Abstract
The identification, in the adult, of cardiomyocyte turnover events and of cardiac progenitor cells (CPCs) has revolutionized the field of cardiovascular medicine. However, the low rate of CPCs differentiation events reported both in vitro and in vivo, even after injury, raised concerns on the biological significance of these subsets. In this Comprehensive Review, we discuss the current understanding of cardiac Lin−Sca-1+ cells in light of what is also known for cellular compartments with similar phenotypes in other organs. The Lin−Sca-1+ heart subset is heterogeneous and displays a mesenchymal profile, characterized by a limited ability to generate cardiomyocytes in vitro and in vivo, even after injury. There is no evidence for Sca-1 expression in embryonic cardiovascular progenitors. In other organs, Sca-1 expression is mainly observed on mesoderm-derived cells, although it is not restricted to stem/progenitor cell populations. It is urgent to determine, at a single cell level, to which extent cardiac Lin−Sca-1+ cells overlap with the fibroblast compartment.
Introduction
Cardiomyocyte (CM) replacement in the adult heart, via the expansion of pre-existing CMs and/or the differentiation of endogenous progenitors, has been extensively discussed [1,2]. The renewal rate and the physiologic conditions that trigger adult CM formation, and therefore its functional relevance, are not consensual.
The modest figures for human CMs renewal derived from the resourceful work by Bergman et al., 1% per annum at the age of 25 and 0.45% at the age 75 [3], contrast with the much higher rates observed in a study enrolling patients submitted to radiotherapy [4]. Likewise, mouse CMs turnover was estimated to reach values of ∼1.3%–4% per year [5].
Recent groundbreaking experiments showed that a massive CMs loss, secondary to experimental heart injury (mechanical and ischemic), inflicted during the first 6 days of life is fully restored in a process that we would designate as myocardial re-genesis. However, starting at day 7 postbirth, this capacity is lost and, like in the adult heart, a fibrotic scar is formed [6,7]. The loss of regenerative capacity coincides with postnatal maturation and cell-cycle arrest of CMs [8,9], suggesting that the identification of the underlying regulators is crucial to unlock the boundaries of cardiac regeneration-repair mechanisms.
In the current state of knowledge it is possible that heart regeneration is more complex than the intrinsic (cell-autonomous) capacity of any cell subset to expand and/or differentiate into functional elements. The reciprocal modulation of cells and of the embedding extracellular matrix (ECM) might be a major process governing regeneration and/or repair of the damaged tissue, at different stages of postnatal life. Having said this, it should also be noted that the apparent loss of regenerative capacity in the adult heart cannot be used as an argument to contest the existence of adult cardiac progenitor cells (CPCs), in the same manner that limited adult neurogenesis is not invoked to refute the recognized stem cell activity in some territories of the central nervous system [10]. What are then the major concerns in the stem cell biology field when discussing the properties of CPCs? What is the basis for the heated dispute concerning the nature and function of the adult CPCs?
This Comprehensive Review critically revisits several aspects of CPC's biology, debating in particular the CPC-subsets that express the stem cell associated-marker stem cell antigen-1 (Sca-1). Sca-1+ CPCs display a mesenchymal phenotype, have limited cardiogenic potential, and are able to improve cardiac remodeling following myocardial infarction (MI) mainly by paracrine mechanisms. The analysis of other organs containing mesodermal derivatives with similar phenotype (Lin−Sca-1+) highlighted the possible fibroblastic nature of this compartment and stressed the need to clarify the eventual overlapping of Sca-1+ CPCs with other Lin−Sca-1+ stromal cells of the heart.
How Have Heart Resident Stem/Progenitor Cells Been Defined?
The possibility that CMs can be generated outside the boundaries of the developing heart emerged back in the 1990s from the identification of interstitial cells displaying stem cell-like properties in adult mammalian heart [11]. Since then, self-renewing, multipotent, and clonogenic cardiac cells, capable of differentiation, in vitro and in vivo, into CMs and cells of the vasculature [endothelial cells (ECs) and smooth muscle cells (SMCs)] were reported by several authors and grouped under the designation of CPCs [12]. CPCs have been isolated based on the expression of surface markers (eg, Sca-1 and c-Kit), on functional properties, such as the ability to efflux dyes (eg, rhodamine and Hoechst 33342)—side population (SP) [13–17], and on the capacity to migrate out of cardiac explants and grow as 3D multicellular clusters—termed cardiospheres (CSs) [18,19].
The strategy to isolate CPCs by the expression of c-Kit [20,21] and Sca-1 [22–25], two surface proteins also present on hematopoietic stem cells (HSCs), coincided with claims that adult hematopoietic progenitors could, under certain conditions, generate cells affiliated with different tissues (neurons and muscle, among others) [26,27]. Despite subsequent evidence that unmanipulated HSCs can only regenerate the hematopoietic system, the notion that c-Kit and Sca-1 could be useful markers to identify the adult stem/progenitor compartment in multiple tissues persisted in the scientific community. In the heart, this strategy led to the identification of an apparent multiplicity of CPC subsets (Table 1). It is not clear whether these various CPC subsets represent distinct and perhaps transient physiological states of a single cell type or belong instead to unrelated lineages [28,29]. The unknown developmental origin of CPCs has also entrenched doubts on whether they constitute remnants of bona fide embryonic cardiovascular progenitors [30–32], circulating cells from the bone marrow [33–35], or simply derive from the surrounding vasculature [25,36]. The multipotency of CPCs has been also questioned because most reports rely on poorly defined protocols of cardiomyogenic differentiation, that is, cellular exposure to demethylating agents [25,37,38] or co-culture with CMs [14,15,18,37,39]; and on a minimalistic verification of cell differentiation based on the upregulated expression of a single lineage-affiliated, but not lineage-restricted, protein [16,22,24,25,40]. Additionally, CPCs on their own are clearly not able to functionally restore the heart in response to an extensive cardiac injury [12,28]. Nevertheless, they have been viewed as the most promising target for cell-based therapies by authors who favor CPCs as innately prone to generate heart tissue and to respond to cardiac molecular cues [41]. Based on this rationale, at least two clinical trials were established in which c-Kit-expressing cells (SCIPIO) [42] and CS-derived Sca-1+ cells (CADUCEUS) [43] have been transplanted in an autologous setting. Short-term MRI assessment indicates reduction of scar size, increased healthy heart muscle mass, and attenuated adverse remodeling.
Table 1.
