Abstract
Background
The goal of this study was to assess the rates of recurrence in the neck for node-positive patients with HPV-associated oropharynx cancer treated with definitive radiation (with or without chemotherapy).
Methods
This is a single institutional retrospective study. Methodology included database search, and statistical testing including frequency analysis, Kaplan-Meier tests, and comparative tests including chi-square, logistic regression and log-rank.
Results
The cohort consisted of 401 node-positive patients irradiated between 2006 – June 2012. Three hundred eighty eight patients had CT restaging, and 251 had PET and/or US as a component of their post radiation staging. Eighty patients (20%) underwent neck dissection, and 21 (26%) had a positive specimen. The rate of neck dissection increased with increasing nodal stage, and was lower in patients who had PET scans or ultrasound in addition to CT restaging. The median follow-up was 30 months. The 2-year actuarial neck recurrence rate was 7% and 5% in all patients and those with local control, respectively. Nodal recurrence rates were greater in current smokers (p=.008). There was no difference in nodal recurrences rates in patients who did or did not have a neck dissection (p = .4)
Conclusions
A treatment strategy of (chemo)radiation with neck dissection performed based on response resulted in high rates of regional disease control in patients with HPV-associated oropharyngeal cancer.
Keywords: Human papilloma virus (HPV), neck dissection, oropharyngeal cancer, IMRT
Introduction
The management of the neck in patients with head and neck cancer has been a topic of controversy for many years.1 Historically, patients who were irradiated with intact nodal disease were treated with a “planned” neck dissection following their radiotherapy. This concept was espoused due to a relatively high rate of neck recurrences in patients treated with radiation alone, and the belief that clinical response was inadequate to determine the efficacy of treatment.2 Imaging was ultimately incorporated into restaging patients, and the predictive value of CT imaging3 and subsequently PET imaging4 in determining if a patient has residual disease has shifted the neck management paradigm from one that routinely incorporates neck dissection to a policy of performing a neck dissection only in patients whose imaging suggests a possibility of viable residual disease.
In addition to improvements in imaging to predict if disease still remains, treatments have been intensified with the incorporation of chemotherapy leading to higher response and disease control rates.5, 6 As an example, Argiris et al. demonstrated that in their patients with head and neck cancer treated with induction chemotherapy in addition to concurrent chemotherapy, there was a reduction in the nodal positivity rate in neck dissection specimens.7 These favorable results with varying strategies of treatment intensification have been accompanied by the observation that more patients with head and neck cancer are presenting with human papilloma virus (HPV) associated disease.8 Several studies have demonstrated that patients with HPV associated head and neck cancer (and specifically oropharyngeal cancer) have a disease that is more chemo- and radio-sensitive, and disease control rates for patients with HPV associated oropharyngeal cancer is significantly better than that seen in patients with oropharyngeal cancer not associated with HPV.9, 10 Thus, improvements in imaging to detect viable disease, more intense treatment regimens, and a disease that is more sensitive to therapy have led many to eliminate a planned neck dissection from the overall treatment algorithm.
Despite the shift in management of the neck in node-positive patients towards one that uses surgery only in those with a high suspicion of residual post radiation disease, few have focused on this strategy in patients with HPV-associated disease, a disease that presents with a very high percentage of node positivity. We therefore performed this study to assess outcomes of patients with HPV-associated oropharyngeal cancer who were managed with radiation (with or without chemotherapy) and neck dissection reserved for patients in whom a concern remained for a high probability of residual disease.
Methods
The database maintained by the Department of Radiation Oncology at The University of Texas M.D. Anderson Cancer Center was searched to identify patients treated with intensity-modulated radiation therapy (IMRT) for oropharyngeal carcinoma (squamous cell, poorly differentiated or undifferentiated, or not otherwise specified) irradiated between January, 2006 – June, 2012. Our institutional review board granted permission to conduct this retrospective study.
The search was further narrowed to exclude patients for the following reasons: distant metastases or concurrent malignancies (exclusive of a second malignancy of the oropharynx) at the time of diagnosis, a previously treated malignancy of the head and neck or previous radiation to the head or neck, a history of any malignancy (excluding non-melanomatous skin cancer) within two years of diagnosis, or treatment with chemotherapy prior to staging at MDACC.
