Abstract
Statins are widely used lipid-lowering drugs that are effective in reducing cardiovascular disease risk. Although they are generally well tolerated, they can cause muscle toxicity, which can lead to severe rhabdomyolysis. Research in this area has been hampered to some extent by the lack of standardized nomenclature and phenotypic definitions. We have used numerical and descriptive classifications and developed an algorithm to define statin-related myotoxicity phenotypes, including myalgia, myopathy, rhabdomyolysis, and necrotizing autoimmune myopathy.
Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are effective in lowering blood cholesterol as well as in primary and secondary prevention of cardiovascular events. Approximately 25 million people worldwide are taking statins.1,2,3 Statins are safe and effective in the majority of patients, but they are associated with muscle toxicity, which, although rare, can be serious and potentially life threatening.4,5 The clinical spectrum of statin-induced myotoxicity varies greatly from asymptomatic elevations of creatine kinase (CK) without muscle pain, to muscle pain or weakness with raised CK levels, myositis with biopsy-proven muscle inflammation, and, finally, rhabdomyolysis with muscle symptoms, high CK, and potential for acute kidney injury (Table 1).6,7 Whether statins cause muscle pain in the absence of CK changes remains controversial. Although milder forms of myotoxicity tend to be self-limiting and disappear after cessation of therapy, they can lead to poor quality of life, poor drug compliance, and, consequently, the failure to prevent cardiovascular events.
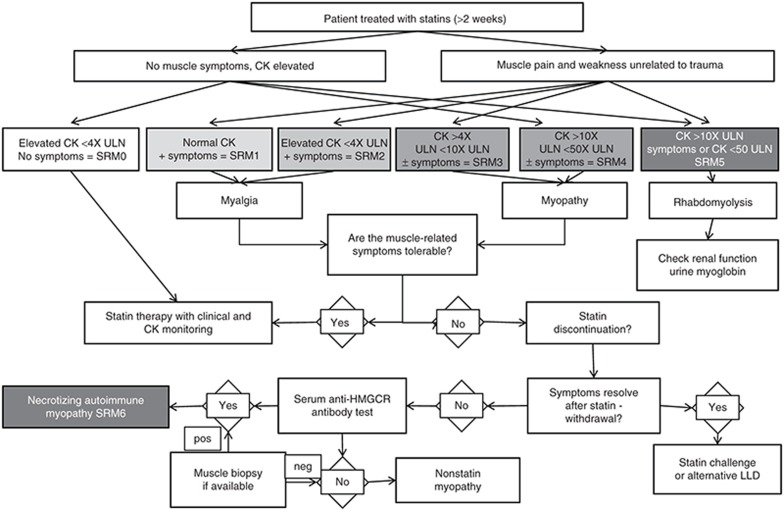
Table 1. Statin-related myotoxicity phenotype classification.
Because statin-induced myotoxicity is uncommon, it is necessary to pool and analyze data from various sources, including multicenter clinical trials and observational studies, to study genetic etiology. In addition, electronic medical records linked to biological repositories or to individual patients have been useful in identifying and recruiting patients with drug-induced toxicities.6,8,9,10,11,12 The Phenotype Standardization Project was started a few years ago to facilitate such multicenter research collaborations on various types of serious adverse drug reactions (ADRs). Phenotype consensus papers on drug-induced liver injury, skin injury, and torsade de pointes have been published by multidisciplinary groups of international experts.13,14,15 The aim of this article is to provide consensus definitions for statin-induced myotoxicity phenotypes, to facilitate cross-study comparisons from the existing cohorts, to aid in the recruitment of retrospective and newly diagnosed patients with statin-induced muscle damage, and to define the phenotype for genomic data analyses.
We convened an international expert workshop on statin-induced myotoxicity in December 2013 in Liverpool, UK, to agree on definitions and a minimum set of criteria to help in identification and recruitment. We report on the deliberations of this workshop: first, we summarize evidence of the clinical and biochemical phenotypes that have been reported, and second, we report on our suggested standardization of the terminology and phenotypes of statin-induced muscle toxicity.
Consensus Process
The PREDICTION-ADR consortium, funded by the EU Seventh Framework Programme, organized a joint meeting on 10 December 2013 in Liverpool with a multidisciplinary team of experts on statin-related myopathy and angiotensin converting enzyme-inhibitor angioedema. An international team with known expertise in the area was assembled by invitation. The group comprised clinical and basic pharmacologists, internists, rheumatology and myopathy experts, immunology and clinical chemistry scientists, allergists, regulatory agency representatives, and managers of electronic medical record databases.