Schematic Representation of the Heart as Composed of a Mosaic of Different Progenitor Cell-Populations Identified in the Myocardium
| In vitro differentiation | In vivo differentiation | |||||||
|---|---|---|---|---|---|---|---|---|
| Ref. | Phenotype | CM | EC | SMC | CM | EC | SMC | Clonogenic |
| [25] | Sca-1+ Ftl-1− Flk-1− CD31+ CD34− CD45− | 4.6% | nd | nd | <1%a | nd | nd | nd |
| [22] | Sca-1+ c-Kit− CD34− CD31− CD45− CD90+ CD105+ CD29+ CD44+ CD106+ CD73+ CD13+ | 1.24% | 12.4% | 31.9% | 100b | 80b | 120b | + |
| [23] | Sca-1+ PDGFRα+ CD31− CD45− CD90+ CD105+ CD29+ CD44+ Flk-1− | + | + | + | + | + | + | + |
| [24] | Sca-1+ c-Kit− CD34− CD31− CD45− CD29+ | <1% | ∼4.5% | ∼30% | + | + | nd | + |
| [39] | Sca-1+ PDGFRα+ CD44+ CD105+ CD29+ CD31− CD45− Flk-1− c-Kit− CD34− CD31− CD45− CD90− | + | ∼1.75% | ∼27% | ∼9c | ∼4c | ∼41c | + |
| Side population | ||||||||
| [13] | Sca-1+ c-Kit− Flk-2− CD34− Thy1.1− | nd | nd | nd | nd | nd | nd | + |
| [14] | Sca-1+ Abcg2+ c-Kitlow CD45low CD34low CD31− | + | nd | nd | nd | nd | nd | nd |
| [15] | Sca-1+ c-Kit− CD31− CD45− CD44− CD34− | + | nd | nd | nd | nd | nd | + |
| [16] | CD29+CD31+/− CD45+/− | 5.5% | nd | nd | 4.4% | 6.7% | 29% | nd |
| [17] | Sca-1+ VE-cadherin− CD34+ CD73+ CD90+ CD105+ CD45− | + | nd | nd | nd | nd | nd | nd |
| Epicardial-derived progenitors | ||||||||
| [92] | Sca-1+ c-Kit− CD31− Flk-1− | nd | nd | nd | + | nd | nd | nd |
| Sphere formation (cardiospheres) | ||||||||
| [18] | Sca-1+ c-Kit+ CD31+ CD34+ Flk-1+ | + | + | + | + | + | + | + |
| [19] | Sca-1+ c-Kit− CD31− CD34− CD45− CD29low CD133− Flk-1− | ∼60% | ∼20% | ∼34% | ∼10% | ∼10% | ∼10% | + |
| c-Kit+ cells | ||||||||
| [20] | c-Kit+ CD45− CD34− CD20− CD45RO− CD8− Ter119− | + | + | + | + | + | + | + |
| [21] | c-Kit+ CD29+ CD44+ CD105+ CD90+ | + | + | + | nd | nd | nd | nd |
A variety of procedures have been adopted to isolate and purify a cardiac progenitor cells panoply, either based on the expression of the stemness-associated surface markers Sca-1 and c-Kit, the ability to efflux dyes (eg, rhodamine and Hoechst 33342)—SP, to grow as 3D multicellular clusters—CSs or by the expression of a specific genetic marker—Wt-1 epicardial progenitors. The isolated population have been subjected to phenotypic characterization and their multipotency, that is, the ability to differentiate in CMs, ECs, and SMCs, has been tested in vitro and/or in vivo, as well as the clonogenic potential. A concise summary of these results is displayed with indication of whether differentiation into a specific cell type was observed (+) in vitro and/or in vivo or if it was nd in the specific study.
Percentage of diffentiated myocytes.
Cells/mm2.
Cells/heart section.
CMs, cardiomyocytes; CSs, cardiospheres; ECs, endothelial cells; nd, not determined; Sca-1, stem cell antigen-1; SMC, smooth muscle cell; SP, side population.
Despite extensive reports on this particular topic, the existence of a prototypical stem/progenitor cell(s) entity in the adult mammalian myocardium is a concept that is far from being consensual.
What Is Known About the Role and the Cardiovascular Potential of Sca-1+ CPCs?
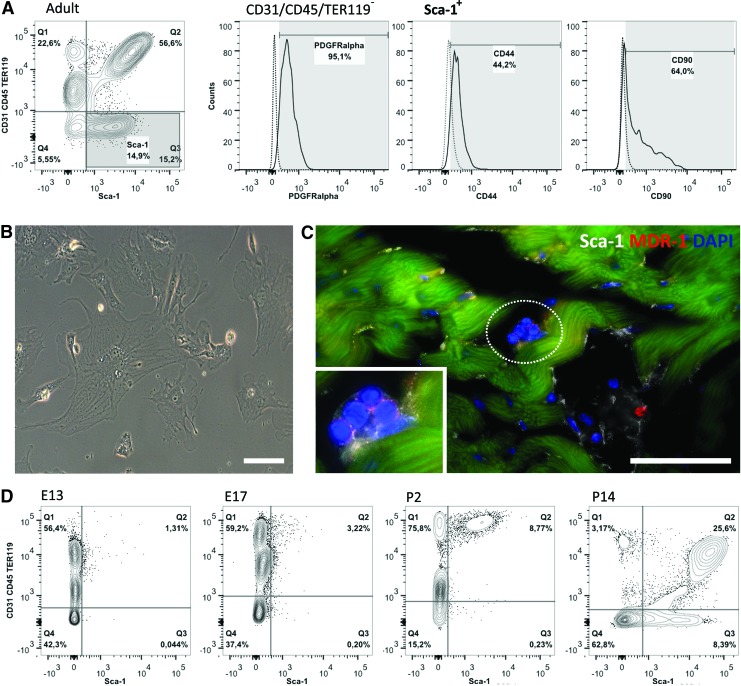
In the adult mouse, ∼70% of heart cells express Sca-1 after depletion of CMs. The majority of these Sca-1+ cells are ECs (Fig. 1A), a finding common to other organs, such as the liver, lung, and brain [44–48]. Oh et al. first reported a population of Sca-1+ cells from murine adult myocardium with telomerase activity analogous to that observed in newborn heart. This Sca-1+ population was considered distinct from HSCs due to their lack of CD45, CD34, c-Kit, Lmo2, GATA-2, and Tal1/Scl proteins, and also distinct from endothelial progenitor/precursor cells since they do not express CD34, Flk-1, or Flt-1. Although transcripts for cardiomyocytic structural genes were absent, these Sca-1+ cells expressed transcriptional regulators indicative of cardiac commitment, for example, GATA-4, Mef2c, and Tef1. Moreover, this population also exhibited the prototypical endothelial marker CD31, possibly due to the presence of contaminating endothelial CD31+Sca-1+ cells. Be that as it may, the Sca-1+ cells were reported to differentiate in CMs, in vitro in response to the demethylating agent 5-azacytidine (5-aza), and in vivo following intravenous injection in a myocardial ischemia-reperfusion system [25].
FIG. 1.
