Abstract
Angioedema is a potentially life-threatening adverse reaction to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. To study the genetic etiology of this rare adverse event, international consortia and multicenter recruitment of patients are needed. To reduce patient heterogeneity, we have standardized the phenotype. In brief, it comprises swelling in the head and neck region that first occurs during treatment. It should not coincide with urticaria or have another likely cause such as hereditary angioedema.
Agents acting on the angiotensin system are commonly used for the treatment of hypertension and heart failure. Angiotensin-converting enzyme (ACE) inhibitors reduce the production of angiotensin II, whereas angiotensin receptor blocker (ARB) drugs inhibit the binding of angiotensin II to the angiotensin II type 1 receptor.1 ACE inhibitors have been on the market since the early 1980s, and more than 40 million patients are treated worldwide.2 ARBs were first licensed in the mid-1990s and are steadily increasing their market share: In 2013, 7% of the Swedish population was treated with an ACE inhibitor and 6% was treated with an ARB.3 ACE inhibitors and ARBs are safe and effective in the majority of patients, but some patients can develop adverse drug reactions (ADRs). For instance, persistent dry cough occurs in about 9% of patients treated with an ACE inhibitor and in 2% treated with an ARB.4 More rarely, patients can develop angioedema affecting the head and neck region; this occurs in 0.1–0.7% of those treated with an ACE inhibitor and in 0.1% of those treated with an ARB.5,6,7
Consensus Process
In order to study the genetic etiology of rare ADRs, international consortia and multicenter recruitment are needed.8 Angioedema of the head and neck region induced by ACE inhibitors or ARBs is being investigated by the international consortium PREDICTION-ADR, which was funded by the European Union's Seventh Framework Programme in 2013. To facilitate multicenter recruitment of patients, a meeting was organized to standardize the phenotype of angioedema induced by agents acting on the angiotensin system. A panel of invited experts comprising clinical and basic pharmacologists, internists, immunologists and clinical chemistry scientists, allergists, oto-rhino-laryngology specialists, regulatory agency representatives, and managers of electronic medical record databases convened in Liverpool on 10 December 2013.
Description of the Phenotype
Angioedema, also known as angioneurotic edema or Quincke's edema, is a transient, localized, potentially life-threatening swelling of the deep reticular dermis, subcutaneous or submucosal tissues, and occasionally the viscera.9 It is caused by vasodilation and increased endothelial permeability, leading to extravasation of fluid into the interstitial compartment.10,11,12,13 Angioedema can be either hereditary or acquired. Diagnosis of angioedema induced by drugs acting on the angiotensin system is based on the case history and clinical features, and other causes of angioedema need to be excluded.14 Supplementary Table S1 online describes the clinical and demographic data recommended to be collected for each patient.
Pathophysiological mechanism
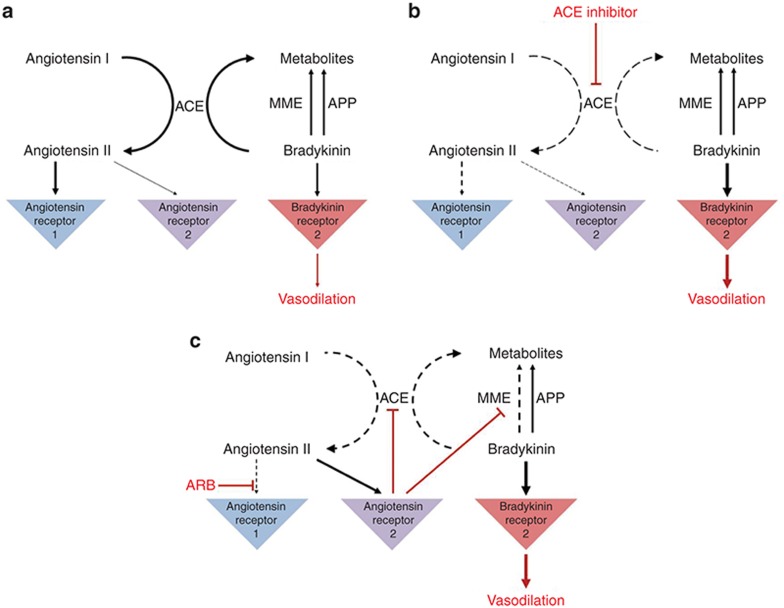
Angioedema induced by ACE inhibitors is thought to be mediated by bradykinin and other vasodilating molecules, but the precise pathophysiology has not been elucidated.13,14,15 ACE has two active sites that are able to generate angiotensin II from angiotensin I and degrade bradykinin to inactive metabolites (Figure 1a).14 Inhibition of ACE will thus decrease both the formation of angiotensin II and the degradation of bradykinin (Figure 1b). Alternative bradykinin inactivating pathways usually step in, but if these pathways are deficient, bradykinin accumulates.15 Genetic variants in these pathways could explain why only a minority of patients develop angioedema.16 Variants have been identified in the first intron of the membrane metallo-endopeptidase gene (MME) and upstream of the X-prolyl aminopeptidase 2 gene (XPNPEP2) that encodes aminopeptidase P (APP).17,18,19,20 However, for the majority of patients, the predisposing factors are unknown.
