they were not informed about the probability of US delivery. The intensity of "highly unpleasant but not painful" electric shock was determined individually for each observer before the conditioning session (mean intensity = 1.77 mA, range 1.1 – 2.3 mA). The trials that included shocks were excluded in subsequent analyses.
Following the conditioning session, the orientation identification task was administered. The trials began with a fixation point jittered to last between 7 to 10 s; then either the CS+ or CS− was played for 0.7 s to manipulate arousal level. Following a 1-s blank screen, one target Gabor grating was presented in the center of the screen for 0.05 s. Observers indicated the target orientation (counterclockwise or clockwise; ± 45°) via a button press during the response period (Figure 1C). A small black square (0.6°) was presented in the center of the target grating to reduce stimulus uncertainty, which could affect the contrast sensitivity function measurement, especially in the high-frequency region (R. L. Woods, 1996). We put a 1-s blank interval between the arousing cue and target onsets to avoid possible temporal competition in processing. Note that each trial’s stimulus type (i.e., contrast and spatial frequency) was determined via the qCSF algorithm for the two CS conditions separately based on the observer’s responses in previous trials (see section below for more details), and therefore each observer was tested with a different combination of target properties. To minimize extinction of conditioned responses, booster trials consisting of a shock after a CS+ tone without any target stimulus on that trial were randomly intermixed with the other trials. Booster trials were excluded from subsequent analyses. The task consisted of 100 CS+ and 100 CS− trials randomly ordered. In addition to these 200 trials, there were approximately 12% additional “booster” CS+ tone trials involving shock to prevent extinction.
qCSF method implementation
In the current study, we estimated individuals’ contrast sensitivity functions in both CS+ and CS− conditions using the qCSF method (Lesmes et al., 2010). Contrast sensitivity is defined as the reciprocal of contrast threshold (1/threshold) at 82% accuracy in the orientation discrimination task. For example, a threshold of 0.02 translates into a sensitivity of 50; and a sensitivity of 50 means that the observer’s threshold is 1/50=.02 (or 2% contrast). Using a well-known functional form of the contrast sensitivity function, the qCSF method applies Bayesian inference to directly estimate the parameters of the contrast sensitivity function by optimal placements of test stimuli based on trial-by-trial response from the observer. The functional form imposed by the qCSF method is a truncated log-parabola, which is specified by four parameters: (1) the peak gain (optimal maximum contrast sensitivity) γmax; (2) the spatial frequency of peak sensitivity fmax; (3) the bandwidth β, which describes the function's full width at half maximum (in octaves); and (4) the low-frequency truncation level δ (Figure 1A). With this functional form, the observer’s response on each trial is used to update the posterior distributions of the parameters (γmax, fmax, β, δ). The contrast sensitivity (or threshold) over the full range of spatial-frequencies is computed based on the estimated parameter values in the end of the procedure. Simultaneously, the trial outcomes (i.e., the updated contrast sensitivity function) are used to select the particular grating frequency and contrast level that maximizes the expected information gain for the next trial. In sum, the goal of the qCSF is to efficiently search the stimulus space to gain information in the parameter space.
Results
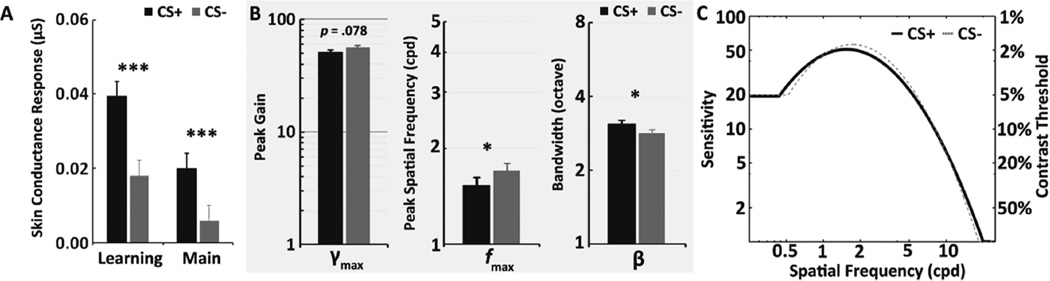
On each trial, skin conductance responses were calculated by subtracting a baseline (average signal between 0 and 1 s) from the peak amplitude during the 1 – 7 s time window following the CS onset. CS+ cues yielded stronger skin conductance responses than CS− cues during both the initial conditioning session, t(26) = 5.67, p < .001, and the orientation identification task, t(26) = 3.83, p < .001, confirming the success of the arousal manipulation via fear conditioning in the current study (Figure 2A).