Heart resident Sca-1+ cells—an overview. Following collagenase digestion, the adult heart single cell suspension is depleted from cardiomyocytes (CMs). Lin− (CD31−, CD45−, and TER119−) cells constitute a minority of the Sca-1+ population within the heart (A), which is consistent with the reported expression of this marker also by mature endothelial cell (EC). Lin−Sca-1+ cells display mesenchymal-affiliated markers (PDGFRα, CD44, and CD90) and plastic-adherence (B). Scale bar 10 μm. Sca-1+ cells that do not integrate the vasculature are scattered in small clusters through the myocardium also displaying the side population (SP)-affiliated protein MDR-1 (C). Scale bar 50 μm. To address the ontogenic origin of the Sca-1+ population we determined the emergence of this population within the developing heart (D) by flow cytometry of whole-heart single cell suspensions. Lin−Sca-1+ cells appear in the heart around E17 although the establishment of both Sca-1+CD31+ and Sca-1+CD31− cellular compartments only occurs following birth. Color images available online at www.liebertpub.com/scd
Following Oh et al., several other authors isolated and characterized heart-derived CPCs on the basis of Sca-1 expression (Table 1). These subsets lack expression of the endothelial- (CD31) and hematopoietic-affiliated (CD45) markers [hereafter referred as lineage negative (Lin−)], display a mesenchymal cell-surface profile (CD34−, CD29+, CD90+, CD105+, and CD44+) and were generally reported to be able to differentiate, to a certain extent, into CM-, endothelial-, and smooth muscle-like cells [22–24,39].
Although a human ortholog of the Sca-1 protein has not so far been identified, Sca-1+-like cells have been isolated from the adult human heart using an anti-mouse Sca-1 antibody. Human Sca-1+-like cells were shown to express early cardiac transcription factors (GATA-4, Mef2c, Isl-1, and Nkx-2.5) and to differentiate into contractile CMs. Notably, the authors reported that CM differentiation was more robust when cells were subjected to 5-aza pretreatment [49].
Cells with the capacity to extrude metabolic dyes through overproduction of ATP-binding cassette transporters (eg, Abcg2 and MDR-1), called SP, were described in several organs as being able to undergo tissue-specific differentiation [50]. Different authors identified SP cells [12,14,15,51] expressing Sca-1 (93.3%) and lacking c-Kit [52] in the heart (Table 1). The cardiac SP was shown to be heterogeneous and composed of ECs, SMCs, and a mesenchymal stromal cell fraction. Thus, similar to the results obtained using Sca-1+ cells, the vast majority of the Sca-1+ cardiac SP were described as being endothelium-affiliated, while the cardiomyogenic potential appeared restricted to the Lin− mesenchymal subset [15,17].
Additionally, isolation and ex vivo expansion of CPCs also revealed their ability to form spheres, designated as CSs. CSs are characterized by a mixed population growing in vitro as 3D clusters, containing Sca-1+ CPCs that are either c-Kit+ [18] or c-Kit− [19], and spontaneously differentiating into CM- and vascular-like cells, depending on the size of the sphere and on the length of time in culture (Table 1). This specific isolation protocol has the advantage of allowing substantial in vitro cell expansion, a critical requirement to obtain enough cells for therapeutic use [18].
A number of experimental animal systems were used to address Sca-1 function in the heart. Recently, Sca-1 reporter and Sca-1 lineage tracer mouse models were used to investigate the contribution of Sca-1-expressing cells to the adult myocardium. Sca-1 expression was not detected in adult CMs, in healthy or injured heart. However, the descendants of Sca-1+ cells appeared to contribute to the CM compartment. This work provides evidence for a low but continuous replacement of Sca-1-derived CMs in aging hearts (0.17±0.06 cells/mm2, 8 months old) and after MI (024±0.04 cells/mm2, 8 months old) [53]. Nevertheless, the low frequency of Sca-1 traced cells (a maximum of 4.5% of CMs) indicates that most CMs derive from Sca-1− progenitors. This observation, together with the data indicating that before 2 months of life Sca-1-derived CMs are virtually undetectable, is compatible with the existence of two distinct CM progenitors: one that generates most CMs during fetal life and another that generates the small number of new CMs detected in adult heart [53]. Alternatively, a few persisting CM progenitors acquire Sca-1 expression after birth. Irrespective of their origin, the adult Sca-1+ CM progenitors did not significantly contribute to myocardial regeneration in the injured heart.
Although the role of Sca-1 in heart repair is still elusive, different models of Sca-1 KO mice consistently reported age-related cardiac hypertrophy, fibrosis and dysfunction after pressure overload. Less clear is the role of Sca-1 in the CPCs compartment, owing to the opposing effects on proliferation/differentiation of these cell subsets that have been claimed [54–56]. Nevertheless, a subset of myocardium-resident Sca-1+CD31− cells was shown to exhibit significant proliferation at 7 days post-MI. While the proliferative response of the endogenous Sca-1+CD31− appears insufficient to prevent adverse MI remodeling, this process is attenuated, with preservation of the left ventricle contractile performance and improvement of bioenergetic properties, when Sca-1+ cells are expanded ex vivo prior to transplantation into the injured myocardium [37]. Furthermore, transplantation of clonally expanded Sca-1+ cell sheets, that is, spontaneously detached monolayer cell grafts, improved cardiac function and attenuated cardiac remodeling following MI. Donor-derived Sca-1+ cells differentiated in CM- and endothelial-like cells and secreted a battery of cytokines and growth factors, including soluble VCAM-1 (CD106), which in its turn promoted angiogenesis, cardioprotection, and CPC migration/survival via VLA-4 binding [57]. The role of the Sca-1+ CPCs secretome in improving cardiac repair at the onset of MI has been emphasized in a series of in vitro and in vivo studies [39,58–60]. In 2012, Ryzhov et al. showed that the accumulation of adenosine in the injured myocardium might activate Sca-1+CD31− cells. These cells exhibit high adenosine receptor density at their surface and secrete pro-angiogenic factors, such as VEGF, CXCL1, and IL-6 via adenosine-dependent activation. At the present time, the beneficial effect of most cell-based therapies for cardiac repair has been assigned to paracrine effects, irrespective of the cell origin. The reported mechanisms encompass a decrease in hypoxia-induced apoptosis, increase of CMs proliferation, enhancement of neovascularization, restraining of the granulation and scar tissue formation, and recruitment of endogenous progenitor cells (reviewed in Refs. [61,62]). Further work is needed to address whether the secretome of CPCs outperform that of, for instance, mesenchymal stem/stromal cells of extra-cardiac origin. It is also conceivable that growth factors and cytokines produced by transplanted cells in general might play a role in the proliferation, mobilization, differentiation, and/or survival of endogenous CPCs. We have recently demonstrated that noncardiac Wharton's jelly mesenchymal stem/stromal cells are able to stimulate Sca-1+ CPCs proliferation and to activate a cardiomyogenic program through secreted factors [63]. Comprehensive investigations are also lacking on the ability of Sca-1+ CPCs to modulate their nesting microenvironment. On this point, there are indications that CPCs produce fibronectin in addition to collagens I, III, and IV, and also matrix metalloproteinases (MMPs) and their inhibitors [64]. The production of ECM and remodeling proteins was further enhanced following stimulation with transforming growth factor (TGF)-β1 [49], which is commonly used to stimulate CM differentiation by CPCs. Remarkably, this cytokine is also a known myofibroblast activator [65].