Figure 1.
Theoretical effect of agents acting on the angiotensin system. (a) A simplified scheme of the angiotensin–bradykinin pathways.1,14 (b) Mechanism of action of angiotensin-converting enzyme (ACE) inhibitors.1,14 ACE inhibitors inhibit two pathways: the formation of angiotensin II from angiotensin I, and the degradation of bradykinin into inactive peptides. Aminopeptidase P (APP) and membrane metallo-endopeptidase (MME) are alternative pathways to inactivate bradykinin. Accumulation of bradykinin may contribute to the development of angioedema. (c) Mechanism of action of angiotensin II receptor type 1 blockers (ARBs).1,14,22 ARBs block the type 1 receptor site for angiotensin II, which instead may bind to the type 2 receptor. Stimulation of the type 2 binding site results in a bradykinin cascade that inhibits ACE and metallo-endopeptidase (MME), which can lead to increased bradykinin levels. Bradykinin contributes to the therapeutic action of ARBs and may also contribute to the development of angioedema.
The pathophysiological mechanism underlying angioedema during ARB treatment is even more unclear (Figure 1c).21 ARBs have no direct effect on ACE or bradykinin breakdown but have been shown to increase bradykinin levels in hypertensive humans, possibly through indirect inhibition of ACE and metallo-endopeptidase.22 It is assumed that by blocking the angiotensin type 1 receptor, more circulating angiotensin II is made available for binding to the type 2 receptor.1 This, in turn, inhibits ACE and metallo-endopeptidase, which can result in increased bradykinin levels.14,22
Incidence and onset
ACE inhibitor treatment is the single most common cause of angioedema in the head and neck region in adults,11,23 accounting for ~30% of angioedema cases in emergency departments.24,25 Angioedema generally occurs within the first 3 months of therapy, but symptoms may appear after many years of treatment.6,7,26 During the first year, the cumulative incidence of angioedema per 1,000 persons was 1.79 (95% confidence interval (CI), 1.73–1.85), with the incidence rate per 1,000 person-years being 4.38 (95% CI, 4.24–4.54) based on an analysis of 1,845,138 patients initiating ACE inhibitor treatment.27 If the use of an ACE inhibitor is continued after an incident, the rate of recurrent angioedema increases to 187 per 1,000 person-years, with, on average, 11 months to recurrence, according to a study covering 51,752 person-years of ACE inhibitor use.28 After drug withdrawal, the tendency to develop angioedema usually abates but may persist for months or even years.29,30 It is difficult to rationalize how such episodes can occur after stopping the ACE inhibitor, and indeed this is not in accordance with the usual criteria for causality assessment for acutely occurring ADRs. It is possible that the episodes that occur after stopping the ACE inhibitor reflect exacerbation of an intrinsic underlying susceptibility to angioedema, the etiology of which is unclear.