Figure 2.
Skin conductance responses during the study (B) Estimated parameters; peak gain (γmax), peak spatial frequency (fmax), and bandwidth (β) (C) Contrast sensitivity function as a function of spatial frequency for CS+ and CS− conditions. Error denotes the standard within-subject error term (Loftus & Masson, 1994); ***p <.001; ** p < .01; * p < .05.
The qCSF method estimated parameters for each CS (i.e., CS+ and CS−) were compared. Early visual perception differed significantly depending on whether the CS was arousing or non-arousing (Figures 2B & C). A maximum contrast sensitivity (γmax) of 51.00 was found for CS+ trials at a spatial frequency (fmax) of 1.53 cpd. In contrast, a γmax of 56.56 was found at fmax of 1.70 cpd for CS− trials. That is, the average contrast to reach 82% accuracy was 1.96% at 1.53 cpd in the CS+ condition, but 1.77% at 1.70 cpd in the CS− condition. Paired t-tests revealed that there was a significant difference in fmax, t(26) = −2.26, p = .03, and a marginal difference in γmax, t(26) = −1.83, p = .079. Also, the bandwidth (β) of the contrast sensitivity function was wider for CS+ trials (3.11 octave) than for CS− trials (2.84 octave), t(26) = 2.79, p =.01. However, there was no significant difference in truncation, δ, of the contrast sensitivity function (p = .44). The area under the curve (AUC) for the contrast sensitivity function curve constructed by estimating these four parameters did not differ by CS (p = .82).
Discussion
To provide a more comprehensive understanding of how emotional arousal influences early visual perception, the current study used conditioned stimuli to manipulate arousal on a trial-by-trial basis while quantifying observers’ contrast sensitivity function, a fundamental measure of early visual system. To our knowledge, this is the first study to measure how arousal influences the contrast sensitivity function. Observers’ arousal levels were manipulated by a fear conditioning procedure that yielded greater skin conductance responses for CS+ cues than for CS− cues in both phases of the experiment. Playing a tone previously associated with shock influenced subsequent visual perception, as reflected in significant changes in the contrast sensitivity function as a function of CS (Figure 2B). First, there was an overall shift of the center of the contrast sensitivity function (fmax) toward lower spatial frequencies during CS+ trials relative to CS− trials (1.53 vs. 1.70 cpd), indicating that arousal led to greater contrast sensitivity for low spatial frequencies. Second, the bandwidth (β) of the contrast sensitivity function—the range of frequencies over which the observer can detect a given level of contrast—increased during CS+ trials compared to CS− trials (3.11 vs. 2.84 octave). In addition, we found a marginally significant arousal-induced contrast sensitivity loss at the peak spatial frequency (γmax;p = .078).
The parametric changes revealed in our study indicates that increasing emotional arousal leads the visual system to more effectively detect coarse visual input information (e.g., scene gist or the overall shape of an object), while losing fine details. In addition, our results help integrate previous psychophysical studies. As can be seen in our contrast sensitivity function (Figure 2C), emotional arousal increases contrast sensitivity at relatively low spatial frequency ranges but reduces it at high ranges. Thus, the current findings offers further support not only for Phelps et al.'s (2006) study that showed emotion-induced enhancement in contrast perception with (fixed) low spatial frequency target (2 cpd) across the experiment, but also for Bocanegra and Zeelenberg's (2009b) study that found emotion enhanced sensitivity for low spatial frequency stimuli but impaired it for high spatial frequency stimuli (see also, Borst & Kosslyn, 2010; Song & Keil, 2013).
The contrast sensitivity function shift during the emotional trials of our study indicates that momentary fluctuations in arousal stimulate dynamic adaptation of neuronal receptive fields (i.e., neuronal tuning) for visual system optimization (Billock & Harding, 1996; Blakemore & Campbell, 1969; F. W. Campbell & J. Robson, 1968; Russell L De Valois et al., 1982; Hubel & Wiesel, 1968; Mallat, 1989; Pantle & Sekuler, 1968; Robert Sekuler et al., 1984). Attentional manipulations can also lead to dynamic adaption of contrast sensitivity (Carrasco, 2011). However, attention leads to a different pattern of contrast sensitivity modulation than seen in the emotion condition of our study; exogenous covert attention enhances overall contrast sensitivity across spatial frequencies (Carrasco et al., 2004; Carrasco et al., 2000). In contrast, in our study, emotion shifted peak sensitivity at the cost of maximal sensitivity without changing the area under the curve. As noted in the Introduction and Methods sections, in the current study, covert attention to the periphery was unnecessary and the location of the targets was always the same. Thus, transient covert attention was not a critical factor in determining detection of the target in the current study, allowing us to evaluate the effect of emotional arousal without this confounding factor.