Further inclusion criteria were then applied. The principal inclusion criterion was that the pathologic specimen was tested for either HPV or p16, and a positive result was obtained for one or the other. The second inclusion criterion was that the patient had lymphadenopathy at the time of diagnosis. Patients who had either excisional biopsies or a neck dissection and presented for their radiation (+/− chemotherapy) without macroscopic disease in the neck were excluded.
Medical records were reviewed to assess patients’ demographic, clinical, radiologic and pathologic data. Based upon the medical history at presentation and as described previously,11 patients were classified as current smokers, former smokers, or never-smokers.
Patients’ disease was staged according to the AJCC 2002 staging system.12 Charts were reviewed to verify tumor size and sites of invasion. Staging variables of interest included T-category, N-category, and overall AJCC group stage. Patients staged Tx were typically those seen post-tonsillectomy and if the tumor size could not be determined after record review, these patients were staged T1 for the purpose of AJCC stage grouping in this analysis.
The overall treatment strategies were individualized for each patient. General treatment strategies were recommended at our multidisciplinary tumor board, though specifics of treatment were carried out by the patients’ treatment team. Our general philosophy during the years of this study was to use concurrent chemotherapy for patients with bulky disease, principally primary site disease (large T2 – T4 disease). Induction therapy was recommended based on nodal status, particularly for patients with multiple nodes and nodal disease in the lower levels of the neck. Selected patients with either prohibitive comorbidities, or non-bulky disease with low T and N stages were treated with radiation alone despite nominally having advanced AJCC group staging.
Our approach to managing the node positive neck in patients with head and neck cancer has been described previously.13 Decisions for neck dissection are deferred till treatment response is assessed clinically and radiographically. Infrequently a planned neck dissection is decided upon prior to initiating radiation. This occurs in the uncommon situation in which a patient presents with bulky adenopathy in level 4 where the nodal disease is in close proximity to the brachial plexus. If necessary, we will accept 66 Gy maximal dose to the brachial plexus, and will treat subcentimeter nodes to this dose, but will recommend a planned neck dissection for bulkier disease in this location.
All patients were irradiated with IMRT. The methodology of treatment delivery, planning including target definition, and dose and fractionation schedules have been described.11 The majority of patients were treated with once-daily fractionation to doses of 66 – 70 Gy, at 2.2 – 2.12 Gy per fraction. Occasionally an accelerated schedule delivering 70 – 72 Gy in 6 weeks with some twice daily fractionation was used. For those treated to 66 Gy to the primary tumor, consideration for a 2–4 Gy boost (with electrons) to the lymph nodes was made on an individual basis.
Chi-squared tests were used to compare proportions between subsets. Binary logistic regression was used to test the relationship between continuous variables and binary responses. The Kaplan-Meier method was used to calculate actuarial curves. Time of diagnosis was used as time zero. Patients without disease recurrence at the site of interest were censored at last follow-up. Comparisons between survival curves were made using the log-rank test. Multivariate analysis was performed using the Cox proportional model.
Results
Patients, Disease and Treatment Characteristics
Patient and tumor characteristics for the 401 patients who met the inclusion criteria are shown in Table 1. Radiation doses ranged from 29 – 74 Gy (median, 70 Gy). Only 4 patients received < 66 Gy; one patient died during treatment, and 3 discontinued their treatment due to toxicity.
Table 1.