Angioedema during ARB treatment is more infrequent.7,21 During the first year, the cumulative incidence of angioedema per 1,000 person-years was 0.62 (95% CI, 0.55–0.69), with the incidence rate per 1,000 person-years being 1.66 (95% CI, 1.47–1.86) in 467,313 patients initiating ARB treatment.27 ARBs are usually well tolerated in patients with previous general ACE inhibitor intolerance.31 However, patients who have experienced ACE inhibitor angioedema have been known to develop angioedema when switched to an ARB.21,32,33,34 Among such patients, the rate of angioedema during ARB treatment is ~4%, according to two meta-analyses, suggesting that a previous episode of ACE inhibitor angioedema can predispose patients to ARB angioedema.31,34
Clinical presentation
Angioedema induced by an ACE inhibitor develops in areas of loose connective tissue over about 4–6 h and usually resolves in 24–48 h.35 The face, lips, tongue, floor of the mouth, and upper airways are most often affected, and the swelling may be asymmetrical.13,21,25 Symptoms such as a lump in the throat, hoarse voice, and difficulties in swallowing and breathing are signs of impending airway obstruction.13,15 ACE inhibitor–induced angioedema rarely presents with urticaria or swelling outside the head and neck region, although gastrointestinal and genital angioedema have been reported.21,36,37 There are no obvious differences in symptoms of angioedema induced by ACE inhibitors and ARBs.26 Supplementary Table S2 online describes further information to be requested with regard to the reaction.
Risk factors
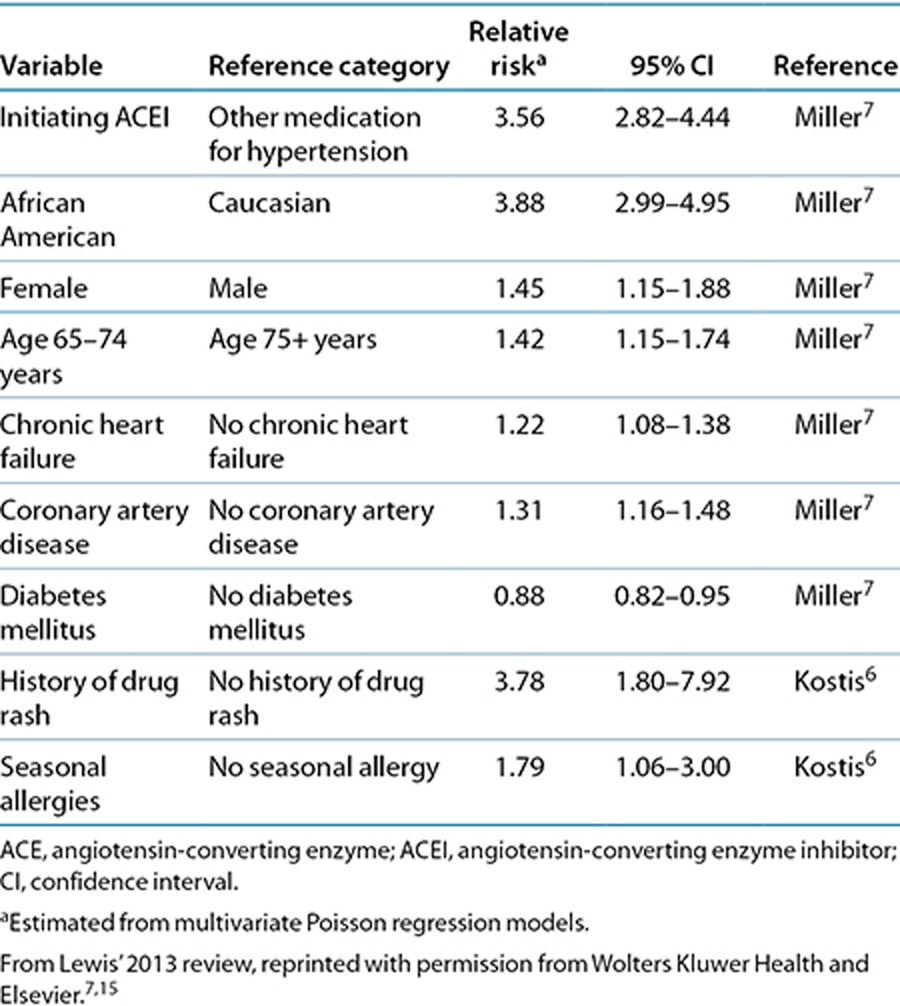
Minor local trauma, surgical procedures, and dental treatment may trigger angioedema during ACE inhibitor treatment.13,33,38 Smoking and a history of ACE inhibitor–induced cough have also been identified as risk factors.39 Other risk factors include female gender, African-American ethnicity, chronic heart failure, coronary artery disease, and a history of drug rash and seasonal allergies (Table 1).6,7,15 Due to the rarity of angioedema induced by ARBs, knowledge about risk factors is poor.40 Neither sex nor age has been identified as a risk factor.27
Table 1. Relative risk estimates for angioedema in patients taking ACE inhibitors as compared with reference category.