Another aspect of our results worth noting is that, across the two conditions, the contrast sensitivity function was shifted towards lower spatial frequency levels than expected under normal circumstances (e.g., Owsley, 2003; R. Sekuler, Hutman, & Owsley, 1980). While we found that the emotionally arousing condition induced significantly greater fmax shifting than the non-arousing condition did, the contrast sensitivity function in the non-arousing condition was also lower than the normal range of fmax (2 – 4 cpd). This might be due to the current design. In the main task, we randomly presented either CS+ or CS− tones prior to each target. Thus, as CS− trials were intermixed with CS+ trials, observers may have had a baseline level of arousal throughout the whole session that was higher than normal. However, the random intermixing of trials was useful in that it revealed that a brief increase in arousal induced by a CS+ tone can alter perception. Future research should compare sessions with fear-conditioned tones to sessions without any fear-conditioned tones to examine whether there are more global effects of arousal (e.g., Lee, Itti, & Mather, 2012) in addition to the transient trial-by-trial effects seen here.
Presenting a fear-conditioned stimulus activates the amygdala (e.g., Lim, Padmala, & Pessoa, 2009), and it has been argued that the amygdala has more of an influence over the processing of low spatial frequency information than high spatial frequency information due to the amygdala activating magnocellular pathways specialized for processing low spatial frequency information (Bocanegra & Zeelenberg, 2009b, 2011a). One interesting study (Kveraga, Boshyan, & Bar, 2007) presented participants with line drawings of neutral objects that were outlined either using light grey on a darker grey background (biased towards magnocellular processing) or outlined in red against an isoluminant green background (biased towards parvocellular processing). The magnocellular-biased stimuli had a lower contrast than the parvocellular-biased stimuli but were recognized faster and more accurately. Furthermore, in comparison with the parvoceullular-biased stimuli they activated a different pathway that included the amygdala.
These findings with neutral stimuli are consistent with findings that the amgydala responds more to faces with low than high spatial frequency (Vuilleumier, Armony, Driver, & Dolan, 2003) and with electrophysiological findings that low-spatial-frequency unpleasant pictures elicited more enhanced initial visual processing, as reflected by the P1 component, than low-spatial-frequency neutral pictures, but that there was no difference for unpleasant and neutral high-spatial-frequency pictures (Alorda, Serrano-Pedraza, Campos-Bueno, Sierra-Vázquez, & Montoya, 2007). Likewise, a network of frontoparietal regions that responded more to emotionally arousing distractors than neutral distractors showed differential responding only for distractors shown in low spatial frequencies, not high spatial frequencies (Carretié, Ríos, Periáñez, Kessel, & Álvarez-Linera, 2012). Thus, whereas previous studies comparing the effects of emotional versus neutral stimuli indicate that the amygdala is more responsive to emotion conveyed via low-spatial-frequency information than via high-spatial-frequency information, our results suggest that the influence can work in the opposite direction as well. Namely, when activated by an independent source of arousal, the amygdala may selectively enhance processing of subsequent low-spatial-frequency information.
In summary, the current results indicate that arousal shifts the contrast sensitivity function such that people find it harder to perceive high spatial frequency stimuli but do not find it harder to perceive low spatial frequency stimuli. The current study did not involve covert peripheral attention and the pattern of the contrast sensitivity function modulation was different from previous studies examining the effects of attention on contrast sensitivity. Thus, emotion effects in visual system appear to be distinct from attention effects. Future work should test whether the amygdala plays an essential role in these changes, or whether some other process, such as direct norepinephrine stimulation of visual cortex, can account for the rapid shifts in contrast sensitivity under arousal.
Acknowledgments
This work was supported by NIH grants RO1AG025340 and K02AG032309.
Footnotes
Full disclosure of interest: Zhong-lin Lu has an intellectual rights (US patent) for the quick-contrast-sensitivity-function (qCSF) toolbox (http://lobes.osu.edu/qMethods.php).
One observer had extremely high max gains in both the arousing and non-arousing conditions (982.60 and 282.31). These values were far greater than three standard deviations above the peak gain mean (56.69 and 59.90 for arousing and non-arousing conditions, respectively). We excluded this observer from the final analysis but note that all significant effects remained significant when he was included.