In summary, different Sca-1+ cell subsets have been identified by using a set of cell surface markers and/or based on functional properties, resulting in the isolation of diverse collections of Sca-1+ cells (Table 1), which are difficult to directly compare. As mentioned previously, this is a recurrent problem in the field of CPCs. The common characteristics of Sca-1+ CPCs are as follows: (i) CPCs belong to the CD31−CD45− compartment; (ii) CPCs display mesenchymal-affiliated markers (Fig. 1A, B); and/or (iii) express proteins whose function is to extrude metabolic dyes (Fig. 1C). Significantly, the cardiomyocytic differentiation of CPCs in vitro has been generally evaluated by methods that, when used separately, are insufficient to assign a cell to the CM pool, for example, immunofluorescence, quantitative PCR and calcium transient analysis. Nevertheless, it is clear that the cardiogenic potential of heart resident Sca-1+ CPCs is either limited or masked by the heterogeneous nature of the isolated subsets (Table 1). Alternatively, the low cardiogenic potential of this population simply reflects the absence of appropriate in vitro culture conditions [66,67]. Also, in vivo Sca-1+ CPCs exhibit a low capacity for de novo CMs generation, even following injury [53]. Likewise, the collective results obtained in the Sca-1+ transplantation experiments, during the initial phases of MI, appear to be a modest replacement of ischemic tissue by newly formed CMs [24,25,39]. Additionally, their capacity to differentiate into cells with SMC-like phenotype has been frequently reported as being more efficient than that of generating ECs and CMs (Table 1) [16,22,24,25,39]. Of note, some of the proteins used to identify SMC differentiation (eg, Smooth Muscle Actin, Calponin, and SM22) are also expressed by myofibroblasts, and thus further discrimination is required to precisely assess whether Sca-1+ CPCs undergo SMC differentiation or acquire a fibroblasts/myofibroblast phenotype.
At this point, it appears too speculative to assume that Sca-1+ CPCs are a source for CMs, ECs, and SMCs either in the steady state or following injury.
Is There a Correlation of Sca-1 Expression and the Distinct Cardiac Cell Types Emerging During Embryonic Development?
The identification of the embryonic origin of the cell subsets that make the heart will certainly provide us with knowledge of potential biomedical impact. After the isolation of putative CPCs from the adult heart it was imperative to enquire their origin and how they correlate with other already known players in cardiogenesis. Thus, we will briefly revisit heart development, with the aim of contextualizing a few pertinent findings.
The heart is essentially a mesodermal organ. During embryonic morphogenesis the cardiovascular progenitors shape a horseshoe-like structure, the cardiac crescent, which progresses to the linear heart tube, elongates, and loops rightward until it reaches the complex four-chambered heart structure. The heart tube starts beating at around embryonic day (E)8.0 [68], while the organ is still under construction.
The first and second heart fields (FHF and SHF, respectively) sequentially originate from early mesodermal progenitors. Current knowledge in cardiovascular development supports the divergence of CM-, SMC-, and EC-lineages from a common progenitor [69,70]. At the onset of the cardiac program, around E7.5, the FHF progenitors colonize the cardiac crescent and immediately differentiate into CMs [70,71]. These primitive CMs are functional, express sarcomeric proteins [72,73], and later contribute to the formation of the left ventricle [74,75]. SHF multipotent cells migrate to both poles of the heart tube, express Isl-1, Nkx-2.5, and Flk-1 and are able to give rise both to CMs and vascular cells (ECs and SMCs) [69,76]. This population contributes to the majority of the right ventricle, atria (left and right), and the outflow tract [77,78].
The myocardium of the looped tube (E9.5–11.5) is highly regionalized and the length and thickness of the wall tissue increases dramatically, by expansion and migration of cardiovascular progenitor cells and proliferation of CMs [79]. What will become the heart chambers suffer a balloon-like growth through the proliferation of CMs, particularly from the compact layer. In contrast, the inner myocardial layer is trabecular, more differentiated, and displays a lower mitotic rate [79–81]. Noteworthy, E9.5 is the later stage at which a cardiovascular progenitor, originated from cardiogenic mesoderm, was isolated. These cells are bipotent, express Nkx-2.5, and show spontaneous differentiation into CM- and SMC-like cells, suggesting that they are more committed to a muscle fate. Nkx-2.5+ progenitors display modest levels of the c-Kit and Sca-1, surface markers used to isolate adult CPCs; however, attempts to clonally expand these cells were unsuccessful [82].
Although the myocardium is the most relevant tissue for heart pumping function, the survival and proper functional maturation of developing CMs would be compromised without signals emanating from both the endocardium (specialized vascular endothelium) [83] and the epicardium (epithelial layer that covers the myocardium) [84–86]. Indeed, both nonmyocardial layers are crucial, contributing with new cell subsets and soluble signals that modulate the progress of myocardial histogenesis [87]. However, as the myocardium becomes thicker, it is unlikely that the signals from both layers can reach all myocardial cells. The epicardial cells arise around E10.5 from a cluster of cells, the proepicardial organ, which are recognized by Tbx18 [88] and Wt-1 expression [89]. After wrapping the myocardial layer, epicardial cells undergo epithelial to mesenchymal transition and contribute to the epicardial-derived cells (EPDCs) that migrate and colonize the muscle wall. EPDCs give rise to cells of myocardial stroma, that is, fibroblasts, pericytes, and coronary vasculature cells (SMCs and ECs) [86,90]; contributing also to the production of ECM components that create the propitious environment for maturation of the late-fetal heart [90]. Irrespective of the well-established contribution of the epicardium to the stromal heart fraction, signs of epicardial-derived CM differentiation have also been reported [88,89].
The ontogenic origin of Sca-1+ CPCs remains largely unknown although is currently under scrutiny by several laboratories, including our own (unpublished work), and conjectures in the field stem from the so far slim body of data available [23,91].