Differential Diagnosis
Other causes of angioedema can be divided by their pathophysiology into (i) hereditary or acquired, mediated by bradykinin or vasoactive molecules; (ii) allergic or pseudoallergic, dependent on direct mast cell degranulation; and (iii) idiopathic angioedema.11,13,14,15,21,41,42
Angioedema mediated by bradykinin or vasoactive molecules
Bradykinin-mediated angioedema is characterized by painless swelling without urticaria that responds to treatment with the selective bradykinin B2 receptor antagonist icatibant.15,43 Bradykinin-mediated angioedema is not associated with urticaria or other manifestations of histamine release such as hypotension, wheezing, or anaphylaxis.42,44
Hereditary angioedema. This is a rare condition with a prevalence of 1 in 50,000.10,11,14 Patients with hereditary angioedema may present with abdominal pain, obstruction of the upper airways, and swelling of the hands and feet in addition to angioedema at other sites.11,15 They do not experience urticaria but may present a transient creeping (serpiginous) skin rash at the leading edge of the angiodematous area.10 Hereditary angioedema should be suspected in young adults with a family history of angioedema.14 There are three subtypes of hereditary angioedema.11,14,15 Both types I and II are caused by mutations in the C1 inhibitor gene serpin peptidase inhibitor clade G member 1 (SERPING1) on chromosome 11.14 Type I is characterized by low circulating levels of C1-inhibitor (C1-INH) and represents 85% of cases. Type II has a functional deficiency of C1-INH, but normal levels, and accounts for 15% of cases. A rare type III is caused by mutations in the coagulation factor XII gene (F12) on chromosome 5.11 It is found in women with normal levels and function of C1-INH, and is thought to be induced by estrogen.
Acquired angioedema. This is an uncommon form of C1-INH deficiency that is usually associated with B-cell lymphoproliferative diseases such as lymphoma and myeloma, and occasionally with connective tissue diseases such as systemic lupus erythematosus.11,14,15 Like hereditary angioedema, it is characterized by low complement C4 and reduced C1-INH levels, and in some patients, autoantibodies against C1q have been detected.11,14
Angioedema dependent on mast cell degranulation
Mast cell degranulation results in the release of histamine and other inflammatory mediators that increase vascular permeability and lead to angioedema, which is often associated with urticaria and pruritus.10,11,12 Angioedema dependent on mast cell degranulation can be divided into allergic and pseudoallergic types.11,15,21 It usually responds to treatment with antihistamines, corticosteroids, and epinephrine.11,15 Elevated levels of histamine and serum tryptase are sometimes found in this type of angioedema when accompanied by systemic features (anaphylaxis).12,44,45
Allergic angioedema. This is the result of immunoglobulin E (IgE)-mediated mast cell activation and degranulation.12,15,21 IgE-mediated reactions are triggered by specific allergens, and previous exposure is required, but this may be covert. Symptoms do not appear in the absence of the allergens such as food, latex, insect stings, and drugs. IgE-mediated angioedema usually subsides within 24 h, but relapses are common and unpredictable.10 Typical localization is to the face, particularly the lips and periorbital area; extremities; and genitals.11 Severe cases manifest with respiratory tract symptoms with rhinitis, wheezing, or stridor, and cardiovascular involvement with tachycardia and hypotension that may evolve into anaphylactic shock.10,12,45
Pseudoallergic angioedema. This mimics an allergic reaction but is not mediated by IgE.15 It can be caused by direct mast cell degranulation due to, for example, intravenous radiocontrast media and opiates.15 Nonsteroidal anti-inflammatory drugs can induce both allergic and more commonly nonallergic angioedema.21 When nonallergic, the mechanism is thought to be mast cell activation that is mediated through the inhibition of cyclooxygenase pathways, resulting in leukotriene synthesis.21,46
Idiopathic angioedema
Idiopathic angioedema has, by definition, no known pathophysiology.15,42 It is often associated with urticaria and appears to be triggered by physical stimuli (heat, cold, pressure, and severe trauma) as well as by infection and autoimmune processes.10,15,42 Some cases of idiopathic angioedema respond to antihistamines and leukotriene receptor antagonists, suggesting a histamine-mediated pathway, whereas others do not, and treatment is largely empiric.42
Concluding Remarks
The adverse event angioedema occurring in the head and neck region and induced by agents acting on the angiotensin system is being investigated by the international consortium PREDICTION-ADR. To enable multicenter recruitment of patients, the phenotype was standardized through a consensus procedure involving discussions with an expert panel and the drafting of this article.
