Abstract
The tetrameric kainate receptors can be assembled from a combination of five different subunit subtypes. While GluK1-3 subunits can form homomeric receptors, GluK4 and GluK5 require a heteromeric partner to assemble, traffic to the membrane surface, and produce a functional channel. Previous studies have shown that incorporation of a GluK4 or GluK5 subunit changes both receptor pharmacology and channel kinetics. We directly compared the functional characteristics of recombinant receptors containing either GluK4 or GluK5 in combination with the GluK1 or GluK2 subunit. In addition, we took advantage of mutations within the agonist binding sites of GluK1, GluK2, or GluK5 to isolate the response of the wild-type partner within the heteromeric receptor. Our results suggest that GluK1 and GluK2 differ primarily in their pharmacological properties, but that GluK4 and GluK5 have distinct functional characteristics. In particular, while binding of agonist to only the GluK5 subunit appears to activate the channel to a non-desensitizing state, binding to GluK4 does produce some desensitization. This suggests that GluK4 and GluK5 differ fundamentally in their contribution to receptor desensitization. In addition, mutation of the agonist binding site of GluK5 results in a heteromeric receptor with a glutamate sensitivity similar to homomeric GluK1 or GluK2 receptors, but which requires higher agonist concentrations to produce desensitization. This suggests that onset of desensitization in heteromeric receptors is determined more by the number of subunits bound to agonist than by the identity of those subunits. The distinct, concentration-dependent properties observed with heteromeric receptors in response to glutamate or kainate are consistent with a model in which either subunit can activate the channel, but in which occupancy of both subunits within a dimer is needed to allow desensitization of GluK2/K5 receptors.
Introduction
Kainate receptors are cation-permeable, ligand-gated channels activated by the excitatory neurotransmitter glutamate. The ionotropic glutamate receptors, which include AMPA, NMDA, and kainate receptors, are tetrameric in structure, and each of the homologous subunits contains three full transmembrane domains and a ligand binding site (Traynelis et al., 2010; Kumar and Mayer, 2013). Kainate receptors are located both pre- and post-synaptically, where they regulate neurotransmitter release and mediate excitatory neurotransmission (Contractor et al., 2011; Lerma and Marques, 2013; Sihra and Rodriguez-Moreno, 2013). Although the post-synaptic current produced by kainate receptors is relatively small in amplitude compared to that of the AMPA receptors, it is characterized by a slow deactivation rate, allowing synaptic integration and temporal summation in response to repetitive stimulation (Castillo et al., 1997; Frerking et al., 1998; Frerking and Ohliger-Frerking, 2002).
Kainate receptors are assembled from a combination of five different subunits (GluK1–GluK5). The GluK1-3 subunits (formerly GluR5–7) are able to produce functional homomeric receptors in heterologous expression systems. These homomeric receptors exhibit relatively low sensitivity to activation by glutamate, and are characterized by rapid and complete desensitization in response to even sub-maximal glutamate levels (Sommer et al., 1992; Heckmann et al., 1996; Schiffer et al., 1997; Paternain et al., 1998). GluK1 subunits are highly expressed in the developing brain (Bahn et al., 1994) and may play an important role in neuronal maturation (Lauri et al, 2005, 2006; Segerstrale et al., 2010; Carta et al, 2014). In the adult, these subunits are predominantly expressed in interneurons, and contribute to both pre- and post-synaptic kainate receptor populations (Carta et al., 2014). GluK2-containing receptors appear to be responsible for most of the kainate receptor mediated post-synaptic response at the mossy fiber-CA3 synapse in the hippocampus, which represents the best characterized of the kainate receptor populations (Carta et al., 2014). The GluK2 subunit is also widely expressed in other brain regions, and in combination with GluK5 is considered to be the most common kainate receptor isoform (Perrais et al., 2010). The GluK3 subunit is likely to contribute primarily to presynaptic kainate receptors, forming heteromeric complexes with GluK2 subunits (Pinheiro et al., 2007). The GluK3-containing receptors have distinct functional characteristics, with a unique pharmacological profile and exceptionally low sensitivity to activation by glutamate, with an EC50 in the mM range (Schiffer et al., 1997; Pinheiro et al. 2007).
The GluK4-5 subunits (formerly KA1 and KA2) are distinct both structurally and functionally from the GluK1-3 subunits. They are obligate heteromers, and must assemble with GluK1-3 subunits to produce functional surface receptors which contain two of each subunit type (Gallyas et al., 2003; Ren et al., 2003; Perrais et al., 2010; Reiner et al., 2012). A high affinity interaction between the amino-terminal domains provides a mechanism for preferential assembly of heteromeric receptors (Kumar et al., 2011) and in neurons, it is likely that most post-synaptic kainate receptors are heteromers, containing GluK1 or GluK2 along with either a GluK4 or GluK5 subunit (Petralia et al, 1994; Darstein et al., 2003; Fernandes et al, 2009). While GluK5 is widely expressed throughout the brain, GluK4 is expressed primarily in the hippocampus (Bahn et al., 1994). Incorporation of a GluK4 or GluK5 subunit changes the functional and pharmacological properties of recombinant receptors, increasing sensitivity to glutamate, allowing activation by AMPA, slowing deactivation, and altering the concentration-dependence of desensitization (Sakimura et al., 1992; Herb et al., 1992; Pinheiro and Mulle, 2006; Barberis et al., 2008, Mott et al., 2010; Fisher and Mott 2011, 2013).
Each subunit within the tetrameric receptor contains an agonist binding site, and thus has the potential to contribute to channel activation and gating. Previous studies demonstrated that, for heteromeric receptors, glutamate binding to the higher affinity GluK4 or GluK5 subunits is able to activate the channel, with higher concentrations required to produce desensitization through the lower affinity GluK2 partner (Mott et al, 2010; Fisher and Mott, 2011). Some studies indicated that binding of just one subunit is sufficient to produce desensitization of AMPA receptors (Robert and Howe, 2003) and that partially bound states of homomeric kainate receptors are more likely to desensitize than to open (Barberis et al., 2008; Perrais et al., 2009a) although others suggested that partly desensitized conducting states could contribute to the current response (Bowie and Lange, 2002). In addition, work with subunit-selective agonists or antagonists (Swanson et al, 2002; Fisher and Mott, 2011; Pinheiro et al., 2013; Fisher 2014) and tethered ligands (Reiner and Isacoff, 2014) suggests that partial occupancy of the binding sites in homomeric or heteromeric receptors may be sufficient for activation of the receptors but is not necessarily sufficient for complete desensitization. Therefore, the relationship between subunit occupancy and channel gating may be dependent upon the subunit composition of the kainate receptor.