Patient and Disease Characteristics
| AllN=401 | Post-XRT Neck Dissection N=80 (20%) | Post-XRT Neck Dissection Positivea N=21 (26%) | ||
|---|---|---|---|---|
| Patient characteristics | ||||
| Sex | Male | 352 (88%) | 72 (90%) | 20 (28%) |
| Age at diagnosis, y | Mean (range) | 57.9 (36–84) | 57.1 (38–84) | 60 (44–84) |
| Smoker | Current | 76 (19%) | 15 (20%) | 4 (27%) |
| Former | 128 (32%) | 25 (31%) | 6 (24%) | |
| Never | 197 (49%) | 40 (49%) | 11 (28%) | |
| Disease characteristics | ||||
| Primary site | Tonsil | 202 (50%) | 35 (44%) | 9 (26%) |
| Base of tongue | 192 (48%) | 43 (53%) | 11 (25%) | |
| Other | 7 (2%) | 2 (3%) | 1 (50%) | |
| T classification | 1 | 121 (30%) | 29 (36%) | 8 (28%) |
| 2 | 159 (40%) | 33 (41%) | 7 (21%) | |
| 3 | 68 (17%) | 10 (13%) | 3 (30%) | |
| 4 | 53 (13%) | 8 (10%) | 3 (30%) | |
| N classification | 1 | 41 (11%) | 4 (5%) | 0 (0%) |
| 2a | 28 (7%) | 11 (10%) | 4 (36%) | |
| 2b | 218 (54%) | 43 (54%) | 10 (23%) | |
| 2c | 87 (21%) | 14 (17%) | 5 (36%) | |
| 3 | 27 (7%) | 8 (10%) | 2 (25%) | |
| Size of largest lymph node, cm | ≥3 | 134 (33%) | 33 (42%) | 9 (27%) |
| Lowest neck level involved | 2 | 214 (53%) | 44 (55%) | 8 (18%) |
| 3 | 140 (35%) | 25 (31%) | 7 (28%) | |
| 4 | 47 (12%) | 11 (14%) | 6 (55%) | |
| Treatment | ||||
| Chemotherapy | None | 47 (12%) | 12 (15%) | 2 (17%) |
| CC | 130 (32%) | 23 (28%) | 6 (26%) | |
| IC | 87 (22%) | 18 (22%) | 3 (17%) | |
| IC+CC | 137 (34%) | 27 (36%) | 10 (37%) | |
| Concurrent drugs | None | 134 (33%) | 30 (38%) | 5 (17%) |
| Cisplatin | 99 (25%) | 13 (16%) | 4 (31%) | |
| Carboplatin | 54 (14%) | 12 (15%) | 2 (17%) | |
| Cetuximab | 93 (23%) | 20 (25%) | 9 (45%) | |
| Other | 22 (5%) | 5 (6%) | 1 (20%) | |
Abbreviations: CC, concurrent chemoradiation; IC, induction chemotherapy; XRT, radiotherapya Percentage is of patients who underwent post-XRT neck dissection
Neoadjuvant chemotherapy was delivered to 224 patients. All patients received regimens that were platin and taxane based. The use of induction therapy was associated with neck stage, size of largest node and lowest neck level (all p < .001). Only 5 patients (7%) with N1-2A received induction therapy. Ninety-two percent of patients with level 4 disease had induction compared to 76% and 35% of patients whose lowest nodal levels were level 3 and 2, respectively. Chemotherapy was administered concurrent with radiation in 267 patients. A singlet of cisplatin, carboplatin or cetuximab was used in 246 patients. Eight additional patients had a singlet, but had their agent changed due to toxicity. Eight patients were treated on a protocol using vandetanib, 3 were treated with docetaxel, and 2 were treated on study with both cetuximab and cisplatin. The use of concurrent chemotherapy was not associated (p > 0.1) with nodal stage, size of largest node, or lowest neck node.
Restaging Post Radiation
Initial restaging was performed with CT in 353 patients (88%), PET/CT in 18 patients (4%), and both contrast CT and PET/CT in 25 patients (6%). Four patients were not restaged, and 1 was reassessed outside MDACC and these data was not available. Initial restaging was performed 5 – 182 days following the completion of radiation (mean, 58.4 days, median, 54 days)
CT tomography was used as a component of restaging within 6 months of completion of radiation in 388 patients. The range of days from completion of radiation to the first restaging CT scan was 5 – 182 days (median, 53 days). CT restaging was the only mode of reimaging in 139 patients (35%). PET restaging was performed 40 – 175 days (median, 91 days) after the completion of radiation in 199 patients, and 126 patients had ultrasound of the neck 34 – 180 days (median, 87 days) following radiation. Ultrasonography was accompanied by fine needle aspiration in 98 patients, and a positive specimen was obtained in 10 patients. Sixty-six patients had all 3 imaging modalities. There was no association between the use of additional imaging (to CT) neither with initial nodal staging nor with the mode of systemic therapy.