Smart et al. showed that a progenitor population resident in the adult heart can be activated with thymosin β4 (Tβ4) to re-express the embryonic epicardial gene Wt-1. The Wt-1+ cell subset expresses Sca-1 (80%) and, when treated with Tβ4 prior to injury, a small fraction of the Tβ4-primed cells (∼0.59%±0.18%) displays a CM phenotype 14 days post-MI. Of note, de novo CMs formation is only observed if the injury is inflicted after Tβ4 priming. Conversely, Tβ4 pretreatment in otherwise healthy hearts appears to lead to an increase in the frequency of Isl-1+ cells at the subepicardial zone [92]. Less exciting, from the therapeutic viewpoint, is that Tβ4 administration following MI is not seen to either mobilize or differentiate the EPDCs [93]. Although the authors were unable to unequivocally disregard a nonepicardial source for the Tβ4-primed Wt-1+ progenitors, the evident restriction of Wt-1 expression in the epicardium and subepicardial region is indicative of an EPDC origin. Harvey and colleagues tracked back to an epicardial origin the PDGFRα+Sca-1+ population of multipotent cells with colony-forming units (cCFU) properties (adherent, clonogenic, and nonphagocytic) [23,94]. While adult cCFU appear to be confined to the PDGFRα+Sca-1+ subset, it is of interest that the authors based the genetic tracing analysis solely in PDGFRα given that Sca-1 expression is not detected in the heart before mid-gestation. This is in agreement with our data when analyzing cardiac cell populations from E13.5, all through postnatal day (P)2, P14 and adult hearts; Sca-1 cells appear late in cardiac development (from E17.5 onward), and both CD31+Sca-1+ and CD31−Sca-1+ subsets increase in frequency after birth (Fig. 1A, D). Therefore, a prominent role for a CPC subset, in this case Sca-1+ CPCs, which emerges after the establishment of the four-chambered heart, is difficult to substantiate. Here again, it is formally possible that an embryonic cardiovascular progenitor acquires Sca-1 expression only after birth to lose it again as it differentiates (discussed in What Is Known About the Role and the Cardiovascular Potential of Sca-1+ CPCs? section). We find this possibility worth considering but unlikely.
Is Sca-1 a Pan Marker for Stem/Progenitor Cell Activity in Mesodermal Derivatives?
Sca-1, or lymphocyte activation protein-6A (Ly-6A) belongs to Ly6 gene family and is a glycosyl phosphatidylinositol-anchored cell surface protein (GPI-AP). Sca-1 was first reported as a cell surface marker of HSCs and, in combination with c-Kit, allows the definition of a population that contains the vast majority of HSCs, although they are still present at low frequency (around 10%) [44,95]. It has also been claimed that Sca-1 is expressed by the progenitor compartment of a number of other tissues, such as the skeletal muscle [95,96], mammary gland [95,97], kidney [95,98], liver [99], and prostate [95,100]. While some of these reports are not consensually accepted, others remain unchallenged. It should also be kept in mind that protocols used to identify progenitors frequently rely on a previous period of cell expansion in vitro. In vitro, cell expansion might result in a population that is not representative of the cell input and cells can also acquire new surface markers due to differentiation or stress in the culture. Therefore, conclusions based on the analysis of cells after culture, rather than on their isolation ex vivo, should be critically evaluated.
Additionally, Sca-1 expression has been observed in the resident fibroblast population of several organs. In the skin, Sca-1+ fibroblasts are restricted to the hypodermal lineage (Sca-1+PDGFRα+Dlk1+/−), display adipogenic potential and are of mesenchymal origin. After injury, these cells participate in the first wave of dermal regeneration, characterized by the production of high levels of collagenous-based ECM [101]. In the lung, Lin−Sca-1+ cells appear to be highly enriched in fibroblast progenitors located in perivascular areas. The latter population expands postbirth and co-express markers typical of mesenchymal affiliation, that is, PDGFRα, and CD90 [102]. Furthermore, after an injury stimulus, lung fibroblasts (Sca-1high) can differentiate into myofibroblasts (Sca-1low) and increase the ECM production toward a more efficient repair process [103].
Nevertheless, and surprisingly in view of the quasi ubiquitous expression of this protein (present in a wide array of cells, including mature cell types and distinct stem/progenitor cell systems), the mechanisms and signaling pathways through which Sca-1 acts remain unclear. Sca-1 protein regulates and activates cell signaling via receptor-ligand binding and protein–protein interactions, playing a role in cell adhesion in the hematopoietic system [95]. It has been reported to play a part in the self-renewal of hematopoietic and mesenchymal progenitors [95,97,104] and, in skeletal muscle, Sca-1+ cells were shown to upregulate directly or indirectly the activity of MMPs, thereby promoting breakdown of the ECM and thus supporting muscle regeneration [105].
To the best of our knowledge, all revised Sca-1 cell subsets are indicative of a mesodermal origin, even considering organs from distinct germ layers, for example, ectoderm (skin) and endoderm (lung). Thus, the answer to the original question is that, while Sca-1 expression is confined to mesoderm-derivatives it is not restricted to a stem/progenitor compartment.
Does the Lin−Sca-1+ Population in the Heart Overlap with Fibroblasts?
The adult cardiac tissue is composed of a syncytial network of CMs and of a noncardiomyocytic fraction, in which fibroblasts are a major constituent. Fibroblasts are recognized mediators of the heart repair response to MI and to other pathological conditions. Several other functions have also been attributed to cardiac fibroblasts, including (i) production of mitogenic stimuli during heart development, (ii) secretion of growth factors, ECM and ECM-remodeling proteins throughout life, and (iii) electrical coupling with CMs [106]. Despite all this, it is still difficult to define a “fibroblast” mainly because the designation is given to a heterogeneous population [107] with a largely undefined molecular signature. While a definitive lineage marker is not established, cardiac fibroblasts are commonly identified by the expression of DDR-2, Fsp-1, PDGFRα, Periostin, CD90 and Vimentin [106].
Lin−Sca-1+ cells, in different organs, seem to overlap with the population of tissue-resident fibroblasts [101–103]. In the heart, Sca-1 expression has not been ascribed to a population of cardiac fibroblasts, although the evidence is that Sca-1-expressing cells are present in the myocardial interstitium. What are then the similarities between cardiac Lin−Sca-1+ cells and fibroblasts? Similar to fibroblasts, Lin−Sca-1+ heart cells (i) display the typical mesenchymal proteins PDGFRα, CD90, CD44, and CD105 [22,23,39]; (ii) appear to be more prone to differentiate in smooth muscle actin-expressing cells [16,22,39]; (iii) augment the production of ECM and MMPs following TGF-β stimulation [65]; (iv) appear to derive from the epicardium late in development, although the population expands mainly after birth [23]; and (v) play an important role in heart repair mainly by paracrine and pro-angiogenic actions [57,108]. Thus, it is tempting to postulate that Lin−Sca-1+ heart cells constitute/generate stromal cell producers of ECM and, importantly, are the main responders following tissue injury. Indeed, this hypothesis has recently been put forward together with the suggestion that cardiac-resident Sca-1+PDGFRα+ mesenchymal progenitors, as defined by Chong et al. [23], could be a source for fibroblasts and adipocytes, characteristic of a fibro-fatty heart condition [109].