Studies with genetically modified mouse models lacking one or more of the kainate subunits indicate that different receptor isoforms have distinct roles in regulating neuronal function and behavioral responses (Mulle et al. 1998; Contractor et al., 2003; Ruiz et al., 2005; Fisahn et al., 2005; Pinheiro et al., 2007; Fernandes et al., 2009; Catches et al., 2012; Lowry et al, 2013). To determine the role of subunit composition in the response of kainate receptors to glutamate, we characterized the properties of recombinant homomeric and heteromeric receptors containing combinations of the GluK1, GluK2, GluK4 and GluK5 subunits. In addition, to determine the contribution of each subunit to receptor activation and desensitization, we examined the impact of binding site mutations that reduced agonist sensitivity. Our results show that each kainate receptor subunit has distinct pharmacological properties, and are consistent with a model in which occupancy of both subunits within a dimer pair may be necessary for complete desensitization of the receptor.
Materials and Methods
Cell culture and transfection of mammalian cells
Full-length cDNAs for rat kainate receptor subunits GluK1a(Q) (obtained from Dr. C. Mulle, Univ. Bordeaux, France), GluK2(Q), GluK4 and GluK5 (all obtained from Dr. S. Heinemann, Salk Institute, San Diego CA) in mammalian expression vectors were transfected into the human embryonic kidney cell line HEK-293T (GenHunter, Nashville, TN). Point mutations were generated using the QuikChange procedure and products (Agilent Technologies, Santa Clara, CA). Residue numbering includes the signal sequence. Oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, IA) and mutations were verified by DNA sequencing (University of South Carolina Environmental Genomics core, Columbia, SC). Cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 IU/ml penicillin and 100 μg/ml streptomycin. Cells were passaged by a 2 min. incubation with 0.025% trypsin/0.01% EDTA solution in phosphate-buffered saline (10 mM Na2HPO4, 150 mM NaCl, pH=7.3).
The cells were transiently transfected using calcium phosphate precipitation. A total of 5 μg of cDNA was transfected into the cells. For homomeric receptors, 4 μg of GluK1 or GluK2 was transfected. To produce heteromeric receptors, plasmids were added to the cells in a 1:3 ratio (GluK1 or 2:GluK4 or 5), previously shown to optimize formation of a homogeneous population (Barberis et al., 2008). To allow identification of positively transfected cells, 1 μg of the plasmid pHook™-1 (Invitrogen Life Technologies, Grand Island NY) which encodes a surface antibody, was also included (Chesnut et al., 1996). The cells were incubated at 3% CO2 for 4–6 hrs with the DNA/calcium phosphate mixture and then treated with a 15% glycerol solution in BES-buffered saline (25 mM BES(N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid), 140 mM NaCl, 0.75 mM Na2HPO4) for 30 sec. The selection procedure for pHook expression was performed 18–28 hrs later. The cells were trypsinized and mixed for 30–60 min. with approximately 6 × 105 magnetic beads coated with antigen for the pHook antibody (Chesnut et al., 1996). Bead-coated cells were isolated using a magnetic stand, resuspended into DMEM, plated onto collagen-coated glass coverslips and incubated overnight prior to electrophysiological recordings. In order to increase surface expression of homomeric GluK1E753D or GluK2E738D receptors with low agonist binding affinity, 100 μM CNQX was added to the feeding media 24 hours prior to recordings, following isolation of the transfected cells (Fleck, 2006).
Electrophysiological recordings
For all recordings the external solution consisted of (in mM): 150 NaCl, 3 KCl, 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 1 CaCl2 and 0.4 MgCl2 at pH 7.4 and osmolarity 295–305 mOsm. Recording electrodes were filled with an internal solution of (in mM); 130 CsGluconate, 5 CsCl, 0.5 CaCl2, 10 HEPES, 5 CsBapta, 2 MgCl2, 2 MgATP and 0.3 NaGTP adjusted to pH 7.4 and 290–300 mOsm. Glutamate and kainate were diluted into external solution from freshly made or frozen stocks in water. Patch pipettes were pulled from borosilicate glass with an internal filament (World Precision Instruments, Sarasota, FL) on a two-stage puller (Narishige, Japan) to a resistance of 5–10 MΩ. Drugs were applied to cells using a stepper solution exchanger with a complete exchange time of <50 msec (open tip, SF-77B, Harvard Apparatus, Holliston, MA). Peak currents may not be fully captured in the whole-cell recording mode due to the onset of fast desensitization in response to high concentrations of agonists. As a result, the measured peak current is likely an underestimate. There was a continuous flow of external solution through the chamber. Currents were recorded with an Axon 200B (Foster City, CA) patch clamp amplifier, filtered at 2 KHz (low-pass Bessel filter) and digitized at 20 KHz.
Data Analysis and statistical tests
Whole-cell currents were analyzed using the programs Clampfit (pClamp10.3 suite, Axon Instruments, Foster City, CA) and Prism (Graphpad, San Diego, CA). Concentration-response data was fit with a four-parameter logistic equation (Current = [Minimum current + (Maximum current - Minimum Current)]/1+(10^(log EC50 – log [agonist])*n) where n represents the Hill number. Fits were made to normalized data with the measured peak current at varying agonist concentrations expressed as a percentage of the maximum response for each cell. In cases where a saturating agonist concentration could not be reached due to low affinity of a mutated subunit, the maximum current was estimated by fitting the current amplitudes while fixing the Hill number to that obtained from the fit to data from wild-type receptors. ANOVA and Tukey-Kramer multiple comparisons tests of logEC50 values were performed using the Instat program (Graphpad) with a significance level of p<0.05.
Results
The GluK1, GluK2, GluK4 and GluK5 subunits can all contribute to the formation of functional kainate receptors in heterologous expression systems, and many studies have demonstrated the distinct pharmacological properties associated with different combinations of these subunits into homomeric or heteromeric receptors (Donevan et al., 1998; Paternain et al., 1998; Alt et al., 2004; Jane et al., 2009). However, there is a great deal of variability in the EC50 values reported for agonists at these receptors, likely due to differences among expression and recording systems and use of modulators such as concanavalin A to reduce desensitization. Therefore, we first examined the response of GluK1- and GluK2- containing homomeric and heteromeric receptors to glutamate, to allow a direct comparison of these different isoforms under the same conditions. The GluK3 subunit does not appear to co-assemble to a large extent with the GluK4 or GluK5 subunits in neurons, at least at the well-studied mossy fiber synapses (Pinheiro et al., 2007). Therefore, we did not include GluK3 in these studies.