A draft manuscript circulated before the meeting contained a brief literature review on statin-related myopathy and a description of main phenotypes. The expert group was asked to consider the minimum set of criteria for patient recruitment. An introductory talk was given to start the discussions. In addition, an algorithm was designed to aid standardization of nomenclature and phenotypes, and recommendations were made about the types of data to be collected from each patient (Figure 1). After the meeting, the revised manuscript comprising the criteria (Table 1) and algorithm was approved by all contributors.
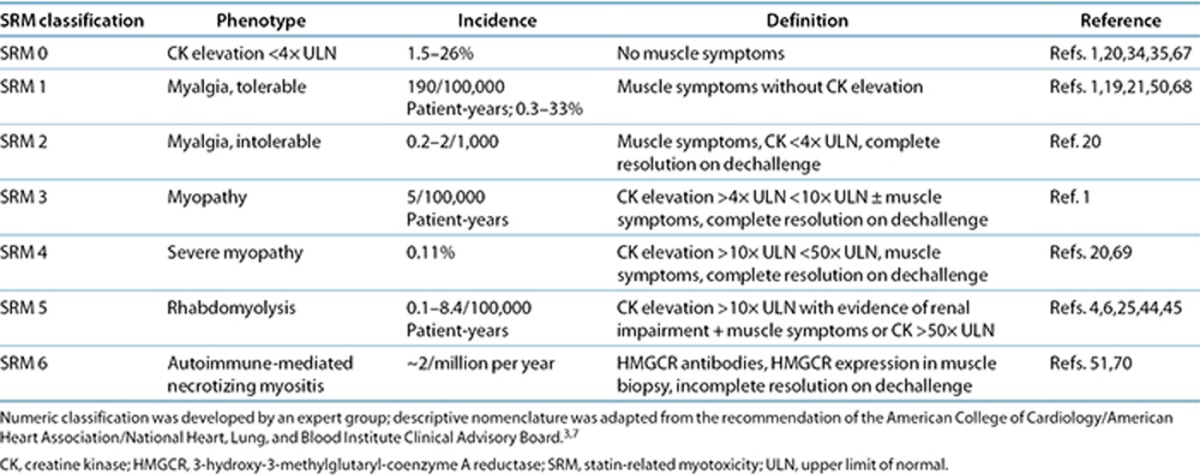
Figure 1.
Algorithm for defining the type of statin-related myotoxicity. New nomenclature was introduced to reflect the phenotype classification and severity (SRM 0 to SRM 6) of statin-related toxicity. CK, creatine kinase; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; LLD, lipid-lowering drug; SRM, statin-related myotoxicity; ULN, upper limit of normal.
We did not discuss causality assessment formally, as the challenges of this task are common for many ADRs.16 They require establishing a temporal relationship, along with dechallenge and rechallenge information, and, particularly important for statin-related myotoxicity, vigilant information on muscle injury from falls, trauma, or vigorous exercise. We also recommend independent assessment of causality by an adjudication panel, using methodology that has been successfully applied to retrospective and prospective recruitment of patients with rare ADRs, including severe drug-induced skin injury.15
Incidence
The rate of statin-induced myotoxicity has been estimated from adverse event data in case series and randomized controlled trials.17,18,19,20,21,22,23,24 However, estimating adverse events from randomized controlled trials may be challenging because (i) they are not usually powered to capture low-frequency ADRs; (ii) patients are exposed to drugs and monitored only for a short period of time when myotoxicity may not yet have developed; (iii) the diagnostic criteria for reaction events may vary across different trials; and (iv) they may have strict inclusion and exclusion criteria that may exclude some populations who are at higher risk of ADRs.
The risk of rhabdomyolysis among hospitalized patients receiving lipid-lowering drugs has recently been estimated in a real-world clinical setting. Claims data from 9 million members of five US health plans were used to confirm 42 cases in >470,000 patients exposed to lipid-lowering drugs. The risk of rhabdomyolysis for individuals on different statin preparations, including combination therapy, and the risk of comorbidities were estimated to be between 0.3 and 8.4 in 10,000 patient-years.25 The authors estimated that the risk in comparison with atorvastatin as a reference was significantly higher when statins were used in combination with cytochrome P450 (CYP)3A4 inhibitors (odds ratio: 7.1, 95% confidence interval: 1.6–31.6) and for cerivastatin monotherapy (odds ratio: 4.7, 95% confidence interval: 1.1–21.1).25
Diagnostic Criteria
The diagnosis of statin-induced myotoxicity is based on medical history, clinical examination, and laboratory tests and can be confirmed by muscle biopsy (Supplementary Table S1 online). Muscle biopsies can reveal muscle fiber necrosis, type II fiber atrophy, and increased lipid stores in muscle fibers or inflammation.26,27,28,29 In clinical practice, a pragmatic approach is adopted: discontinuation of the culprit drug and avoidance of its future use.30 Monitoring of statin therapy for muscle toxicity includes CK measurements, although routine laboratory testing is recommended only for symptomatic patients.31 The potential harm of introducing routine CK monitoring in all patients who take statins may outweigh the benefits from several perspectives, including false-positive results with potentially ensuing invasive investigations, a psychological effect on patients that may result in reduction of statin use, and an increased cost to the health-care system. A recent study on patients' perceptions of statin therapy has demonstrated reduced adherence to statin therapy in those concerned about ADRs.32,33
Clinical Presentations of Statin-Induced Muscle Toxicity
Statin-induced muscle toxicity can present in many different ways, but the recognized phenotypes and degrees of severity are classified into seven different categories, as represented in Table 1 (statin-related myotoxicity (SRM) 0–SRM 6).