Of note, and because of the clear heterogeneity of the heart Lin−Sca-1+ compartment, it is conceivable that several functionally distinct fractions may be present in this population. The latter may possibly embrace a smaller subset of CPCs that are actually responsible for the multipotent phenotype described by many authors [22–25,39]. In our view, a direct comparison between heart-derived Lin−Sca-1+ cells and fibroblasts, at the single cell level, is urgently needed, to establish the functional and phenotypic differences, if they exist, of these populations in vitro and in vivo.
Conclusion
The urge to manage the burden of an escalating number of severe cardiovascular disorders led to the premature therapeutic use of CPCs. CPC-based therapy was initiated even before a consensus was reached in the scientific community to the function of CPCs in the healthy and diseased heart.
The cardiac Lin−Sca-1+ compartment, which has been considered as a multipotent population of CPCs (capable of differentiating in CMs, ECs, and SMCs in vitro and in vivo), is heterogeneous and displays some characteristics commonly associated with fibroblasts. A combination of reporter and lineage-tracer animal models recently demonstrated Sca-1 contribution to the low, but continuous, myocardial turnover during adulthood and after injury. This work supports the potential of Sca-1-expressing cells in heart regeneration. Thus, it is of major importance to study the differences that may exist between Sca-1+ CPCs and other Lin−Sca-1+ stromal cells of the heart. This will be only possible with a detailed characterization (at the single cell level) of Lin−Sca-1+ heart-resident cells, combined with robust clonal assays to test stem cell activity, both in vitro and in vivo.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia [SFRH/BD/74218/2010] to M.V., [SFRH/BPD/42254/2007] and QREN/ON.2 [NORTE-07-0124-FEDER-000005] to D.S.N., and Fundo Europeu de Desenvolvimento Regional, Programa Operacional Factores de Competitividade-COMPETE, Quadro de Referência Estratégico Nacional, Fundo Social Europeu [PEst-C/SAU/LA0002/2013, PTDC/SAU-ORG/118297/2010, and NORTE-07-0124-FEDER-000005]. By the Pasteur Institute, INSERM, ANR through a grant “Lymphopoiesis” and through the REVIVE Future Investment Program, La Ligue contre le Cancer with grants to A.C. The authors are thankful to Dr. Tatiana P. Resende for critical discussion during the article preparation and to Dr. Paulo Vieira for the fine editing work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J. and Lee RT. (2007). Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13:970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP. and Lee RT. (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, et al. (2009). Evidence for cardiomyocyte renewal in humans. Science 324:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, et al. (2010). Cardiomyogenesis in the adult human heart. Circ Res 107:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M. and Marban E. (2013). Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med 5:191–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN. and Sadek HA. (2011). Transient regenerative potential of the neonatal mouse heart. Science 331:1078–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN. and Sadek HA. (2013). Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A 110:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clubb FJ, Jr., and Bishop SP. (1984). Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Lab Invest 50:571–577 [PubMed] [Google Scholar]
- 9.Soonpaa MH, Kim KK, Pajak L, Franklin M. and Field LJ. (1996). Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol 271:H2183–H2189 [DOI] [PubMed] [Google Scholar]
- 10.Goldman S. (2005). Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol 23:862–871 [DOI] [PubMed] [Google Scholar]
- 11.Warejcka DJ, Harvey R, Taylor BJ, Young HE. and Lucas PA. (1996). A Population of cells isolated from rat heart capable of differentiating into several mesodermal phenotypes. J Surg Res 62:233–242 [DOI] [PubMed] [Google Scholar]
- 12.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR. and Marban E. (2007). Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115:896–908 [DOI] [PubMed] [Google Scholar]
- 13.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA. and Megeney LA. (2002). The post-natal heart contains a myocardial stem cell population. FEBS Lett 530:239–243 [DOI] [PubMed] [Google Scholar]
- 14.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD. and Garry DJ. (2004). Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 265:262–275 [DOI] [PubMed] [Google Scholar]
- 15.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS. and Liao R. (2005). CD31- but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97:52–61 [DOI] [PubMed] [Google Scholar]
- 16.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, et al. (2007). Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol 176:329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamahara K, Fukushima S, Coppen SR, Felkin LE, Varela-Carver A, Barton PJR, Yacoub MH. and Suzuki K. (2008). Heterogeneic nature of adult cardiac side population cells. Biochem Biophys Res Commun 371:615–620 [DOI] [PubMed] [Google Scholar]
- 18.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MVG, et al. (2004). Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95:911–921 [DOI] [PubMed] [Google Scholar]
- 19.Ye J, Boyle A, Shih H, Sievers RE, Zhang Y, Prasad M, Su H, Zhou Y, Grossman W, Bernstein HS. and Yeghiazarians Y. (2012). Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One 7:e30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776 [DOI] [PubMed] [Google Scholar]
- 21.Gambini E, Pompilio G, Biondi A, Alamanni F, Capogrossi MC, Agrifoglio M. and Pesce M. (2011). C-kit+ cardiac progenitors exhibit mesenchymal markers and preferential cardiovascular commitment. Cardiovasc Res 89:362–373 [DOI] [PubMed] [Google Scholar]
- 22.Tateishi K, Ashihara E, Takehara N, Nomura T, Honsho S, Nakagami T, Morikawa S, Takahashi T, Ueyama T, Matsubara H. and Oh H. (2007). Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J Cell Sci 120:1791–1800 [DOI] [PubMed] [Google Scholar]
- 23.Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, et al. (2011). Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell 9:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takamiya M, Haider KH. and Ashraf M. (2011). Identification and characterization of a novel multipotent sub-population of Sca-1(+) cardiac progenitor cells for myocardial regeneration. PLoS One 6:e25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, et al. (2003). Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 100:12313–12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL. and Robbins RC. (2004). Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 428:668–673 [DOI] [PubMed] [Google Scholar]
- 27.Wagers AJ. and Weissman IL. (2004). Plasticity of adult stem cells. Cell 116:639–648 [DOI] [PubMed] [Google Scholar]
- 28.Lyngbæk S, Schneider M, Hansen J. and Sheikh S. (2007). Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res Cardiol 102:101–114 [DOI] [PubMed] [Google Scholar]
- 29.Segers VF. and Lee RT. (2008). Stem-cell therapy for cardiac disease. Nature 451:937–942 [DOI] [PubMed] [Google Scholar]