Wild-type homomeric and heteromeric receptors
GluK1 and GluK2 homomers
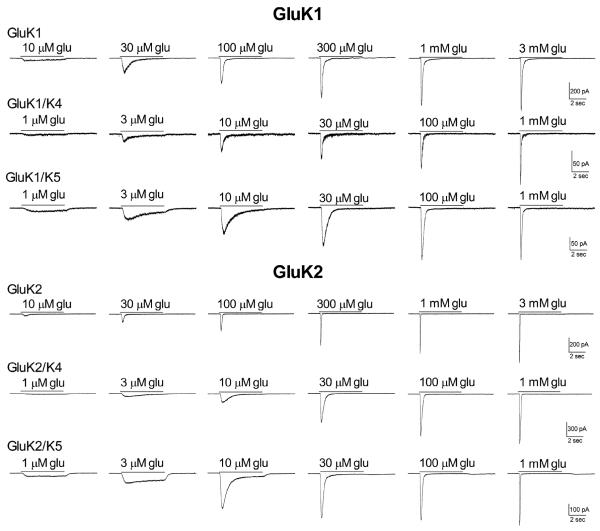
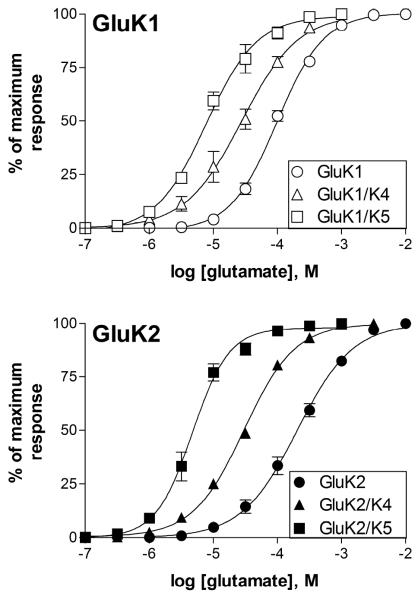
Both GluK1 and GluK2 readily produced functional homomeric receptors in transiently transfected HEK-293T cells. While both isoforms showed rapid and nearly complete desensitization even at relatively low glutamate levels, the GluK1 receptors appeared to have a slower onset of desensitization at all concentrations compared to GluK2 homomers under these whole-cell recording conditions (Fig 1). We also found that GluK1 homomers were slightly more sensitive to glutamate, with an average EC50 of 99.2 ± 7.4 μM (n=5) compared to 208.3 ± 28.1 μM (n=5) for the GluK2 homomers (Fig 2), although this difference was not significant (p>0.5). These EC50 values are in the general range (200 μM - 800 μM) of those reported by others using electrophysiological techniques to measure current responses in the whole-cell or outside-out patch configurations (Raymond et al., 1993; Heckman et al, 1996; Paternain et al, 1998, Barberis et al., 2008), although relatively few studies have examined the glutamate sensitivity of GluK1 homomeric receptors in the absence of modulators like concanavalin A (Sommer et al., 1992; Han et al., 2012). While the whole-cell configuration does not permit accurate quantification of fast kinetic properties like desensitization, the appearance of the GluK2 receptor currents is consistent with several previous studies, and that of the GluK1 homomers is most similar to the responses most commonly observed from these receptors and designated S-type by other investigators (Swanson and Heinemann, 1998).
Figure 1.
Effect of subunit composition on the response of recombinant receptors to glutamate. HEK-293T cells were transiently transfected with the subunits indicated. Glutamate was applied for 5 sec (solid line) to cells voltage-clamped at −70 mV in the whole-cell configuration. For each subunit combination, all current traces shown were obtained from the same cell.
Figure 2.
Comparison of the glutamate sensitivity of GluK1- and GluK2-containing homomeric and heteromeric receptors. Concentration-response relationships for glutamate were constructed by measuring the peak current amplitude and normalizing to the maximal peak response in response to a saturating concentration of glutamate for each cell. Symbols represent mean ± SEM (n=5–11 cells) and lines show the fit of a four-parameter logistic equation to the averaged data.
Formation of heteromeric receptors containing GluK4 or GluK5
Co-transfection of GluK4 with either GluK1 or GluK2 caused a change in the onset of whole-cell desensitization and an increase in glutamate sensitivity compared to the homomeric receptors, with average EC50s of 30.2 ± 5.9 μM (GluK1/K4, n=5) and 30.3 ± 1.2 μM (GluK2/K4, n=11) (Figs 1, 2). These EC50 values were significantly different from the GluK1 or GluK2 homomeric receptors (p≤0.001), but the two GluK4-containing isoforms were not different from one another (p>0.5). This is consistent with our previous study, which showed that the EC50 for activation of GluK5-containing receptors by glutamate was not affected by the agonist sensitivity of its GluK2 partner (Fisher and Mott, 2011) and demonstrates that the GluK4 subunit shares this characteristic with GluK5. Addition of the GluK4 subunit had modest effects on the onset and extent of desensitization (Fig 1). At glutamate concentrations below 10 μM, a level which produced minimal activation of homomeric receptors, the heteromeric GluK4-containing channels exhibited slower and less complete desensitization. However, at higher concentrations, the onset of desensitization for GluK2/K4 heteromers was comparable to that observed with GluK2 homomers and that of GluK1/K4 heteromers appeared to be faster and more complete compared to GluK1 homomers at the same glutamate concentration. This is similar to the pattern we reported using rapid glutamate application to GluK2/K4 receptors in outside-out patch recordings (Mott et al., 2010).
As was observed with GluK4, co-transfection with GluK5 produced receptors with similar glutamate sensitivity, regardless of the GluK1/2 partner, with average EC50's of 8.8 ± 1.4 μM (GluK1/K5, n=5) and 4.9 ± 0.7 μM (GluK2/K5, n=5) (Fig 2). However, when compared to the GluK4 subunit, co-assembly with GluK5 had a greater impact on the extent of desensitization produced by sub-maximal glutamate concentrations (Fig 1, 4D). At mM glutamate concentrations, a small rebound current could be observed with GluK2/K5 that was not seen with GluK2/K4. This rebound has been attributed to the unbinding of glutamate from the lower affinity GluK2 subunit, permitting brief channel activity through the higher affinity and non-desensitizing GluK5 subunit (Barberis et al., 2008; Mott et al., 2010; Fisher and Mott, 2011). When combined with the GluK1 subunit, onset of desensitization was observed at slightly lower glutamate concentrations compared to GluK2/K5 (Fig 1). This is consistent with the higher agonist sensitivity of the GluK1 subunit and with the general model that activation the GluK1 or GluK2 partner is required to produce desensitization of the GluK5-containing heteromer. These results indicate that the GluK4 and GluK5 subunit have similar roles in the activation of the channel, but that agonist binding to GluK4 may contribute to receptor desensitization.