The clinical criteria presented in Table 2 can be used both for ACE inhibitor–induced angioedema and for the rarer condition of ARB angioedema. The main feature is symptomatic swelling in the head and neck region, clinically judged to be angioedema during treatment with an ACE inhibitor or ARB. There are reports of angioedema recurring after cessation of treatment, the mechanism of which is unknown. If a follow-up of patients is made after the initial event, we would recommend that any recurrence of the angioedema is noted. This will enable a subgroup analysis of these patients in order to identify distinct genetic predisposing factors.
Table 2. Phenotype definition for angioedema induced by an ACE inhibitor or ARB.
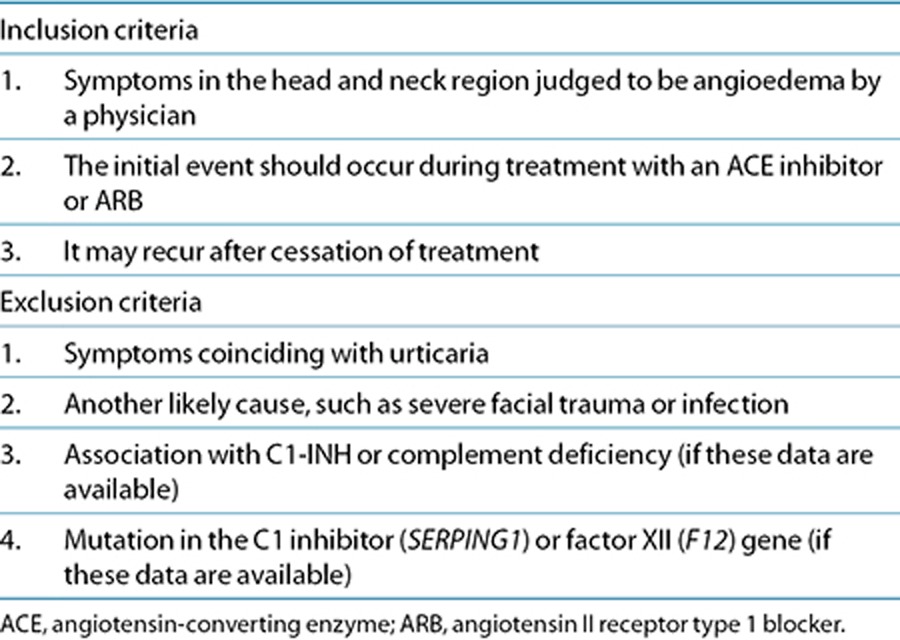
Patients recruited should not have another likely cause such as severe facial trauma or infection, as seen in idiopathic angioedema (Supplementary Table S1 online). The angioedema should not co-occur with urticaria, which is more suggestive of angioedema due to mast cell degranulation (Supplementary Table S2 online). Patients with a known hereditary angioedema should be excluded. However, when an ACE inhibitor or ARB angioedema is suspected, it is not obligatory to analyze for C1-INH or complement factor deficiency or to genotype for C1 inhibitor or factor XII mutations. Should coding mutations in these genes be present in angioedema cases in PREDICTION-ADR, they will be caught during exome sequencing.