Muscle symptoms
Statin-induced muscle toxicity may present with a wide variety of symptoms, including fatigue, muscle pain, muscle weakness, muscle tenderness, and cramps. These symptoms are usually proximal and symmetrical but may be generalized. They can be aggravated by vigorous exercise and sometimes by addition of a new medication.2 Pain tolerance varies greatly in different individuals, and many studies have relied on self-reporting of muscle symptoms. If patients can tolerate mild muscle pain, statins are not usually discontinued (SRM 1, Table 1).
Plasma CK elevation
Asymptomatic serum CK elevations (SRM 0) and muscle pain without an increase in CK (SRM 1) have been the two most commonly (occurring in up to 33% of patients) described features of statin-induced toxicity.20,34,35 With the increasing use of electronic medical records, it may be possible to use CK elevations to identify patients with statin myotoxicity. However, because the use of CK as a screening test for muscle injury is not recommended, the identification of an isolated CK elevation in an electronic (or paper-based) record may indicate suspicion of the clinician of muscle toxicity, even if symptoms are variably recorded. CK levels are often used as a crude estimate of severity, but the correlation between muscle symptoms and CK levels is imperfect, and the clinical interpretation of CK levels is complex.36 There is considerable variability in the inclusion criteria and CK levels in the literature, particularly in genetic susceptibility studies. Some authors investigate patients with self-reported myalgia and CK levels from 1 to 3× the upper limit of normal (ULN) (SRM 2), whereas others apply more stringent criteria with CK elevations >4× (ref. 1) (SRM 3) or >10× the ULN (SRM 4) for myopathy and ≥50× the ULN for rhabdomyolysis (SRM 5),9,35,37,38,39,40,41,42 which are the criteria used in some recent industry-funded studies. To prevent inclusion of patients with CK elevations from causes other than statin myotoxicity, we have adopted the >4× the ULN for myopathy (SRM 3) and >10× the ULN for severe myopathy (SRM 4). Although these cutoff points have been commonly used in clinical trials and in several guidelines, they are somewhat arbitrary. However, future analyses based on these cutoff points (<4× the ULN, 4–10× the ULN, and >10× the ULN) will help in defining the sensitivity and specificity of different genetic markers, and provide a first step in future refinement of cutoff levels. Some investigators have also used alanine aminotransferase elevation to identify muscle injury;35 this may be of use when measured in combination with CK. Isolated elevation of alanine aminotransferase, in the absence of an increase in CK levels, should raise the suspicion of liver injury, which can also rarely occur with statins.43
Rhabdomyolysis
Rhabdomyolysis (SRM 5), the most serious ADR associated with statins,4,6,25,44,45 is characterized by muscle necrosis, release of myoglobin into the bloodstream, and sometimes acute renal failure.4,6,26,45,46 Muscle symptoms are accompanied by marked CK elevation, typically greater than 50× the ULN. Pigment nephropathy with brown urine is typically evident due to myoglobinuria and is consistent with serum creatinine elevation.7
HMGCR autoantibodies
A number of studies (e.g., ref.46) have reported the occurrence of an autoimmune-mediated necrotizing myopathy (SRM 6) after statin exposure, with symptoms that continued after drug withdrawal. Serum autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), the pharmacological target of statins, have been reported in patients with statin-induced autoimmune myopathy.47 Muscle biopsies can also be diagnostically useful for the detection of HMGCR expression on the cell surface of regenerating muscle fibers.47 In 51 patients with self-limited statin intolerance, anti-HMGCR antibodies were not observed in a single individual.48 This suggests that self-limited statin myopathy has a distinct biological mechanism from statin-induced autoimmune myopathy. In addition, atorvastatin and simvastatin promote a proinflammatory or Th1 response in activated peripheral blood mononuclear cells by increasing the number of interferon-γ-secreting T cells.49 It should be noted, however, that many autoimmune myopathy cases are likely to be excluded from studies because withdrawal of the statins does not alleviate symptoms and thus statins may be discounted as the causal agents for myopathy pathogenesis.