Figure 4.
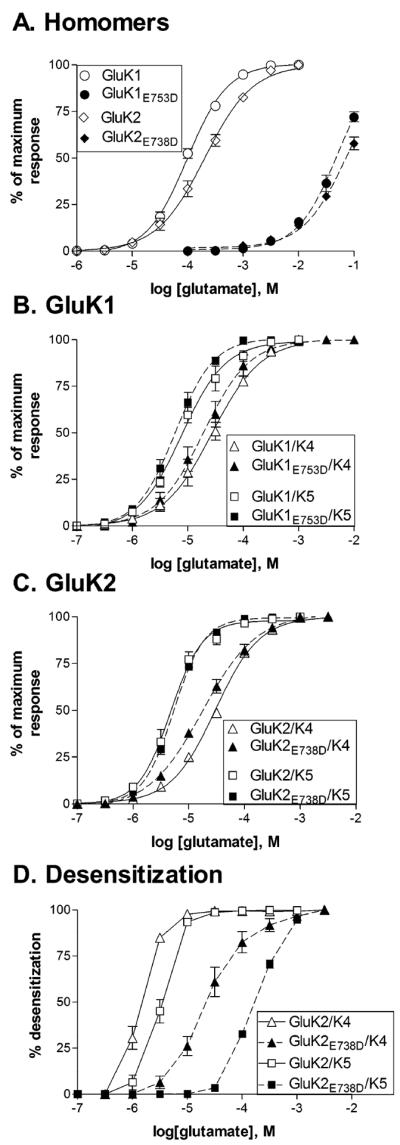
Mutations that reduce glutamate binding to GluK1 and GluK2 subunit alter desensitization, but not activation of heteromeric receptors.
A., B., C. Concentration-response relationships for glutamate were constructed by measuring the peak current amplitude and normalizing to the maximal peak response to a saturating concentration of glutamate for each cell. A saturating glutamate concentration could not be reached for mutated homomeric receptors, and therefore the peak current was estimated by fitting the current amplitudes for each cell with a logistic equation with a fixed Hill slope taken from the fit of the wild-type data. The maximum current from this fit was then used for normalization. Symbols represent mean ± SEM (n=3–8 cells) and solid (wild-type) or dashed (mutated) lines show the fit of a four-parameter logistic equation to the averaged data.
D. Effect of glutamate concentration on the extent of whole-cell desensitization. The steady state current measured at the end of the 5 second glutamate application was divided by the peak current. This steady state/peak ratio was then subtracted from 1 and multiplied by 100 to give the % desensitization.
Reducing agonist sensitivity of GluK1 or GluK2
GluK1 and GluK2 homomers
In order to functionally isolate the contribution from each of the different subunits in the heteromeric receptor, we used a mutation within the S2 domain of the agonist binding site to reduce sensitivity to glutamate (Mah et al., 2005). This fairly conservative glutamate to aspartate mutation produced dramatic shifts in glutamate sensitivity for both GluK1E753D and GluK2E738D (Fig 4A). Because of the extremely low agonist affinities of these receptors, saturating concentrations could not be reached, and therefore the EC50's can only be estimated using parameters from the fits to wild-type receptors. The average EC50s were 53.5 ± 11.2 mM for GluK1E753D (n=3) and 74.3 ± 9.7 mM for GluK2E738D (n=4). The value for GluK2E738D is similar to previous reports (Mah et al. 2005, Fisher and Mott, 2011), and this data suggests that the mutation has a similar effect in both GluK1 and GluK2 subunits. We co-expressed these mutated, low affinity subunits with wild-type GluK4 and GluK5 subunits to generate heteromeric receptors containing two types of subunits with vastly different agonist affinities.
Co-expression with GluK5
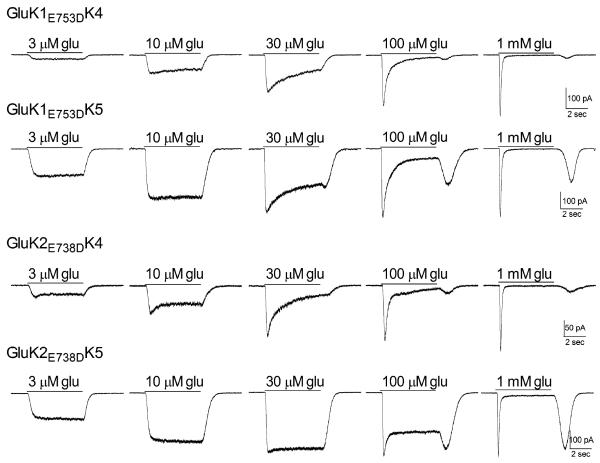
When expressed with wild-type GluK5 subunits, the heteromeric receptors had glutamate sensitivity like that of the wild-type receptors, with average EC50's of 6.6 ± 1.3 μM (GluK1E753D/K5, n=8) and 5.4 ± 0.2 μM (GluK2E738D/K5, n=7) (p≤0.05 compared to the respective wild-type heteromeric receptor) (Figs 4B,4C). The impact of the reduced glutamate sensitivity of the mutated GluK1/K2 subunits was seen only in the onset of desensitization, which was shifted to higher glutamate concentrations (Figs 3, 4D). A larger `rebound' current was also observed for both isoforms upon removal of glutamate, consistent with the greater difference in agonist sensitivity between the mutated GluK1/2 and the wild-type GluK5 (Mott et al., 2010). These results confirm our previous work with GluK2/GluK5 (Fisher and Mott, 2011) and indicate that the ability of the GluK5 to open the channel is not dependent on the identity of its partner within the heteromer.
Figure 3.
Effect of mutations within the glutamate binding sites of GluK1 and GluK2 on the response of heteromeric receptors. Representative current traces from transfected HEK-293T cells. Glutamate at the concentration indicated was applied for 5 sec (solid line) to cells voltage-clamped (−70 mV) in the whole-cell configuration.