We hope this guidance will be of use to others studying angioedema induced by ACE inhibitors or ARBs, and invite researchers and consortia interested in collaborating with regard to this rare, but potentially dangerous, ADR.
Acknowledgments
The authors acknowledge the critical input of members of the International Consortium for the Personalisation of Treatment in Cardiovascular Disease Through Next-Generation Sequencing in Adverse Drug Reactions (PREDICTION-ADR), funded by the European Union's Seventh Framework Programme for research, technological development, and demonstration under grant agreement no 602108. M.W. is supported by the Swedish Research Council (Medicine 523-2008-5568), the Swedish Heart and Lung Foundation, and the Clinical Research Support (ALF) at Uppsala University. M.P. is supported by the UK Department of Health (National Health Service chair of Pharmacogenetics), Medical Research Council Center for Drug Safety Science, and the Serious Adverse Event Consortium and is a National Institute for Health Research senior investigator.
The authors declared no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Supplementary Material
References
- Weir M.R., Henrich W.L. Theoretical basis and clinical evidence for differential effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor subtype 1 blockers. Curr. Opin. Nephrol. Hypertens. 2000;9:403–411. doi: 10.1097/00041552-200007000-00012. [DOI] [PubMed] [Google Scholar]
- Sánchez-Borges M., González-Aveledo L.A. Angiotensin-converting enzyme inhibitors and angioedema. Allergy. Asthma Immunol. Res. 2010;2:195–198. doi: 10.4168/aair.2010.2.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Board of Health and Welfare. Swedish Prescribed Drug Register . < http://www.socialstyrelsen.se/statistik/statistikdatabas/lakemedel > ( 2013
- Powers B.J., et al. Updated report on comparative effectiveness of ACE inhibitors, ARBs, and direct renin inhibitors for patients with essential hypertension: much more data, little new information. J. Gen. Intern. Med. 2012;27:716–729. doi: 10.1007/s11606-011-1938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israili Z.H., Hall W.D. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann. Intern. Med. 1992;117:234–242. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- Kostis J.B., et al. Incidence and characteristics of angioedema associated with enalapril. Arch. Intern. Med. 2005;165:1637–1642. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- Miller D.R., Oliveria S.A., Berlowitz D.R., Fincke B.G., Stang P., Lillienfeld D.E. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51:1624–1630. doi: 10.1161/HYPERTENSIONAHA.108.110270. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M., Aithal G.P., Behr E., Daly A., Roden D. The phenotype standardization project: improving pharmacogenetic studies of serious adverse drug reactions. Clin. Pharmacol. Ther. 2011;89:784–785. doi: 10.1038/clpt.2011.30. [DOI] [PubMed] [Google Scholar]
- Borzova E., Grattan C.E.H.ngioedema Urticaria and Angioedemaeds. Zuberbier T., Grattan C.E.H., Maurer M.117–127.Springer Berlin, Germany; 2010 [Google Scholar]
- Kaplan A.P., Greaves M.W. Angioedema. J. Am. Acad. Dermatol. 2005;53:373–388; quiz 389. doi: 10.1016/j.jaad.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Kaplan A.P. Angioedema. World Allergy Organ. J. 2008;1:103–113. doi: 10.1097/WOX.0b013e31817aecbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiganesh T., et al. Acute angioedema: recognition and management in the emergency department. Eur. J. Emerg. Med. 2013;20:10–17. doi: 10.1097/MEJ.0b013e328356f76e. [DOI] [PubMed] [Google Scholar]