Time to Onset
In the PRIMO (Prediction of Muscular Risk in Observational Conditions) study, the median time to onset in the 832 of 7,924 patients who developed muscular symptoms was one month after statin initiation.50 A study of 45 patients reported a mean duration of statin therapy before myopathic symptoms of 6.3 months, with a maximum of 9.8 months.19
A large study, using a case-crossover design to determine statin myotoxicity in two primary-care databases comprising 93,831 patients, suggested that most cases occur within the first 12 weeks of statin exposure.21 The authors recommended a 26-week cutoff to enable fewer misclassifications of exposed cases.21 A 26-week cutoff seems sensible for those patients who have been on a stable dose of statin monotherapy; however, this may need to be extended. It is important to note that some patients can develop statin myotoxicity either after a statin dose increase or after the concomitant administration of an interacting drug, which may occur anytime during the life cycle of statin use. Furthermore, autoimmune myopathy may take longer to develop, with a time to onset as long as 3 years.51
Risk Factors
Concomitant medications
Fibrates. A significant body of evidence suggests an increased risk of statin myopathy, particularly rhabdomyolysis, in patients taking statins in combination with fibrates.4 Analysis of the US Food and Drug Administration Adverse Event Reporting System between 1998 and 2002 was used to determine reporting rates for rhabdomyolysis in patients taking fenofibrate and gemfibrozil in combination with statins.52 Gemfibrozil, a fibric acid derivative, increases systemic exposure to active simvastatin acid by inhibiting both glucuronidation and organic anion-transporting polypeptide (OATP)1B1 transporter–mediated uptake into the liver.53,54
Overall rates of rhabdomyolysis for any statin medication users coprescribed fenofibrate or gemfibrozil were 4.5 and 8.7 per million prescriptions, respectively. When stratified to those patients receiving cerivastatin only, the rates increased to 140 and 4,600 per million prescriptions with fenofibrate/cerivastatin and gemfibrozil/cerivastatin combination therapy, respectively.52 Although the effect of fibrate coadministration on statin bioavailability exists for the majority of statins,53,55,56,57 the increase in myopathy risk is especially pronounced with cerivastatin.5,58 Although cerivastatin was withdrawn in 2001, the identification of predisposing pharmacogenomics factors may still be of use for elucidating predisposing factors of muscle toxicity with the statins more commonly used nowadays.
CYP3A4 inhibition. CYP3A4 inhibitors such as azole antifungals, protease inhibitors, amiodarone, cyclosporine, calcium channel blockers, and macrolide antibiotics, to name a few, increase risk of myopathy for statins that undergo CYP3A4 metabolism (simvastatin, atorvastatin, and lovastatin).59,60,61,62 In addition, some foods such as grapefruit juice, which contains furanocoumarins, irreversibly inhibit CYP3A4 in the gut. The effect is reduced gut wall metabolism of statins (particularly simvastatin) and increased systemic exposure, which can lead to adverse effects.60,63
Comorbidities
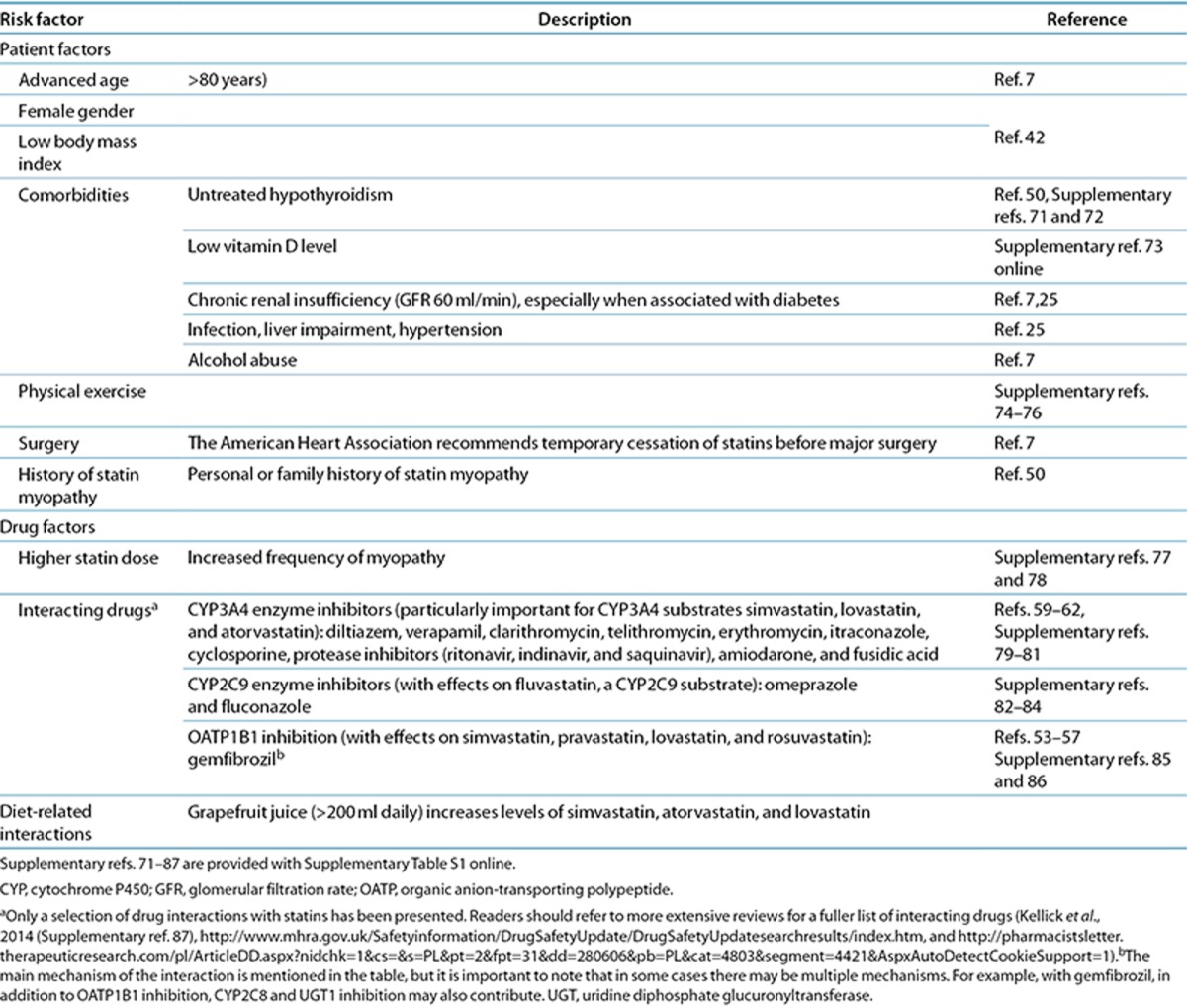
A list of comorbidities that are associated with an increased risk of developing statin-related myotoxicity is shown in Table 2. They include hypothyroidism, chronic renal insufficiency, infection, impaired liver function, hypertension, physical exertion, and diabetes.