Co-expression with GluK4
In a similar fashion, co-expression of the mutated GluK1 or GluK2 subunits with GluK4 also had a little effect on glutamate sensitivity compared to the wild-type heteromers, with average EC50's of 22.9 ± 7.8 μM (GluK1E753D/K4, n=5) and 19.1 ± 2.7 μM (GluK2E738D/K4, n=6) (Figs 4B, 4C). The slight change in glutamate EC50 compared to wild-type heteromers may be an indication of heterogeneity in the receptor population, as others have suggested that the GluK4 subunit is poorly incorporated into recombinant receptors (Perrais et al, 2010). However, the glutamate EC50s of all the GluK4-containing receptors were not significantly different from one another (p>0.05), indicating that any contribution from homomeric receptors was likely to be modest. Although onset of whole-cell desensitization was right-shifted to higher concentrations, when compared to GluK5-containing receptors desensitization occurred at lower glutamate concentrations and the rebound current was not as pronounced (Figs 3, 4D). Under some conditions, although an actual increase in current was not apparent, channel activity was prolonged following agonist removal (i.e. 30 μM glutamate, GluK2E738D/K4). The smaller rebound current is consistent with GluK4 having a slightly lower glutamate affinity than GluK5. In addition, the onset of desensitization at glutamate levels unable to activate the mutated GluK1/2 subunit suggests that glutamate binding only to the GluK4 subunit within the tetramer is capable of producing some desensitization, in contrast to GluK5. These results are comparable to earlier studies in oocytes (Mott et al., 2010), but demonstrate a contribution of the GluK4 subunit to the onset of desensitization that the GluK5 subunit does not appear to share. It may be that GluK5 contributes to a stronger dimer interface with its GluK1/2 partner, and therefore requires both subunits in the dimer to be bound by agonist to undergo conformational changes associated with desensitization (Kumar and Mayer, 2013).
Reducing agonist sensitivity of GluK5
Responses to glutamate
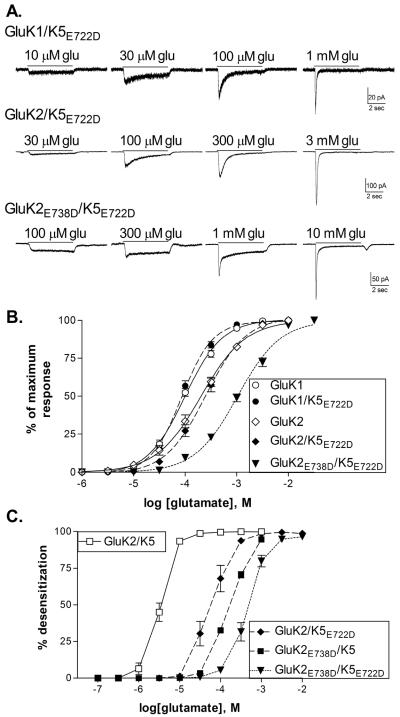
To further examine the relative roles of each type of subunit within the heteromer, we mutated the site in the GluK5 subunit homologous to GluK2E738. Since GluK5 homomers cannot be formed, the impact of this mutation can only be inferred from the properties of the heteromeric receptor. We found that co-expression of the mutated GluK5E722D subunit with wild-type GluK1 or GluK2 subunits produced receptors with glutamate sensitivity similar to the respective GluK1 or -K2 homomer, with average EC50's of 87.1 ± 7.1 (GluK1/K5E722D, n=6) and 258.5 ± 36.7 μM (GluK2/K5E722D, n=5) (Fig. 5B). Although the glutamate EC50 for activation of the receptor was consistent with a homomeric receptor, incorporation of the GluK5 subunit was apparent through the change in onset of desensitization (Figs 5A, 5C). At glutamate concentrations below EC50 values for each receptor isoform, whole-cell desensitization was slow and incomplete, and comparable to that of the wild-type heteromers at the same effective concentration. This was clearly distinct from the behavior of the homomeric receptors at the same glutamate concentrations (Fig 1). These results suggest that the identity of the bound subunit (i.e. GluK2 or GluK5) is not as important as the number of bound subunits, and that activation of heteromeric receptors occurs through the higher affinity subunits while desensitization is initiated by activation of the lower affinity subunit.
Figure 5.
A mutation in the agonist binding site of GluK5 alters the concentration-dependence of channel activation and desensitization.
A. Representative current traces from HEK-293T cells transfected with the indicated subunits in response to 5 sec applications of glutamate.
B. Concentration-response relationships for glutamate were constructed by measuring the peak current amplitude and normalizing this to the maximal peak response to a saturating concentration of glutamate for each cell. Symbols represent mean ± SEM (n=5–6 cells) and solid (wild-type) or dashed (mutated) lines show the fit of a four-parameter logistic equation to the averaged data. Wild-type data is reproduced from Figure 2.
C. Effect of glutamate concentration on the extent of whole-cell desensitization. The steady state current measured at the end of the 5 second glutamate application was divided by the peak current. This steady state/peak ratio was then subtracted from 1 and multiplied by 100 to give the % desensitization.
Since the properties of the GluK5E722D subunit could not be easily isolated from that of the wild-type GluK1/2 subunits, we attempted to estimate the effect of the mutation on glutamate sensitivity of the GluK5E722D subunit by co-expressing it with the very low affinity GluK2E738D subunit (Fig 5). This combination produced receptors with an average EC50 of 1.07 ± 0.09 mM (n=5), significantly different than that of the GluK2E738D homomers (p≤0.001), and therefore likely representing the activation of the GluK5E722D subunit. The desensitization properties of the receptor also support this conclusion, as responses to glutamate at concentrations up to 100 μM glutamate produced little desensitization and a small rebound current could be observed at mM concentrations (Fig 5A). This is comparable to the characteristics of GluK5-containing receptors, and suggests that the heteromeric receptor containing both mutated subunits essentially recapitulates the properties of the wild-type receptor, while shifting both activation and desensitization to higher glutamate concentrations.
Effect of binding site mutations on responses to kainate
The wild-type GluK2 and GluK5 subunits have a relatively large separation in their sensitivity to activation by the endogenous agonist glutamate (Fig 2). However, not all kainate receptor agonists show such discrimination among subunits. The marine neurotoxin kainate activates both GluK2 and GluK2/K5 receptors with similar EC50s, in the range of 1–5 μM (Jones et al., 1997; Alt et al., 2004; Jane et al., 2009). Therefore, we examined the effect of the mutations in either the GluK2 or GluK5 agonist binding sites on the characteristics of the response of heteromeric receptors to kainate.