References
- Alorda C, Serrano-Pedraza I, Campos-Bueno JJ, Sierra-Vázquez V, Montoya P. Low spatial frequency filtering modulates early brain processing of affective complex pictures. Neuropsychologia. 2007;45(14):3223–3233. doi: 10.1016/j.neuropsychologia.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Billock VA, Harding TH. Evidence of spatial and temporal channels in the correlational structure of human spatiotemporal contrast sensitivity. The Journal of physiology. 1996;490(Pt 2):509–517. doi: 10.1113/jphysiol.1996.sp021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore Ct, Campbell F. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. The Journal of physiology. 1969;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra BR, Huijding J, Zeelenberg R. Beyond Attentional Bias: A Perceptual Bias in a Dot-Probe Task. Emotion. 2012 doi: 10.1037/a0028415. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Dissociating emotion-induced blindness and hypervision. Emotion. 2009a;9(6):865–873. doi: 10.1037/a0017749. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotion improves and impairs early vision. Psychol Sci. 2009b;20(6):707–713. doi: 10.1111/j.1467-9280.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotion-induced trade-offs in spatiotemporal vision. J Exp Psychol Gen. 2011a;140(2):272–282. doi: 10.1037/a0023188. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotional cues enhance the attentional effects on spatial and temporal resolution. Psychon Bull Rev. 2011b;18(6):1071–1076. doi: 10.3758/s13423-011-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst G, Kosslyn SM. Fear selectively modulates visual mental imagery and visual perception. The Quarterly Journal of Experimental Psychology. 2010;63(5):833–839. doi: 10.1080/17470211003602420. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Cameron EL, Tai JC, Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vision Research. 2002;42(8) doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Robson J. Application of Fourier analysis to the visibility of gratings. The Journal of physiology. 1968;197(3):551. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vision Res. 2011;51(13):1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7(3) doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein M. Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement. Vision Research. 2000;40(10–12):10–12. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Ríos M, Periáñez JA, Kessel D, Álvarez-Linera J. The role of low and high spatial frequencies in exogenous attention to biologically salient stimuli. PloS one. 2012;7(5):e37082. doi: 10.1371/journal.pone.0037082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Legge GE, Tjan BS. Spatial-frequency characteristics of letter identification in central and peripheral vision. Vision research. 2002;42(18):2137–2152. doi: 10.1016/s0042-6989(02)00092-5. [DOI] [PubMed] [Google Scholar]
- Cornsweet TN. Visual Perception. New York: Academic Press; 1970. [Google Scholar]
- De Cesarei A, Codispoti M. Spatial frequencies and emotional perception. Reviews in the Neurosciences. 2013;24(1):89–104. doi: 10.1515/revneuro-2012-0053. [DOI] [PubMed] [Google Scholar]
- De Valois KK. Spatial frequency adaptation can enhance contrast sensitivity. Vision Research. 1977;17(9):1057–1065. doi: 10.1016/0042-6989(77)90010-4. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Albrecht DG, Thorell LG. Spatial frequency selectivity of cells in macaque visual cortex. Vision research. 1982;22(5):545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Morgan H, Snodderly DM. Psychophysical studies of monkey vision. 3. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974;14(1):75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- Delplanque S, N’diaye K, Scherer K, Grandjean D. Spatial frequencies or emotional effects?: A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. Journal of neuroscience methods. 2007;165(1):144–150. doi: 10.1016/j.jneumeth.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JM, Schwarz W. Spatial attention: effect of position uncertainty and number of distractor patterns on the threshold-versus-contrast function for contrast discrimination. J. Opt. Soc. Am. A. 1998;15(5):1036–1047. [Google Scholar]
- Georgeson MA, Sullivan GD. Contrast constancy: deblurring in human vision by spatial frequency channels. J Physiol. 1975;252(3):627–656. doi: 10.1113/jphysiol.1975.sp011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Huang CB, Lesmes L, Feng LX, Tao L, Zhou YF, Lu ZL. qCSF in clinical application: efficient characterization and classification of contrast sensitivity functions in amblyopia. Invest Ophthalmol Vis Sci. 2010;51(10):5365–5377. doi: 10.1167/iovs.10-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. The Journal of physiology. 1968;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Strasburger H, Cerutti DT, Sabel BA. Assessing spatial vision - automated measurement of the contrast-sensitivity function in the hooded rat. J Neurosci Methods. 2000;97(2):103–110. doi: 10.1016/s0165-0270(00)00173-4. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M. Magnocellular projections as the trigger of top-down facilitation in recognition. The Journal of Neuroscience. 2007;27(48):13232–13240. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M, Legge GE. Spatial-frequency cutoff requirements for pattern recognition in central and peripheral vision. Vision research. 2011;51(18):1995–2007. doi: 10.1016/j.visres.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-H, Itti L, Mather M. Evidence for arousal-biased competition in perceptual learning. Frontiers in psychology. 2012;3 doi: 10.3389/fpsyg.2012.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Foley JM. Contrast masking in human vision. JOSA. 1980;70(12):1458–1471. doi: 10.1364/josa.70.001458. [DOI] [PubMed] [Google Scholar]
- Lesmes LA, Lu ZL, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. J Vis. 2010;10(3):11–21. doi: 10.1167/10.3.17. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lu ZL, Xu P, Jin J, Zhou Y. Generating high gray-level resolution monochrome displays with conventional computer graphics cards and color monitors. J Neurosci Methods. 2003;130(1):9–18. doi: 10.1016/s0165-0270(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proceedings of the National Academy of Sciences. 2009;106(39):16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1(4):476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A, Bisti S. Neural correlate of perceptual adaptation to gratings. Science. 1973;182(4116):1036–1038. doi: 10.1126/science.182.4116.1036. [DOI] [PubMed] [Google Scholar]
- Mallat SG. Multifrequency channel decompositions of images and wavelet models. Acoustics, Speech and Signal Processing, IEEE Transactions on. 1989;37(12):2091–2110. [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. The Journal of Neuroscience. 1999;19(1):431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Thompson ID, Tolhurst DJ. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C. Contrast sensitivity. Ophthalmol Clin North Am. 2003;16(2):171–177. doi: 10.1016/s0896-1549(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. The Journal of Neuroscience. 2008;28(24):6202–6210. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantle A, Sekuler R. Size-detecting mechanisms in human vision. Science. 1968;162(3858):1146–1148. doi: 10.1126/science.162.3858.1146-a. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Merigan WH. The luminance dependence of spatial vision in the cat. Vision Res. 1981;21(9):1333–1339. doi: 10.1016/0042-6989(81)90240-6. [DOI] [PubMed] [Google Scholar]
- Pelli DG. Uncertainty explains many aspects of visual contrast detection and discrimination. Journal of the Optical Society of America. 1985;2(9) doi: 10.1364/josaa.2.001508. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17(4):292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclar G, Lennie P, DePriest DD. Contrast adaptation in striate cortex of macaque. Vision research. 1989;29(7):747–755. doi: 10.1016/0042-6989(89)90087-4. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Hutman LP, Owsley CJ. Human aging and spatial vision. Science. 1980;209(4462):1255–1256. doi: 10.1126/science.7403884. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Wilson HR, Owsley C. Structural modeling of spatial vision. Vision research. 1984;24(7):689–700. doi: 10.1016/0042-6989(84)90210-4. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. The circuitry of V1 and V2: integration of color, form, and motion. Annu. Rev. Neurosci. 2005;28:303–326. doi: 10.1146/annurev.neuro.28.061604.135731. [DOI] [PubMed] [Google Scholar]
- Solomon JA, Lavie N, Morgan MJ. Contrast discrimination function: spatial cuing effects. J Opt Soc Am A Opt Image Sci Vis. 1997;14(9):2443–2448. doi: 10.1364/josaa.14.002443. [DOI] [PubMed] [Google Scholar]
- Song I, Keil A. Affective Engagement and Subsequent Visual Processing: Effects of Contrast and Spatial Frequency. 2013 doi: 10.1037/a0031553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci. 2003;6(6):624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Watson AB, Ahumada AJ., Jr A standard model for foveal detection of spatial contrast. J Vis. 2005;5(9):717–740. doi: 10.1167/5.9.6. [DOI] [PubMed] [Google Scholar]
- Watson AB, Solomon JA. Model of visual contrast gain control and pattern masking. JOSA A. 1997;14(9):2379–2391. doi: 10.1364/josaa.14.002379. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Humanski R. Spatial frequency adaptation and contrast gain control. Vision Res. 1993;33(8):1133–1149. doi: 10.1016/0042-6989(93)90248-u. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Philbeck JW, Wirtz P. Hyper-arousal decreases human visual thresholds. PloS one. 2013;8(4):e61415. doi: 10.1371/journal.pone.0061415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RL. Spatial frequency dependent observer bias in the measurement of contrast sensitivity. Ophthalmic Physiol Opt. 1996;16(6):513–519. [PubMed] [Google Scholar]
- Zeelenberg R, Bocanegra BR. Auditory emotional cues enhance visual perception. Cognition. 2010;115(1):202–206. doi: 10.1016/j.cognition.2009.12.004. [DOI] [PubMed] [Google Scholar]