Table 2. Reported risk factors for developing statin-induced myopathy.
Exercise
It is commonly believed that vigorous exercise increases the risk of statin-induced myopathy. Indeed, it is thought that myopathic symptoms may occur in 25% of statin users who exercise, as compared with a population incidence estimated at 1–5% for those who exercise but do not take statins.64 In professional athletes, it is estimated that as many as 75% of those taking statins may develop muscular symptoms;65 however, this may be overestimated.
Conclusions
Given the high prevalence of statin use worldwide and their significance in the prevention of cardiovascular and cerebrovascular disease, extensive research on the prediction and prevention of serious adverse effects such as statin-related myotoxicity is justified. Improved patient tolerability and adherence to statins is crucial because it reduces the incidence and the cost to any health-care system of treating cardiovascular disease. Large prospective studies of patient cohorts treated with statins are required to identify new genetic susceptibility biomarkers for statin-related myotoxicity that could be implemented into clinical practice. To date, one of the problems in comparing the results of observational studies on statin myotoxicity has been the lack of phenotype classification and standardization of nomenclature. In addition, given the rarity of the most severe phenotypes, a small sample size of several studies has hampered genetic biomarker discovery.
We have adapted a previously described consensus approach15 to define phenotypic criteria that can be used in an effort to standardize statin-related myotoxicity phenotypes using the numerical and descriptive nomenclature given in Table 1. Our standardization was based on expert opinion from a multidisciplinary group and the literature. The following are key criteria to be used in the deep phenotyping of patients with suspected statin-induced muscle injury:
A CK level that is >4× the ULN in the presence or absence of clinical symptoms for the definition of myopathy. We have adopted the >4× the ULN level pragmatically, as we feel, based on the literature, that this cutoff provides the right balance in preventing inclusion of patients with CK elevation due to normal variation or other causes, while simultaneously ensuring that we do not unnecessarily exclude valuable patients in studies investigating genetic factors predisposing to statin myotoxicity. A CK >10× the ULN should be categorized as rhabdomyolysis if accompanied by renal impairment. The adoption of the classification shown in Table 1 may help in more clearly defining the various phenotypes that are recruited in different studies. Papers reporting genetic factors predisposing to statin-induced muscle injury should show the effect size of the genetic polymorphism based on the degree of CK elevation, as has been done in recent studies.9,66
Clinical symptoms need to be carefully recorded in the patients; these should include not only the muscle symptoms but also whether there is any involvement of the kidneys, which might indicate rhabdomyolysis. Causality assessment as to whether the statin was responsible should include details of the temporal relationship between onset of statin use and the occurrence of myotoxicity, the effect of dechallenge, and the effect of any rechallenge. Dechallenge may not always be successful, for example, in patients with autoimmune myopathy, and should not necessarily be used to exclude statins as etiological agents.