Kainate activated both homomeric and heteromeric wild-type receptors (Fig 6). As previously shown, kainate activated homomeric GluK2 receptors and induced rapid desensitization of the whole-cell response (Herb et al. 1992; Jones et al., 1997; Swanson et al, 1997). Addition of the GluK5 subunit slowed the onset of desensitization (Herb et al., 1992) and modestly increased sensitivity to kainate (average EC50 = 1.3 ± 0.4 μM, n=4) compared to the homomers (average EC50= 4.8 ± 0.7 μM, n=5)(p≤0.001 compared to GluK2). When the mutated GluK2E738D subunit was co-expressed with wild-type GluK5, the kainate EC50 was unchanged compared to the wild-type heteromers (average EC50 = 0.8 ± 0.1 μM, n=5, p>0.05 compared to GluK2/K5, p≤0.001 compared to GluK2) (Fig 6B). As we observed with responses to glutamate however, higher kainate concentrations were required to produce desensitization in these receptors, and rebound currents were observed at agonist removal (Fig 6A). A comparable pattern occurred with the incorporation of the GluK5E722D subunit. The kainate EC50 was shifted to that of the GluK2 homomers (average EC50 = 3.5 ± 0.5 μM, n=4, p≤0.01 compared to GluK2/K5, p>0.5 compared to GluK2), suggesting that the wild-type GluK2 subunits within the heteromer were responsible for activation of the receptor. The concentration-dependence of the onset of desensitization was also right-shifted compared to the wild-type heteromers, producing responses comparable to those observed with GluK2E738D/K5 receptors (Fig 6A). While the results using either kainate or glutamate as the agonist are consistent with the same mechanistic interpretation, the role of the GluK2 subunit is more easily observed with kainate. Because of the similar kainate sensitivity of each of the subunits, the activation of the receptor (associated with the wild-type partner) and the desensitization of the receptor (associated with the mutated partner) occur at nearly the same kainate concentrations respectively, regardless of which subunit is mutated.
Figure 6.
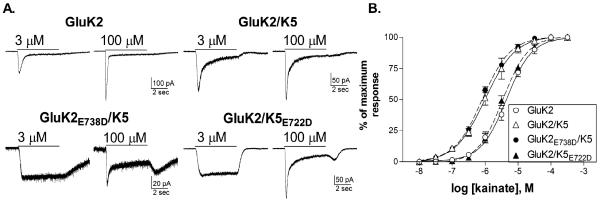
Effect of binding site mutations in GluK2 or GluK5 on the activation of homomeric and heteromeric receptors by kainate.
A. Representative current traces in response to 5 sec applications of kainate from receptors containing wild-type or mutated subunits as indicated.
B. Concentration-response relationships for kainate were constructed by measuring the peak current amplitude and normalizing this to the maximal peak response to a saturating concentration of kainate for each cell. Symbols represent mean ± SEM (n=4–5 cells) and solid (wild-type) or dashed (mutated) lines show the fit of a four-parameter logistic equation to the averaged data.
Discussion
In this study we examined the responses of recombinant kainate receptors to glutamate. In agreement with earlier work, we found that the subunit composition influenced glutamate sensitivity. Homomeric GluK1 or GluK2 receptors had relatively low sensitivity to glutamate, with EC50's in the 100–200 μM range. Addition of either the GluK4 or GluK5 subunit to form heteromeric receptors increased glutamate sensitivity, with EC50's 3–50x lower than the homomeric receptors. This is consistent with the long-standing description of GluK1-3 subunits as `low affinity' and the GluK4-5 subunits as `high-affinity' which arose from their radioligand binding properties. To isolate the functional contribution of each subunit within the tetrameric receptor, we created mutations in the binding site of the GluK1, GluK2 and GluK5 subunits. Because the GluK4 and GluK5 subunits must combine with a GluK1-3 subunit for assembly, these heteromeric receptors have a fixed stoichiometry, with each dimer within the tetrameric structure containing one GluK4/5 subunit (Reiner et al., 2012). This work confirmed our earlier conclusion that occupancy of only the GluK5 subunits by agonist activates the receptor without inducing desensitization (Fisher and Mott, 2011). However, GluK4-containing heteromers were able to desensitize at glutamate concentrations unable to activate the low affinity, mutated GluK1/K2 subunits, suggesting that the GluK4 and GluK5 subunits differ in this characteristic. When the mutated, low affinity GluK5 subunit was incorporated into heteromeric receptors, glutamate or kainate binding to the wild-type GluK2 partner was also able to activate the receptor without desensitization. These results clearly demonstrate that the identity of the bound subunit is not the key factor in regulating heteromeric channel activity. Instead, the number and location of the activated subunits determines the functional response. This is somewhat surprising as structural studies suggest that the GluK2 and GluK5 subunits have asymmetric contributions to channel structure, at least at the level of the amino-terminal domains (Kumar et al., 2011) and likely at the level of the ligand-binding domains. In heteromeric receptors, the GluK1-3 subunits have been suggested to contribute the `distal' pair of subunits, while GluK4-5 subunits assemble in the `proximal' conformations (Kumar et al. 2011; Kumar and Mayer, 2013). Our results suggest that the subunits in these two different locations within the dimer structure are actually similar in their functional contributions to activation and desensitization.
This model also predicts that subunit-selective agonists or antagonists that act at only one of the subunits within a dimer pair would be expected to reduce the onset and extent of desensitization. This predicted outcome has been observed in a number of previously published studies. For example, the antagonist kynurenate is more effective at GluK2 subunits compared to GluK5 subunits, while the antagonist UBP310 inhibits GluK5 but not GluK2. In both of these cases, application of the antagonist to GluK2/K5 receptors caused a dramatic reduction in desensitization, with minimal effect on the amplitude of the response (Fisher and Mott, 2011; Pinheiro et al., 2013). Two different marine neurotoxins have unique effects on heteromeric kainate receptors that also provide supportive evidence for this mechanism. Dysiherbaine binds with high affinity to the GluK1 subunit, and can activate GluK1/K5 heteromers without activity at GluK5 (Swanson et al. 2002). Dysiherbaine also caused a long-lasting inhibition of the GluK1 subunit, but agonists binding to GluK5 were still able to activate and desensitize the receptor. A similar action was also recently reported for domoic acid, which was found to produce a long-lasting occupation of the GluK5 subunit binding site (Fisher, 2014). While agonists acting through GluK5 were no longer effective at this domoate-bound receptor, heteromeric GluK2/K5 receptors could still be activated by glutamate, kainate or domoate binding to the GluK2 subunit. Although we did not examine the GluK3 subunit in this study, the previously reported properties of GluK3-containing heteromeric receptors are also consistent with this proposed mechanism. GluK3 homomeric receptors require high glutamate concentrations for activation, and are likely to desensitize at low agonist levels (Perrais et al., 2009a). In many ways, therefore, they are similar to the mutated GluK2E738D subunit. Indeed, the GluK3/K5 heteromer exhibits properties analogous to those of the GluK2E738D/K5 receptors, with a glutamate EC50 of 6 mM, compared to 10 mM for GluK3 homomers (Perrais et al, 2009a), and a prominent rebound current following agonist removal (Fisher and Mott, 2013). In addition, the antagonist UBP310, which inhibits GluK3 subunits but not GluK2 subunits, causes a slowing of desensitization of GluK2/K3 receptors without changing the peak response (Perrais et al., 2009b). The interpretation of results from heteromeric receptors composed of GluK1-3 subunits is more complicated because the stoichiometry is not fixed, as it is for GluK4/K5-containing heteromers. However, this impact of a competitive antagonist on desensitization rather than on current amplitude is consistent with a general mechanism that has applicability to all types of kainate receptors.