- 30.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, et al. (2007). Human cardiac stem cells. Proc Natl Acad Sci U S A 104:14068–14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira-Martins J, Ogorek B, Cappetta D, Matsuda A, Signore S, D'Amario D, Kostyla J, Steadman E, Ide-Iwata N, et al. (2012). Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res 110:701–715 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Eberhard D. and Jockusch H. (2005). Patterns of myocardial histogenesis as revealed by mouse chimeras. Dev Biol 278:336–346 [DOI] [PubMed] [Google Scholar]
- 33.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A. and Anversa P. (2001). Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 98:10344–10349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A. and Anversa P. (2002). Chimerism of the transplanted heart. N Engl J Med 346:5–15 [DOI] [PubMed] [Google Scholar]
- 35.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A. and Li R-K. (2006). Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest 116:1865–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimmeler S, Zeiher AM. and Schneider MD. (2005). Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest 115:572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH. and Zhang J. (2006). The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem cells 24:1779–1788 [DOI] [PubMed] [Google Scholar]
- 38.van Vliet P, Roccio M, Smits A, van Oorschot A, Metz C, van Veen T, Sluijter J, Doevendans P. and Goumans MJ. (2008). Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J 16:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freire AG, Nascimento DS, Forte G, Valente M, Resende TP, Pagliari S, Abreu C, Carvalho I, Di Nardo P. and Pinto-do-O P. (2014). Stable phenotype and function of immortalized Lin-Sca-1+ cardiac progenitor cells in long-term culture: a step closer to standardization. Stem Cells Dev 23:1012–1026 [DOI] [PubMed] [Google Scholar]
- 40.Reinecke H, Minami E, Zhu WZ. and Laflamme MA. (2008). Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res 103:1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzales C. and Pedrazzini T. (2009). Progenitor cell therapy for heart disease. Exp Cell Res 315:3077–3085 [DOI] [PubMed] [Google Scholar]
- 42.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, et al. (2011). Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, et al. (2012). Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Rijn M, Heimfeld S, Spangrude GJ. and Weissman IL. (1989). Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A 86:4634–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotton DN, Summer RS, Sun X, Ma BY. and Fine A. (2003). Stem cell antigen-1 expression in the pulmonary vascular endothelium. Am J Physiol Lung Cell Mol Physiol 284:L990–L996 [DOI] [PubMed] [Google Scholar]
- 46.Luna G, Paez J. and Cardier JE. (2004). Expression of the hematopoietic stem cell antigen Sca-1 (LY-6A/E) in liver sinusoidal endothelial cells: possible function of Sca-1 in endothelial cells. Stem Cells Dev 13:528–535 [DOI] [PubMed] [Google Scholar]
- 47.Tsuchiya A, Heike T, Baba S, Fujino H, Umeda K, Matsuda Y, Nomoto M, Ichida T, Aoyagi Y. and Nakahata T. (2008). Sca-1+ endothelial cells (SPECs) reside in the portal area of the liver and contribute to rapid recovery from acute liver disease. Biochem Biophys Res Commun 365:595–601 [DOI] [PubMed] [Google Scholar]
- 48.Kang SG, Shinojima N, Hossain A, Gumin J, Yong RL, Colman H, Marini F, Andreeff M. and Lang FF. (2010). Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery 67:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA. and Goumans MJ. (2009). Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc 4:232–243 [DOI] [PubMed] [Google Scholar]
- 50.Asakura A. and Rudnicki MA. (2002). Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30:1339–1345 [DOI] [PubMed] [Google Scholar]
- 51.Challen GA. and Little MH. (2006). A side order of stem cells: the SP phenotype. Stem Cells 24:3–12 [DOI] [PubMed] [Google Scholar]
- 52.Oh H, Chi X, Bradfute SB, Mishina Y, Pocius J, Michael LH, Behringer RR, Schwartz RJ, Entman ML. and Schneider MD. (2004). Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann N Y Acad Sci 1015:182–189 [DOI] [PubMed] [Google Scholar]
- 53.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K. and Braun T. (2013). Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports 1:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey B, Fransioli J, Gude NA, Alvarez R, Zhan X, Gustafsson ÅB. and Sussman MA. (2012). Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ Res 111:750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenblatt-Velin N, Ogay S, Felley A, Stanford WL. and Pedrazzini T. (2012). Cardiac dysfunction and impaired compensatory response to pressure overload in mice deficient in stem cell antigen-1. FASEB J 26:229–239 [DOI] [PubMed] [Google Scholar]
- 56.Zhou H, Bian Z-Y, Zong J, Deng W, Yan L, Shen D-F, Guo H, Dai J, Yuan Y, et al. (2012). Stem cell antigen 1 protects against cardiac hypertrophy and fibrosis after pressure overload. Hypertension 60:802–809 [DOI] [PubMed] [Google Scholar]
- 57.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N. and Komuro I. (2009). Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest 119:2204–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang C, Gu H, Yu Q, Manukyan MC, Poynter JA. and Wang M. (2011). Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS One 6:e29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maxeiner H, Krehbiehl N, Muller A, Woitasky N, Akinturk H, Muller M, Weigand MA, Abdallah Y, Kasseckert S, et al. (2010). New insights into paracrine mechanisms of human cardiac progenitor cells. Eur J Heart Fail 12:730–737 [DOI] [PubMed] [Google Scholar]
- 60.Ryzhov S, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I. and Feoktistov I. (2012). Role of A2B adenosine receptors in regulation of paracrine functions of stem cell antigen 1-positive cardiac stromal cells. J Pharmacol Exp Ther 341:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gnecchi M, Zhang Z, Ni A. and Dzau VJ. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103:1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burchfield JS. and Dimmeler S. (2008). Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nascimento DS, Mosqueira D, Sousa LM, Teixeira M, Filipe M, Resende TP, Araujo AF, Valente M, Almeida J, et al. (2014). Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling following myocardial infarction by pro-angiogenic, anti-apoptotic and endogenous cell activation mechanisms. Stem Cell Res Ther 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bax NA, van Marion MH, Shah B, Goumans MJ, Bouten CV. and van der Schaft DW. (2012). Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J Mol Cell Cardiol 53:497–508 [DOI] [PubMed] [Google Scholar]
- 65.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM. and Thomas PE. (2003). Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278:12384–12389 [DOI] [PubMed] [Google Scholar]
- 66.Forte G, Carotenuto F, Pagliari F, Pagliari S, Cossa P, Fiaccavento R, Ahluwalia A, Vozzi G, Vinci B, et al. (2008). Criticality of the biological and physical stimuli array inducing resident cardiac stem cell determination. Stem Cells 26:2093–2103 [DOI] [PubMed] [Google Scholar]
- 67.Mosqueira D, Pagliari S, Uto K, Ebara M, Romanazzo S, Escobedo-Lucea C, Nakanishi J, Taniguchi A, Franzese O, et al. (2014). Hippo pathway effectors control cardiac progenitor cell fate by acting as dynamic sensors of substrate mechanics and nanostructure. ACS Nano 8:2033–2047 [DOI] [PubMed] [Google Scholar]