Apart from CK, measurement of alanine aminotransferase, urine myoglobin levels (when clinically indicated), and renal function may be useful. In patients with a suspected autoimmune myopathy, the measurement of anti-HMGCR antibodies and muscle biopsy should be considered.
Predisposing factors, including interacting drugs, comorbidities, and exercise, should be evaluated in all patients. Factors such as trauma that lead to muscle injury irrespective of statin use need to be excluded.
The time to onset can be variable and can be delayed as long as 3 years in patients with autoimmune myopathy. However, in general, most cases of statin-induced myotoxicity occur within 6 months to 1 year of statin onset, an increase in the dose of the statin, or the concomitant administration of an interacting drug.
We have also developed an algorithm that will help assign phenotypes to individual patients based on clinical and biochemical parameters (Figure 1).
We hope the criteria described in this article will help clinicians and researchers to categorize phenotypes in patients with statin-related myotoxicity in order to facilitate research in this area.
Acknowledgments
This project has received funding from the European Union's Seventh Framework Programme for research, technological development, and demonstration under grant agreement 602108. This work was presented at the Phenotype Standardization Workshop, Liverpool, UK, 10 December 2013.
The authors declared no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Supplementary Material
References
- Law M., Rudnicka A.R. Statin safety: a systematic review. Am. J. Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Sathasivam S. Statin induced myotoxicity. Eur. J. Intern. Med. 2012;23:317–324. doi: 10.1016/j.ejim.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Stone N.J., et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Circulation 2013. e-pub ahead of print 12 November 2013.
- Graham D.J., et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- Staffa J.A., Chang J., Green L. Cerivastatin and reports of fatal rhabdomyolysis. N. Engl. J. Med. 2002;346:539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- Floyd J.S., Heckbert S.R., Weiss N.S., Carrell D.S., Psaty B.M. Use of administrative data to estimate the incidence of statin-related rhabdomyolysis. JAMA. 2012;307:1580–1582. doi: 10.1001/jama.2012.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak R.C., Smith S.C., Jr, Bairey-Merz C.N., Grundy S.M., Cleeman J.I., Lenfant C. American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002;106:1024–1028. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- Aithal G.P., Daly A.K. Preempting and preventing drug-induced liver injury. Nat. Genet. 2010;42:650–651. doi: 10.1038/ng0810-650. [DOI] [PubMed] [Google Scholar]
- Carr D.F., et al. SLCO1B1 genetic variant associated with statin-induced myopathy: a proof-of-concept study using the clinical practice research datalink. Clin. Pharmacol. Ther. 2013;94:695–701. doi: 10.1038/clpt.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Kusama M., Ono S., Sugiyama Y., Orii T., Akazawa M. Assessment of statin-associated muscle toxicity in Japan: a cohort study conducted using claims database and laboratory information. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmohamed M., Aithal G.P., Behr E., Daly A., Roden D. The phenotype standardization project: improving pharmacogenetic studies of serious adverse drug reactions. Clin. Pharmacol. Ther. 2011;89:784–785. doi: 10.1038/clpt.2011.30. [DOI] [PubMed] [Google Scholar]
- Sai K., et al. Development of a detection algorithm for statin-induced myopathy using electronic medical records. J. Clin. Pharm. Ther. 2013;38:230–235. doi: 10.1111/jcpt.12063. [DOI] [PubMed] [Google Scholar]
- Aithal G.P., et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- Behr E.R., et al. The International Serious Adverse Events Consortium (iSAEC) phenotype standardization project for drug-induced torsades de pointes. Eur. Heart J. 2013;34:1958–1963. doi: 10.1093/eurheartj/ehs172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmohamed M., et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin. Pharmacol. Ther. 2011;89:896–901. doi: 10.1038/clpt.2011.79. [DOI] [PubMed] [Google Scholar]
- Agbabiaka T.B., Savović J., Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31:21–37. doi: 10.2165/00002018-200831010-00003. [DOI] [PubMed] [Google Scholar]