In many of the subunit combinations examined in this study, a rebound or tail current was observed following the termination of application of high agonist concentrations. These currents can occur when the recovery from desensitization is relatively fast compared to the rate of deactivation and are commonly observed in kainate receptors when subunits with different agonist affinities are co-assembled into the same receptor, allowing differential activation of a subset of subunits within the tetramer. The greater the difference in affinity between subunits, the more pronounced the rebound current becomes (Mott et al, 2010; Fisher and Mott, 2011; current study). Rebound current can also be observed when the rate of recovery from desensitization is enhanced, as it is by the auxiliary subunit Neto1 (Fisher and Mott, 2013). Rebound current has also been observed in recordings from neuronal kainate receptors, where it may be associated with co-assembly with Neto1 (Puthussery et al, 2014). While the appearance of the rebound current is most obvious under conditions of slower agonist removal, and is therefore greatest with oocyte recordings (Mott et al., 2010), it is still apparent with rapid application recordings from excised patches (Fisher and Mott, 2011, 2013), demonstrating that this phenomenon is a characteristic of the receptor, and not just a result of solution exchange.
A recent study used tethered ligands to address the relationship between subunit occupancy of kainate receptors and their activation and desensitization (Reiner and Isacoff, 2014). They found that the degree, but not the rate, of desensitization was dependent on the number of activated subunits in GluK2 homomeric receptors. They were unable to differentiate between receptors in which both subunits within a dimer were bound or those in which one subunit in each dimer were bound, however. They utilized subunit-selective agonists to test GluK2/K5 heteromers, and found that binding to either GluK2 or GluK5 could activate the receptor, although a full response was not generated in either case. In agreement with our findings, neither of these agonists alone was able to induce desensitization of the heteromeric receptors.
Our results also show that, although they have many common features, the GluK4 and GluK5 subunits appear to make distinct contributions to the onset of desensitization. Kainate receptors are generally considered to undergo at least two major conformational changes associated with desensitization which involve a disruption of the dimer interface and separation of the four ligand binding domains (Sun et al., 2002; Armstrong et al., 2006; Chaudhry et al., 2009; Kumar and Mayer, 2013; Schauder et al., 2013). The structural basis for desensitization has primarily been examined using homomeric protein assemblies, and may occur differently in heteromeric, GluK4/K5-containing receptors. The desensitization produced by occupancy of the GluK4 subunits might result from between-dimer interactions among the two bound subunits, or it may be due to a weaker dimer interface permitting occupancy of only one subunit to produce desensitization. Examination of the structural differences between GluK4 and GluK5 at sites of heterogeneity within the proposed interfaces may differentiate between these possibilities.
The rates of onset and recovery from desensitization can regulate the responses of post-synaptic receptors to repeated stimuli. In addition, many kainate receptors appear to be located in peri-synaptic or extra-synaptic regions, where agonist sensitivities and desensitization properties determine their ability to respond to tonic exposure to low glutamate levels (Carta et al., 2014). Therefore the subunit-dependence of these characteristics will influence the response of neurons expressing different combinations of kainate receptor subunits. In addition to the pore-forming subunits, kainate receptors are also regulated by the auxiliary subunits, Neto1 and Neto2, which influence desensitization of the receptors in a subunit-dependent manner (Copits and Swanson, 2012). Thus, contributions from auxiliary subunits add an additional layer of complexity to characterizing the functional properties of neuronal kainate receptors with varying subunit composition. The distinct contributions of different subunits within the tetrameric receptor must also be considered when developing subunit-selective drugs. For example, antagonists with high selectivity may paradoxically produce an enhancement of kainate receptor activity, through a reduction in desensitization of heteromeric receptors. An understanding of the unique characteristics of each subunit, and their contributions to channel function, will allow a more rational approach to modulation of kainate receptor activity.
Highlights.
Different kainate receptor subunits confer distinct pharmacological properties.
In heteromeric receptors, agonist binding to either type of subunit can activate the channel.
Desensitization of GluK2/K5 heteromers requires occupancy of both subunits in a dimer
Acknowledgments
This work was supported by a grant from NIH-NINDS (R01-NS065869 to JLF), an ASPIRE grant from the Office of the Vice President for Research at the University of South Carolina (to JLF) and a Magellan Scholar Award from the University of South Carolina Office of Undergraduate Research (to MTF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors. Thanks to Dr. Morris Benveniste (Morehouse School of Medicine) and Dr. David Mott (University of South Carolina School of Medicine) for helpful comments regarding this study and to Dr. James Warren (University of South Carolina School of Medicine) for technical assistance with site-directed mutagenesis and cell culture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharm. 2004;46:793–806. doi: 10.1016/j.neuropharm.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A, Sachidhanandam S, Mulle C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci. 2008;28:6402–6406. doi: 10.1523/JNEUROSCI.1204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD. Functional stoichiometry of glutamate receptor desensitization. J Neurosci. 2002;22:3392–3403. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Fievre S, Gorlewicz A, Mulle C. Kainate receptors in the hippocampus. Eur J Neurosci. 2014;39:1835–1844. doi: 10.1111/ejn.12590. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Catches JS, Xu J, Contractor A. Genetic ablation of the GluK4 kainate receptor subunit causes anxiolytic and antidepressant-like behavior in mice. Behav Brain Res. 2012;228:406–414. doi: 10.1016/j.bbr.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry C, Weston MC, Schuck P, Rosenmund C, Mayer ML. Stability of ligand-binding domain dimer assembly controls kainate receptor desensitization. EMBO J. 2009;28:1518–1530. doi: 10.1038/emboj.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut JD, Baytan AR, Russell M, Chang MP, Bernard A, Maxwell IH, Hoeffler JP. Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J Immunol Methods. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2 −/− mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Swanson GT. Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nature Rev Neurosci. 2012;13:675–686. doi: 10.1038/nrn3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci. 2003;23:8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Beg A, Gunther JM, Twyman RE. The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J Pharmacol Exp Ther. 1998;285:539–545. [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Heinemann SF, McBain CJ. The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurons. J Physiol. 