- 68.Navaratnam V, Kaufman MH, Skepper JN, Barton S. and Guttridge KM. (1986). Differentiation of the myocardial rudiment of mouse embryos: an ultrastructural study including freeze-fracture replication. J Anat 146:65–85 [PMC free article] [PubMed] [Google Scholar]
- 69.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, et al. (2006). Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127:1151–1165 [DOI] [PubMed] [Google Scholar]
- 70.Kattman SJ, Adler ED. and Keller GM. (2007). Specification of multipotential cardiovascular progenitor cells during embryonic stem cell differentiation and embryonic development. Trends Cardiovasc Med 17:240–246 [DOI] [PubMed] [Google Scholar]
- 71.Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, et al. (2007). An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 128:947–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sassoon DA, Garner I. and Buckingham M. (1988). Transcripts of alpha-cardiac and alpha-skeletal actins are early markers for myogenesis in the mouse embryo. Development 104:155–164 [DOI] [PubMed] [Google Scholar]
- 73.Nishii K. and Shibata Y. (2006). Mode and determination of the initial contraction stage in the mouse embryo heart. Anat Embryol (Berl) 211:95–100 [DOI] [PubMed] [Google Scholar]
- 74.Lints TJ, Parsons LM, Hartley L, Lyons I. and Harvey RP. (1993). Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 119:419–431 [DOI] [PubMed] [Google Scholar]
- 75.Kubalak SW, Miller-Hance WC, O'Brien TX, Dyson E. and Chien KR. (1994). Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis. J Biol Chem 269:16961–16970 [PubMed] [Google Scholar]
- 76.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ. and Chien KR. (2009). Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 460:113–117 [DOI] [PubMed] [Google Scholar]
- 77.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J. and Evans S. (2003). Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5:877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelly RG, Brown NA. and Buckingham ME. (2001). The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 1:435–440 [DOI] [PubMed] [Google Scholar]
- 79.de Boer BA, van den Berg G, de Boer PA, Moorman AF. and Ruijter JM. (2012). Growth of the developing mouse heart: an interactive qualitative and quantitative 3D atlas. Dev Biol 368:203–213 [DOI] [PubMed] [Google Scholar]
- 80.Sedmera D, Reckova M, DeAlmeida A, Coppen SR, Kubalak SW, Gourdie RG. and Thompson RP. (2003). Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec A Discov Mol Cell Evol Biol 274:773–777 [DOI] [PubMed] [Google Scholar]
- 81.Moorman A, Webb S, Brown NA, Lamers W. and Anderson RH. (2003). Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart 89:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien C-L, Schultheiss TM. and Orkin SH. (2006). Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 127:1137–1150 [DOI] [PubMed] [Google Scholar]
- 83.Bersell K, Arab S, Haring B. and Kuhn B. (2009). Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138:257–270 [DOI] [PubMed] [Google Scholar]
- 84.Manasek FJ. (1969). Embryonic development of the heart. II. Formation of the epicardium. J Embryol Exp Morphol 22:333–348 [PubMed] [Google Scholar]
- 85.Vincent SD. and Buckingham ME. (2010). How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol 90:1–41 [DOI] [PubMed] [Google Scholar]
- 86.Dettman RW, Denetclaw W, Jr., Ordahl CP. and Bristow J. (1998). Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 193:169–181 [DOI] [PubMed] [Google Scholar]
- 87.Rosenthal N. and Harvey RP. (2010). Heart Development and Regeneration. Elsevier, Inc., London, United Kingdom [Google Scholar]
- 88.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, et al. (2008). A myocardial lineage derives from Tbx18 epicardial cells. Nature 454:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR. and Pu WT. (2008). Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM. and Srivastava D. (2009). Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell 16:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu SM, Chien KR. and Mummery C. (2008). Origins and fates of cardiovascular progenitor cells. Cell 132:537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, et al. (2011). De novo cardiomyocytes from within the activated adult heart after injury. Nature 474:640–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou B, Honor LB, Ma Q, Oh JH, Lin RZ, Melero-Martin JM, von Gise A, Zhou P, Hu T, et al. (2012). Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J Mol Cell Cardiol 52:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chong JJ, Reinecke H, Iwata M, Torok-Storb B, Stempien-Otero A. and Murry CE. (2013). Progenitor cells identified by PDGFR-alpha expression in the developing and diseased human heart. Stem Cells Dev 22:1932–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holmes C. and Stanford WL. (2007). Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25:1339–1347 [DOI] [PubMed] [Google Scholar]
- 96.Asakura A, Seale P, Girgis-Gabardo A. and Rudnicki MA. (2002). Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159:123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM. and Goodell MA. (2002). Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol 245:42–56 [DOI] [PubMed] [Google Scholar]
- 98.Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R. and Reisner Y. (2006). Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol 17:3300–3314 [DOI] [PubMed] [Google Scholar]
- 99.Clayton E. and Forbes SJ. (2009). The isolation and in vitro expansion of hepatic Sca-1 progenitor cells. Biochem Biophys Res Commun 381:549–553 [DOI] [PubMed] [Google Scholar]
- 100.Xin L, Lawson DA. and Witte ON. (2005). The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A 102:6942–6947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC. and Watt FM. (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ. and Bertoncello I. (2009). Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the Sca-1 positive cell fraction. Stem Cells 27:623–633 [DOI] [PubMed] [Google Scholar]
- 103.Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, Shibata K, Suda T, Chida K. and Iwashita T. (2013). Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ito CY, Li CYJ, Bernstein A, Dick JE. and Stanford WL. (2003). Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood 101:517–523 [DOI] [PubMed] [Google Scholar]
- 105.Kafadar KA, Yi L, Ahmad Y, So L, Rossi F. and Pavlath GK. (2009). Sca-1 expression is required for efficient remodeling of the extracellular matrix during skeletal muscle regeneration. Dev Biol 326:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lajiness JD. and Conway SJ. (2013). Origin, development, and differentiation of cardiac fibroblasts. J Mol Cell Cardiol 70:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D. and Brown PO. (2002). Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 99:12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Bo H, Meng X, Wu Y, Bao Y. and Li Y. (2006). A simple and fast experimental model of myocardial infarction in the mouse. Tex Heart Inst J 33:290–293 [PMC free article] [PubMed] [Google Scholar]
- 109.Paylor B, Fernandes J, McManus B. and Rossi F. (2013). Tissue-resident Sca1+ PDGFRalpha+ mesenchymal progenitors are the cellular source of fibrofatty infiltration in arrhythmogenic cardiomyopathy. F1000Res 2:141. [DOI] [PMC free article] [PubMed] [Google Scholar]