- Cham S., Evans M.A., Denenberg J.O., Golomb B.A. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy. 2010;30:541–553. doi: 10.1592/phco.30.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos J.A., et al. Investigators Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- Hansen K.E., Hildebrand J.P., Ferguson E.E., Stein J.H. Outcomes in 45 patients with statin-associated myopathy. Arch. Intern. Med. 2005;165:2671–2676. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- Kashani A., et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- Molokhia M., McKeigue P., Curcin V., Majeed A. Statin induced myopathy and myalgia: time trend analysis and comparison of risk associated with statin class from 1991-2006. PLoS One. 2008;3:e2522. doi: 10.1371/journal.pone.0002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the U.S. Food and Drug Administration. Intern. Med. 2011;50:845–853. doi: 10.2169/internalmedicine.50.4484. [DOI] [PubMed] [Google Scholar]
- Pfeffer M.A., et al. Safety and tolerability of pravastatin in long-term clinical trials: prospective Pravastatin Pooling (PPP) Project. Circulation. 2002;105:2341–2346. doi: 10.1161/01.cir.0000017634.00171.24. [DOI] [PubMed] [Google Scholar]
- Ridker P.M., et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Cziraky M.J., et al. Risk of hospitalized rhabdomyolysis associated with lipid-lowering drugs in a real-world clinical setting. J. Clin. Lipidol. 2013;7:102–108. doi: 10.1016/j.jacl.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Gilad R., Lampl Y. Rhabdomyolysis induced by simvastatin and ketoconazole treatment. Clin. Neuropharmacol. 1999;22:295–297. [PubMed] [Google Scholar]
- Meriggioli M.N., Barboi A.C., Rowin J., Cochran E.J. HMG-CoA Reductase Inhibitor Myopathy: Clinical, Electrophysiological, and Pathologic Data in Five Patients. J. Clin. Neuromuscul. Dis. 2001;2:129–134. [PubMed] [Google Scholar]
- Phillips P.S., et al. Scripps Mercy Clinical Research Center Statin-associated myopathy with normal creatine kinase levels. Ann. Intern. Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- Pierce L.R., Wysowski D.K., Gross T.P. Myopathy and rhabdomyolysis associated with lovastatin-gemfibrozil combination therapy. JAMA. 1990;264:71–75. [PubMed] [Google Scholar]
- Hohenegger M. Drug induced rhabdomyolysis. Curr. Opin. Pharmacol. 2012;12:335–339. doi: 10.1016/j.coph.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhayany A., Mishaal R.A., Vinker S. Is there clinical benefit to routine enzyme testing of patients on statins. Expert Opin. Drug Saf. 2012;11:185–190. doi: 10.1517/14740338.2012.630659. [DOI] [PubMed] [Google Scholar]
- Fung E.C., Crook M.A. Statin myopathy: a lipid clinic experience on the tolerability of statin rechallenge. Cardiovasc. Ther. 2012;30:e212–e218. doi: 10.1111/j.1755-5922.2011.00267.x. [DOI] [PubMed] [Google Scholar]
- Lemstra M., Blackburn D., Crawley A., Fung R. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can. J. Cardiol. 2012;28:574–580. doi: 10.1016/j.cjca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Blaier O., Lishner M., Elis A. Managing statin-induced muscle toxicity in a lipid clinic. J. Clin. Pharm. Ther. 2011;36:336–341. doi: 10.1111/j.1365-2710.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Link E., et al. SEARCH Collaborative Group SLCO1B1 variants and statin-induced myopathy–a genomewide study. N. Engl. J. Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- Wilke R.A., et al. Clinical Pharmacogenomics Implementation Consortium (CPIC) The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin. Pharmacol. Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham L.R., et al. Differential effect of the rs4149056 variant in SLCO1B1 on myopathy associated with simvastatin and atorvastatin. Pharmacogenomics J. 2012;12:233–237. doi: 10.1038/tpj.2010.92. [DOI] [PubMed] [Google Scholar]
- Donnelly L.A., et al. Common nonsynonymous substitutions in SLCO1B1 predispose to statin intolerance in routinely treated individuals with type 2 diabetes: a go-DARTS study. Clin. Pharmacol. Ther. 2011;89:210–216. doi: 10.1038/clpt.2010.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly L.A., et al. Robust association of the LPA locus with low-density lipoprotein cholesterol lowering response to statin treatment in a meta-analysis of 30 467 individuals from both randomized control trials and observational studies and association with coronary artery disease outcome during statin treatment. Pharmacogenet. Genomics. 2013;23:518–525. doi: 10.1097/FPC.0b013e3283642fd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde R., Peng L., Desai M., Feldman D. The role of vitamin D and SLCO1B1*5 gene polymorphism in statin-associated myalgias. Dermatoendocrinol. 2010;2:77–84. doi: 10.4161/derm.2.2.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciante K.D., et al. Cerivastatin, genetic variants, and the risk of rhabdomyolysis. Pharmacogenet. Genomics. 2011;21:280–288. doi: 10.1097/FPC.0b013e328343dd7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voora D., et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J. Am. Coll. Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C., Tsai J., Szarek M., Luo D., Gibson E. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am. J. Cardiol. 2006;97:61–67. doi: 10.1016/j.amjcard.2005.07.108. [DOI] [PubMed] [Google Scholar]