2005;562:199–203. doi: 10.1113/jphysiol.2004.077412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. Distinct functional roles of subunits within the heteromeric kainate receptor. J Neurosci. 2011;31:17113–17122. doi: 10.1523/JNEUROSCI.3685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. Modulation of homomeric and heteromeric kainate receptors by the auxiliary subunit Neto1. J Physiol. 2013;591:4711–4724. doi: 10.1113/jphysiol.2013.256776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL. The neurotoxin domoate causes long-lasting inhibition of the kainate receptors GluK5 subunit. Neuropharmacol. 2014;85:9–17. doi: 10.1016/j.neuropharm.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck MW. Glutamate receptors and endoplasmic reticulum quality control: looking beneath the surface. Neuroscientist. 2006;12:232–244. doi: 10.1177/1073858405283828. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nature Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P. AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci. 2002;22:7434–7443. doi: 10.1523/JNEUROSCI.22-17-07434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallyas F, Ball SM, Molnar E. Assembly and cell surface expression of KA-2 subunit-containing kainate receptors. J Neurochem. 2003;86:1414–1427. doi: 10.1046/j.1471-4159.2003.01945.x. [DOI] [PubMed] [Google Scholar]
- Han Y, Wang C, Park JS, Niu L. Channel-opening kinetic mechanism of wild-type GluK1 kainate receptors and a C-terminal mutant. Biochem. 2012;51:761–768. doi: 10.1021/bi201446z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann M, Bufler J, Franke C, Dudel J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacol. 2009;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Jones KA, Wilding TJ, Huettner JE, Costa AM. Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacol. 1997;36:853–863. doi: 10.1016/s0028-3908(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Kumar J, Schuck P, Mayer ML. Structure and assembly mechanisms for heteromeric kainate receptors. Neuron. 2011;71:319–331. doi: 10.1016/j.neuron.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Mayer ML. Functional insights from glutamate receptor ion channel structures. Ann Rev Physiol. 2013;75:313–337. doi: 10.1146/annurev-physiol-030212-183711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Segerstrale M, Vesikansa A, Maingret F, Mulle C, Collingridge GL, Isaac JTR, Taira T. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J Neurosci. 2005;25:4473–4484. doi: 10.1523/JNEUROSCI.4050-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JTR, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80:292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- Lowry ER, Kruyer A, Norris EH, Cederroth CR, Strickland S. The GluK4 kainate receptor subunit regulates memory, mood, and excitotoxic neurodegeneration. Neurosci. 2013;235:215–225. doi: 10.1016/j.neuroscience.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah SJ, Cornell E, Mitchell NA, Fleck MW. Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. J Neurosci. 2005;25:2215–2225. doi: 10.1523/JNEUROSCI.4573-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Rojas A, Fisher JL, Dingledine RJ, Benveniste M. Subunit-specific desensitization of heteromeric kainate receptors. J Physiol. 2010;588:683–700. doi: 10.1113/jphysiol.2009.185207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacol. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Perrais D, Coussen F, Mulle C. Atypical functional properties of GluK3-containing kainate receptors. J Neurosci. 2009a;29:15499–15510. doi: 10.1523/JNEUROSCI.2724-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Pinheiro PS, Jane DE, Mulle C. Antagonism of recombinant and native GluK3-containing kainate receptors. Neuropharmacol. 2009b;56:131–140. doi: 10.1016/j.neuropharm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Perrais D, Veran J, Mulle C. Gating and permeation of kainate receptors: differences revealed. Trends Pharmacol Sci. 2010;31:516–522. doi: 10.1016/j.tips.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro PS, Lanore F, Veran J, Artinian J, Blanchet C, Crepel V, Perrais D, Mulle C. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb Cortex. 2013;23:323–331. doi: 10.1093/cercor/bhs022. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Percival KA, Venkataramani S, Gayet-Primo J, Grunert U, Taylor WR. Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. J Neurosci. 2014;34:7611–7621. doi: 10.1523/JNEUROSCI.4855-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond LA, Blackstone CD, Huganir RL. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993;361:637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Reiner A, Arant RJ, Isacoff EY. Assembly stoichiometry of the GluK2/GluK5 kainate receptor complex. Cell Reports. 2012;1:234–240. doi: 10.1016/j.celrep.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Isacoff EY. Tethered ligands reveal glutamate receptor desensitization depends upon subunit occupancy. Nature Chem Biol. 2014;10:273–280. doi: 10.1038/nchembio.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Riley NJ, Garcia EP, Sanders JM, Swanson GT, Marshall J. Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J Neurosci. 2003;23:6608–6616. doi: 10.1523/JNEUROSCI.23-16-06608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends upon receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci. 2005;25:11710–11718. doi: 10.1523/JNEUROSCI.4041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Morita T, Kushiya E, Mishina M. Primary structure and expression of the γ2 subunit of the glutamate receptor channel selective for kainate. Neuron. 1992;8:267–274. doi: 10.1016/0896-6273(92)90293-m. [DOI] [PubMed] [Google Scholar]
- Schauder DM, Kuybeda O, Zhang J, Klymko K, Bartesaghi A, Borgnia MJ, Mayer ML, Subramaniam S. Glutamate receptor desensitization is mediated by changes in quaternary structure of the ligand binding domain. Proc Natl Acad Sci. 2013;110:5921–5926. doi: 10.1073/pnas.1217549110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Segerstrale M, Juuri J, Lanore F, Piepponen P, Lauri SE, Mulle C, Taira T. High firing rate of neonatal hippocampal interneurons is caused by attenuation of afterhyperpolarizing potassium currents by tonically active kainate receptors. J Neurosci. 2010;30:6507–6514. doi: 10.1523/JNEUROSCI.4856-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihra TS, Rodriguez-Moreno A. Presynaptic kainate receptor-mediated bidirectional modulatory actions: mechanisms. Neurochem Int. 2013;62:982–987. doi: 10.1016/j.neuint.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinanen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Gereau RW, Green T, Heinemann SF. Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron. 1997;19:913–926. doi: 10.1016/s0896-6273(00)80972-1. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Heinemann SF. Heterogeneity of homomeric GluR5 kainate receptor desensitization expressed in HEK293 cells. J Physiol. 1998;513:639–646. doi: 10.1111/j.1469-7793.1998.639ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Green T, Sakai R, Contractor A, Che W, Kamiya H, Heinemann SF. Differential activation of individual subunits in heteromeric kainate receptors. Neuron. 2002;34:589–598. doi: 10.1016/s0896-6273(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]