- Rasmussen E.R., Mey K., Bygum A. Angiotensin-converting enzyme inhibitor-induced angioedema–a dangerous new epidemic. Acta Derm. Venereol. 2014;94:260–264. doi: 10.2340/00015555-1760. [DOI] [PubMed] [Google Scholar]
- Bas M., Adams V., Suvorava T., Niehues T., Hoffmann T.K., Kojda G. Nonallergic angioedema: role of bradykinin. Allergy. 2007;62:842–856. doi: 10.1111/j.1398-9995.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- Lewis L.M. Angioedema: etiology, pathophysiology, current and emerging therapies. J. Emerg. Med. 2013;45:789–796. doi: 10.1016/j.jemermed.2013.03.045. [DOI] [PubMed] [Google Scholar]
- Mahmoudpour S.H., et al. Pharmacogenetics of ACE inhibitor-induced angioedema and cough: a systematic review and meta-analysis. Pharmacogenomics. 2013;14:249–260. doi: 10.2217/pgs.12.206. [DOI] [PubMed] [Google Scholar]
- Duan Q.L., et al. A variant in XPNPEP2 is associated with angioedema induced by angiotensin I-converting enzyme inhibitors. Am. J. Hum. Genet. 2005;77:617–626. doi: 10.1086/496899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard-Grice A.V., Lucisano A.C., Byrd J.B., Stone E.R., Simmons W.H., Brown N.J. Sex-dependent and race-dependent association of XPNPEP2 C-2399A polymorphism with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet. Genomics. 2010;20:532–536. doi: 10.1097/FPC.0b013e32833d3acb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia La Corte A.L., et al. A functional XPNPEP2 promoter haplotype leads to reduced plasma aminopeptidase P and increased risk of ACE inhibitor-induced angioedema. Hum. Mutat. 2011;32:1326–1331. doi: 10.1002/humu.21579. [DOI] [PubMed] [Google Scholar]
- Pare G., et al. Genetic variants associated with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet. Genomics. 2013;23:470–478. doi: 10.1097/FPC.0b013e328363c137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata N. Recent advances in drug-induced angioedema. Allergol. Int. 2012;61:545–557. doi: 10.2332/allergolint.12-RAI-0493. [DOI] [PubMed] [Google Scholar]
- Campbell D.J., Krum H., Esler M.D. Losartan increases bradykinin levels in hypertensive humans. Circulation. 2005;111:315–320. doi: 10.1161/01.CIR.0000153269.07762.3B. [DOI] [PubMed] [Google Scholar]
- Hoover T., Lippmann M., Grouzmann E., Marceau F., Herscu P. Angiotensin converting enzyme inhibitor induced angio-oedema: a review of the pathophysiology and risk factors. Clin. Exp. Allergy. 2010;40:50–61. doi: 10.1111/j.1365-2222.2009.03323.x. [DOI] [PubMed] [Google Scholar]
- Banerji A., Clark S., Blanda M., LoVecchio F., Snyder B., Camargo C.A., Jr Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann. Allergy Asthma Immunol. 2008;100:327–332. doi: 10.1016/S1081-1206(10)60594-7. [DOI] [PubMed] [Google Scholar]
- Bluestein H.M., Hoover T.A., Banerji A.S., Camargo C.A., Jr, Reshef A., Herscu P. Angiotensin-converting enzyme inhibitor-induced angioedema in a community hospital emergency department. Ann. Allergy Asthma Immunol. 2009;103:502–507. doi: 10.1016/S1081-1206(10)60267-0. [DOI] [PubMed] [Google Scholar]
- Malde B., Regalado J., Greenberger P.A. Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ann. Allergy Asthma Immunol. 2007;98:57–63. doi: 10.1016/S1081-1206(10)60860-5. [DOI] [PubMed] [Google Scholar]
- Toh S., et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch. Intern. Med. 2012;172:1582–1589. doi: 10.1001/2013.jamainternmed.34. [DOI] [PubMed] [Google Scholar]
- Brown N.J., Snowden M., Griffin M.R. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA. 1997;278:232–233. doi: 10.1001/jama.278.3.232. [DOI] [PubMed] [Google Scholar]
- Beltrami L., Zanichelli A., Zingale L., Vacchini R., Carugo S., Cicardi M. Long-term follow-up of 111 patients with angiotensin-converting enzyme inhibitor-related angioedema. J. Hypertens. 2011;29:2273–2277. doi: 10.1097/HJH.0b013e32834b4b9b. [DOI] [PubMed] [Google Scholar]