- Antons K.A., Williams C.D., Baker S.K., Phillips P.S. Clinical perspectives of statin-induced rhabdomyolysis. Am. J. Med. 2006;119:400–409. doi: 10.1016/j.amjmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Black C., Jick H. Etiology and frequency of rhabdomyolysis. Pharmacotherapy. 2002;22:1524–1526. doi: 10.1592/phco.22.17.1524.34130. [DOI] [PubMed] [Google Scholar]
- Needham M., Fabian V., Knezevic W., Panegyres P., Zilko P., Mastaglia F.L. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul. Disord. 2007;17:194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Mammen A.L., et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen A.L., et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res. (Hoboken) 2012;64:269–272. doi: 10.1002/acr.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward W.R., Marei A., Yang A., Vasa-Nicotera M.M., Chow S.C. Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of caspase-1 and IL-18 secretion in monocytes. J. Immunol. 2006;176:5284–5292. doi: 10.4049/jimmunol.176.9.5284. [DOI] [PubMed] [Google Scholar]
- Bruckert E., Hayem G., Dejager S., Yau C., Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc. Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- Grable-Esposito P., Katzberg H.D., Greenberg S.A., Srinivasan J., Katz J., Amato A.A. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41:185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- Jones P.H., Davidson M.H. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am. J. Cardiol. 2005;95:120–122. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- Backman J.T., Kyrklund C., Kivistö K.T., Wang J.S., Neuvonen P.J. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin. Pharmacol. Ther. 2000;68:122–129. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- Shitara Y., Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Kyrklund C., Backman J.T., Kivistö K.T., Neuvonen M., Laitila J., Neuvonen P.J. Plasma concentrations of active lovastatin acid are markedly increased by gemfibrozil but not by bezafibrate. Clin. Pharmacol. Ther. 2001;69:340–345. doi: 10.1067/mcp.2001.115542. [DOI] [PubMed] [Google Scholar]
- Kyrklund C., Backman J.T., Neuvonen M., Neuvonen P.J. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin. Pharmacol. Ther. 2003;73:538–544. doi: 10.1016/S0009-9236(03)00052-3. [DOI] [PubMed] [Google Scholar]
- Schneck D.W., et al. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin. Pharmacol. Ther. 2004;75:455–463. doi: 10.1016/j.clpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Ozdemir O., Boran M., Gökçe V., Uzun Y., Koçak B., Korkmaz S. A case with severe rhabdomyolysis and renal failure associated with cerivastatin-gemfibrozil combination therapy–a case report. Angiology. 2000;51:695–697. [PubMed] [Google Scholar]
- Ray G.M. Antiretroviral and statin drug-drug interactions. Cardiol. Rev. 2009;17:44–47. doi: 10.1097/CRD.0b013e3181903b7f. [DOI] [PubMed] [Google Scholar]
- Sorokin A.V., Duncan B., Panetta R., Thompson P.D. Rhabdomyolysis associated with pomegranate juice consumption. Am. J. Cardiol. 2006;98:705–706. doi: 10.1016/j.amjcard.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Zhou S., Chan E., Li X., Huang M. Clinical outcomes and management of mechanism-based inhibition of cytochrome P450 3A4. Ther. Clin. Risk Manag. 2005;1:3–13. doi: 10.2147/tcrm.1.1.3.53600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 2005;44:279–304. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]
- Mazokopakis E.E. Unusual causes of rhabdomyolysis. Intern. Med. J. 2008;38:364–367. doi: 10.1111/j.1445-5994.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- Dirks A.J., Jones K.M. Statin-induced apoptosis and skeletal myopathy. Am. J. Physiol. Cell Physiol. 2006;291:C1208–C1212. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- Sinzinger H., O'Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br. J. Clin. Pharmacol. 2004;57:525–528. doi: 10.1111/j.1365-2125.2004.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara H., et al. Electronic health records for biological sample collection: feasibility study of statin-induced myopathy using the Clinical Practice Research Datalink. Br. J. Clin. Pharmacol. 2014;77:831–838. doi: 10.1111/bcp.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays H. Statin safety: an overview and assessment of the data--2005. Am. J. Cardiol. 2006;97:6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Moghadasian M.H., Mancini G.B., Frohlich J.J. Pharmacotherapy of hypercholesterolaemia: statins in clinical practice. Expert Opin. Pharmacother. 2000;1:683–695. doi: 10.1517/14656566.1.4.683. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial Lancet 3607–22.2002. 12114036 [Google Scholar]
- Mohassel P., Mammen A.L. Statin-associated autoimmune myopathy and anti-HMGCR autoantibodies. Muscle Nerve. 2013;48:477–483. doi: 10.1002/mus.23854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.