Neck Dissection
A neck dissection was performed in 80 (20%) of the 401 patients. Only one of these 80 patients had surgery on the primary tumor in addition to a neck dissection. Four patients had a planned neck dissection as part of the overall treatment strategy. The performance of neck dissection was associated with initial nodal staging (p = .021), as a neck dissection was performed in 10% of N1 patients, 21% of N2 patients, and 30% of N3 patients. While associated with nodal stage, there was no association of frequency of neck dissection with nodal levels involved, even taking into account the 4 planned dissections that were done because of concerns of high radiation dose to level 4 (and the brachial plexus). Neck dissection was performed in 27% of patients restaged with CT only compared to 17% of patients that had additional imaging (p=.002). Neck dissection was related to the time of initial restaging (p=.032), as the median number of days from completion of therapy to restaging was 48.5 days for those having a neck dissection compared with 54 days for those who did not have a neck dissection.
Positive specimens (viable tumor) were found in 21 (26%) of the 80 patients who underwent a post-radiation neck dissection. Nodal size (largest node greater than or less than 3cm), nodal stage and the chemotherapy mode were not associated with the finding of positive disease.
Outcomes
The median followup time was 30 months (range 2 – 76 months) for all patients, and 33 months (range 4 – 76 months) for surviving patients. Fifty-two surviving patients (15%) had less than two- year followup, and only 4 of these patients had less than one-year followup.
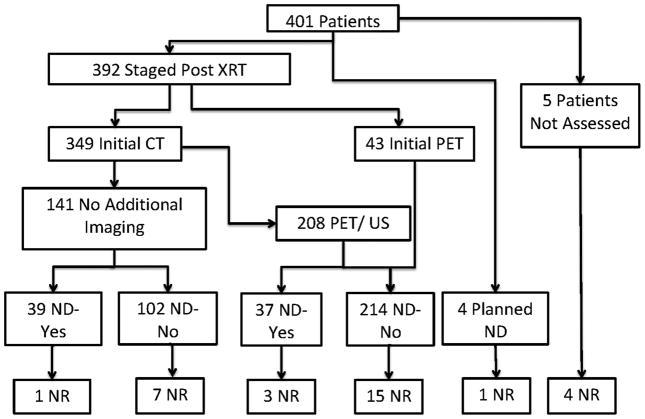
Thirty-one patients (8%) had disease recur in the neck. Figures 1 charts the patients from entry, through initial restaging, surgical management and neck control. The actuarial 2- and 5-year nodal control rates were 93% and 90%, respectively. Nineteen of 378 patients (5%) with primary site control had neck recurrence with actuarial 2- and 5-year nodal control rates of 95% and 94%, respectively. Initial nodal stage, size or level were not associated with recurrence. Neither chemotherapy mode (including none, p = .11) nor neck dissection (p = .4) were associated with recurrence. Four of the nineteen patients with isolated neck recurrence had a neck dissection; 2 of the 4 patients had positive neck dissection specimens, and 2 had negative specimens. There was an association (p= .008) with smoking status, as the 2-year nodal control rates for current, former and never smokers were 86%, 94% and 95%, respectively.
Figure 1.
The nineteen patients with primary control and neck recurrence included 4 patients who had post-radiation neck dissections. All 4 had recurrences in the dissected neck. Among the remaining 15 patients who had neck recurrences without neck surgery, 10 had recurrences in the epicenter of the principal dominant node. One of these patients had persistent disease, but toxicity and comorbidities did not allow for neck dissection. Four patients presented with recurrence in sites that were irradiated to subclinical doses (50 – 63 Gy), as these sites were felt to be at risk but did not harbor disease. These sites included, level 4 (and lung), level 1b, retropharynx and contralateral neck. One patient had recurrence in an unirradiated and initially node negative contralateral neck.
Discussion
The principle goal of this study was to assess a strategy of neck dissection in patients with HPV-associated oropharyngeal cancer only for those who are believed to have residual disease following radiation. This strategy was followed in over 99% of over 400 patients, and the rate of disease recurrence in the neck was 8%, and only 5% if the primary tumor was controlled. Not surprisingly, the incidence of neck dissection post-radiation did increase with tumor bulk (as defined by N-category), but the overall incidence even for patients with N3 disease was 30%.