- Fitzharris P., Jordan A. Investigating recurrent angio-oedema. BMJ. 2011;343:d6607. doi: 10.1136/bmj.d6607. [DOI] [PubMed] [Google Scholar]
- Caldeira D., David C., Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am. J. Cardiovasc. Drugs. 2012;12:263–277. doi: 10.1007/BF03261835. [DOI] [PubMed] [Google Scholar]
- Howes L.G., Tran D. Can angiotensin receptor antagonists be used safely in patients with previous ACE inhibitor-induced angioedema. Drug Saf. 2002;25:73–76. doi: 10.2165/00002018-200225020-00001. [DOI] [PubMed] [Google Scholar]
- Grant N.N., Deeb Z.E., Chia S.H. Clinical experience with angiotensin-converting enzyme inhibitor-induced angioedema. Otolaryngol. Head. Neck Surg. 2007;137:931–935. doi: 10.1016/j.otohns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Haymore B.R., Yoon J., Mikita C.P., Klote M.M., DeZee K.J. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann. Allergy Asthma Immunol. 2008;101:495–499. doi: 10.1016/S1081-1206(10)60288-8. [DOI] [PubMed] [Google Scholar]
- Andrew N., Gabb G., Del Fante M. ACEI associated angioedema - a case study and review. Aust. Fam. Physician. 2011;40:985–988. [PubMed] [Google Scholar]
- Miller D.G., Sweis R.T., Toerne T.S. Penile angioedema developing after 3 years of ACEI therapy. J. Emerg. Med. 2012;43:273–275. doi: 10.1016/j.jemermed.2011.05.102. [DOI] [PubMed] [Google Scholar]
- Benson B.C., Smith C., Laczek J.T. Angiotensin converting enzyme inhibitor-induced gastrointestinal angioedema: a case series and literature review. J. Clin. Gastroenterol. 2013;47:844–849. doi: 10.1097/MCG.0b013e318299c69d. [DOI] [PubMed] [Google Scholar]
- Wakefield Y.S., Theaker E.D., Pemberton M.N. Angiotensin converting enzyme inhibitors and delayed onset, recurrent angioedema of the head and neck. Br. Dent. J. 2008;205:553–556. doi: 10.1038/sj.bdj.2008.982. [DOI] [PubMed] [Google Scholar]
- Morimoto T., et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J. Eval. Clin. Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- Shino M., Takahashi K., Murata T., Iida H., Yasuoka Y., Furuya N. Angiotensin II receptor blocker-induced angioedema in the oral floor and epiglottis. Am. J. Otolaryngol. 2011;32:624–626. doi: 10.1016/j.amjoto.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Powell R.J., et al. British Society for Allergy and Clinical Immunology (BSACI) BSACI guidelines for the management of chronic urticaria and angio-oedema. Clin. Exp. Allergy. 2007;37:631–650. doi: 10.1111/j.1365-2222.2007.02678.x. [DOI] [PubMed] [Google Scholar]
- Sher J., Davis-Lorton M. Angioedema with normal laboratory values: the next step. Curr. Allergy Asthma Rep. 2013;13:563–570. doi: 10.1007/s11882-013-0383-7. [DOI] [PubMed] [Google Scholar]
- Colás C., Montoiro R., Fraj J., Garcés M., Cubero J.L., Caballero T. Nonhistaminergic idiopathic angioedema: clinical response to icatibant. J. Investig. Allergol. Clin. Immunol. 2012;22:520–521. [PubMed] [Google Scholar]
- Lin R.Y., et al. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J. Allergy Clin. Immunol. 2000;106:65–71. doi: 10.1067/mai.2000.107600. [DOI] [PubMed] [Google Scholar]
- Rutkowski K., Dua S., Nasser S. Anaphylaxis: current state of knowledge for the modern physician. Postgrad. Med. J. 2012;88:458–464. doi: 10.1136/postgradmedj-2011-130634. [DOI] [PubMed] [Google Scholar]
- Sánchez-Borges M., Capriles-Hulett A., Caballero-Fonseca F. The multiple faces of nonsteroidal antiinflammatory drug hypersensitivity. J. Investig. Allergol. Clin. Immunol. 2004;14:329–334. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.