One observation is that compared with our prior experience of oropharyngeal cancer patients treated with a similar strategy between 1996 – 2004,13 the incidence of neck dissection post radiation has decreased from 32% to 19%. Statistical testing of nodal staging differences between the 2 experiences was not performed; however, 72% of our former patients had stage N2b or greater compared with 82% of the current cohort. The use of differing modes of integration of chemotherapy between this historic cohort and the current study population were comparable, thus it is unlikely that treatment factors impacted the neck dissection rate. In all likelihood, the favorable response rate of HPV-associated tumors is the explanation for a lower rate of neck dissection. A second hypothesis for the decrease in incidence may be the increase in post therapy imaging with PET/CT and ultrasound complementing CT. We did note a decrease in the neck dissection rate of patients who had additional imaging to complement CT. However, this observation could be biased in that the data from the CT scans may have been sufficiently compelling to decide on neck dissection while additional imaging could have been obtained in cases where the decision for neck dissection was equivocal. Further, in a prospective study of post treatment PET/CT scan we concluded that in patients with HPV-associated disease the gains of PET/CT in addition to conventional CT were small.14
A third possibility is the timing of reimaging may impact the use of neck dissection. Historically, neck dissection was recommended for residual disease 6 to 8 weeks following treatment.15 Liauw et al. describe basing their decision for neck dissection on the findings of CT scans obtained 30 days following radiation.3 The concept of delaying neck dissection till after PET was obtained 10 to 12 weeks following treatment16 was initially met with concerns, but the preponderance of data regarding the use of PET scans post therapy has obviated those fears.17 Our data suggests a relationship between the timing of imaging and the performance of a neck dissection. HPV-associated adenopathy often presents with a cystic, inflammatory component, and progression of nodal size during therapy has been noted to occur,18 often due to an increase in this inflammatory component rather than tumor growth. Thus it may take longer for these nodes to regress19 and our current surveillance program attempts to obtain initial imaging no less than 8 weeks post radiation.
The patterns of disease recurrence raise further questions regarding the value of a uniform practice of neck dissection post radiation. Ten of the 31 patients with nodal recurrence either had or were unable to have neck dissection. Among the remaining 21, 7 had primary recurrence, 1 had distant disease, and 5 had disease recur in areas of the neck that would unlikely have been included in a selective neck dissection. Thus, only 9 (2%) may have benefited from a neck dissection.
While our results for patients with HPV-associated disease support not performing a neck dissection in over 80% of patients based on their response, integration of neck dissection for these patients remains controversial. Specifically, the addition of neck dissection is being espoused in programs of treatment de-intensification advocating a decrease in radiation dose by 5 to 10% combined with neck dissection may be better from a toxicity standpoint than 66 – 70 Gy (often with concurrent chemotherapy).20 Frank et al. describe a limited experience of planned neck dissection in all their patients, but limiting the radiation dose to 60Gy.21 Controls rates were excellent and short-term toxicity was low, but longer followup has not been reported. More commonly, advocates of minimally invasive surgery for the primary tumor are combining this with neck dissection.22 Infrequently, a patient with minimal disease can forgo radiation, but the vast majority of patients with HPV-associated disease are node positive, and will have either extracapsular disease or multiple nodes that require radiation,22 so it remains moot if this approach has a lower toxicity profile.
In conclusion we describe an experience with a large cohort of patients with HPV-associated, node positive oropharyngeal cancer who are treated with radiation (and often chemotherapy) and have neck dissections performed based of response. The overall control rate of disease in the neck, and the patterns of recurrence support this approach. The neck dissection rate was only 20%, and there was a suggestion that complementary imaging in addition to CT scan, and the timing of the initial restaging imaging may have impacted this relatively low rate, though the relative radiosensitivity of HPV-associated disease was likely contributory as well.
Based on our experiences, we have attempted to formalize the post-radiation followup schedule. Currently we see patients for their first post-therapy tumor assessment at 8–10 weeks with a contrast CT scan. Complementary imaging (ultrasound and/or PET ) are obtained 4–6 weeks later if a nodal remnant of concern is present on contrast CT, and decisions regarding operating or placing the patient on a routine surveillance program are made at that time.
References
- 1.Ferlito A, Corry J, Silver CE, Shaha AR, Thomas Robbins K, Rinaldo A. Planned neck dissection for patients with complete response to chemoradiotherapy: a concept approaching obsolescence. Head Neck. 2010;32(2):253–61. doi: 10.1002/hed.21173. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Million RR, Cassisi NJ. Squamous cell carcinoma of the head and neck treated with radiation therapy: the role of neck dissection for clinically positive neck nodes. Int J Radiat Oncol Biol Phys. 1986;12(5):733–40. doi: 10.1016/0360-3016(86)90030-1. [DOI] [PubMed] [Google Scholar]
- 3.Liauw SL, Mancuso AA, Amdur RJ, et al. Postradiotherapy neck dissection for lymph node-positive head and neck cancer: the use of computed tomography to manage the neck. J Clin Oncol. 2006;24(9):1421–7. doi: 10.1200/JCO.2005.04.6052. [DOI] [PubMed] [Google Scholar]
- 4.Schoder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med. 2009;50 (Suppl 1):74S–88S. doi: 10.2967/jnumed.108.057208. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Leopold KA, Fisher SR, et al. A phase I/II trial of twice daily irradiation and concurrent chemotherapy for locally advanced squamous cell carcinoma of the head and neck. International Journal of Radiation Oncology, Biology and Physics. 1994;28:213– 20. doi: 10.1016/0360-3016(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 6.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. Journal of Clinical Oncology. 2003;21(1):92– 98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Argiris A, Stenson KM, Brockstein BE, et al. Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck. 2004;26(5):447–55. doi: 10.1002/hed.10394. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the united states. J Clin Oncol. 2008;26:612–19. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New England Journal of Medicine. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02. 02 phase III trial. J Clin Oncol. 2010;28(27):4142–8. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garden AS, Dong L, Morrison WH, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(4):941–7. doi: 10.1016/j.ijrobp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Greene F, Page D, Fleming I, et al., editors. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. pp. 33–57. [Google Scholar]
- 13.Thariat J, Ang KK, Allen PK, et al. Prediction of neck dissection requirement after definitive radiotherapy for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(3):e367–74. doi: 10.1016/j.ijrobp.2011.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moeller BJ, Rana V, Cannon BA, et al. Prospective risk-adjusted [18F]Fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol. 2009;27(15):2509–15. doi: 10.1200/JCO.2008.19.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher GH, Jesse RH, Jr, Lindberg RD, Westbrook KC. Neck nodes. In: Fletcher GH, editor. Textbook of Radiotherapy. Philadelphia: Lea & Febiger; 1980. pp. 249–71. [Google Scholar]
- 16.Yao M, Smith RB, Graham MM, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2005;63(4):991–9. doi: 10.1016/j.ijrobp.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 17.Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33(3):210–22. doi: 10.1111/j.1749-4486.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 18.Sanguineti G, Ricchetti F, Wu B, et al. Volumetric change of human papillomavirus-related neck lymph nodes before, during, and shortly after intensity-modulated radiation therapy. Head Neck. 2012;34(11):1640–7. doi: 10.1002/hed.21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SH, O’Sullivan B, Xu W, et al. Temporal Nodal Regression and Regional Control After Primary Radiation Therapy for N2–N3 Head-and-Neck Cancer Stratified by HPV Status. Int J Radiat Oncol Biol Phys. 2013;87(5):1078–85. doi: 10.1016/j.ijrobp.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Adelstein DJ, Ridge JA, Brizel DM, et al. Transoral resection of pharyngeal cancer: summary of a National Cancer Institute Head and Neck Cancer Steering Committee Clinical Trials Planning Meeting, November 6–7, 2011, Arlington, Virginia. Head Neck. 2012;34(12):1681–703. doi: 10.1002/hed.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank DK, Hu KS, Culliney BE, et al. Planned neck dissection after concomitant radiochemotherapy for advanced head and neck cancer. Laryngoscope. 2005;115(6):1015–20. doi: 10.1097/01.MLG.0000162648.37638.76. [DOI] [PubMed] [Google Scholar]
- 22.Sinha P, Lewis JS, Jr, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118(14):3519–30. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]