Abstract
Purpose
The aim of this work is to determine if a relationship exists between thyroid dose and incidence of primary hypothyroidism (PH) in children undergoing craniospinal irradiation (CSI).
Methods
A total of 22 patients received CSI with evaluable thyroid dose information. All patients received concurrent chemotherapy and 21 patients (95%) received adjuvant chemotherapy. Median follow-up was 42.9 months.
Results
The incidence of PH in our cohort was 59% at a median time after radiotherapy of 3.5 years (range: 8 months to 7.5 years). Mean thyroid dose appeared to best predict for PH, with a median of 2080 cGy for patients with PH versus 1736 cGy for children without PH (p=0.057). There was no association between the rate of PH and sex, age, CSI dose, minimum thyroid dose and maximum thyroid dose.
Conclusions
A relationship may exist between the mean thyroid dose and incidence of PH in patients undergoing CSI. Thus, new strategies to protect the thyroid gland may be warranted.
Keywords: children, craniospinal irradiation, hypothyroidism, late effects, medulloblastoma
Introduction
Primary hypothyroidism is a known consequence of external beam radiotherapy due to direct irradiation of the thyroid gland. For children undergoing craniospinal irradiation (CSI), the thyroid gland is irradiated in the exit dose of the spinal field. Previous reports estimated that approximately one-third of pediatric patients experience primary hypothyroidism as a result of CSI, with crude rates reported between 28% and 62% (1–6). However, the relationship between thyroid dose and the occurrence of PH in children undergoing CSI has not yet been extensively reported.
The relationship between thyroid dose and the occurrence of primary hypothyroidism has been explored in other disease sites. In pediatric patients undergoing radiotherapy for Hodgkin's disease, there appears to be a significant threshold effect in the development of primary hypothyroidism for patients who received a total radiation dose >2600–3500 cGy (7–10). However, such data did not describe dose-volume parameters particular to the thyroid gland. For adults undergoing head and neck radiotherapy, there is evidence of relationship between increasing radiation dose and the rate of development of hypothyroidism, although the dosimetric parameters that best define the risk of primary hypothyroidism have not been clearly identified in a meta-analysis (11). Presently, a normal tissue complication probability (NTCP) model for radiation-induced primary hypothyroidism does not exist (12). Models evaluating normal tissue complication rates estimate that the radiation dose corresponding to a 50% incidence of hypothyroidism (D50) is 4400 cGy (13). Emami estimated that, for the endpoint of clinical hypothyroidism, an 8% complication rate within 5 years after irradiation of the whole gland (TD 8/5) would occur at 4500 cGy, the TD 13/5 is 6000 cGy and TD 35/5 is 7000 cGy (14). The Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) data did not describe radiation-induced hypothyroidism.
Over the past several decades, there has been a significant increase in the number of long-term survivors of standard risk medulloblastoma, with 5-year overall survival rates of approximately 85% (15). As the number of long-term survivors increase, the need for elucidation of the relationship between thyroid radiation dose and radiation-induced primary hypothyroidism becomes increasingly important.
Materials and methods
A retrospective review was conducted of pediatric oncology patients diagnosed at our institution between 1994 and 2011, who received CSI with evaluable thyroid dose volume histogram (DVH) information, had at least 12 months of follow-up with thyroid function testing at least once, following completion of radiation, and were alive at last follow-up (Table 1). A total of 11 males and 11 females ranging in age from 3 to 21 years at the time of CSI were identified. All patients received concurrent chemotherapy with CSI, and 21 patients (95%) received adjuvant chemotherapy. All of the 22 patients received CSI, with total CSI doses ranging between 1800 and 3600 cGy.
Table 1.
Patient and treatment characteristics according to diagnosis of primary hypothyroidism.
| No primary hypothyroidism (n=9) | Primary hypothyroidism (n=13) | p-Value | |
|---|---|---|---|
| Gender | |||
| Male | 3 | 8 | 0.3870 |
| Female | 6 | 5 | |
| Age at End of CSI, years | |||
| Median | 15.0 | 10.7 | 0.2277 |
| Range | 6.8–20.5 | 3.7–21.7 | |
| CSI Dose (cGy) | |||
| Median | 2340 | 2340 | 0.9782 |
| Range | 1800–3600 | 2340–3600 | |
| Minimum Thyroid Dose (cGy) | |||
| Median | 1438 | 1614 | 0.0949 |
| Range | 287–2766 | 1001–2994 | |
| Maximum Thyroid Dose (cGy) | |||
| Median | 2233 | 2254 | 0.5332 |
| Range | 1833–3362 | 2072–3335 | |
| Mean Thyroid Dose (cGy) | |||
| Median | 1736 | 2080 | 0.0570 |
| Range | 1023–3088 | 1895–3156 | |
| Follow-up, months | |||
| Median | 24.0 | 52.2 | 0.0048 |
| Range | 16.6–48.9 | 21.1–90.1 | |
The dose-volume parameters of the thyroid were quantified on 3D-dose distributions derived from planning-CT images and dose-volume histograms. Serum free or total thyroxine and thyroid-stimulating hormone (TSH) levels were measured at variable intervals after the completion of CSI by conventional commercial assays. Primary hypothyroidism was defined when the TSH levels were higher than 4.2 μIU/mL, with low or normal thyroxine levels (16). Subclinical hypothyroidism was diagnosed when patients demonstrated elevated TSH levels with normal free T4 (FT4) concentrations (17).
Median follow-up for all patients was 42.9 months. For children without hypothyroidism, median follow-up was 24 months (range: 16.6–48.9 months), whereas for those diagnosed with primary hypothyroidism, follow-up time was 52.2 months (range: 21.1–90.1 months). Descriptive statistics regarding patient demographics and thyroid-specific dosimetric parameters were compared between those that developed hypothyroidism to those that did not. Continuous variables were compared based on a 2-sample t-test, and categorical variables were analyzed with Fisher's exact test for proportions.
Results
The patients' median age at time of CSI was 15.0 years for patients without hypothyroidism, and 10.7 years for children who developed primary hypothyroidism. Median follow-up time was 24 months for patients without hypothyroidism, whereas that for patients with hypothyroidism was 52.2 months (p=0.0048).
Primary hypothyroidism was diagnosed in 13 patients (59%). Of these, 12 patients were diagnosed with subclinical hypothyroidism (92%), and one child was diagnosed with clinical hypothyroidism (8%). Primary hypothyroidism was detected at a median time of 3.5 years after CSI. Hypothyroidism occurred in 72.2% of males and in 45.5% of females (p=0.387).
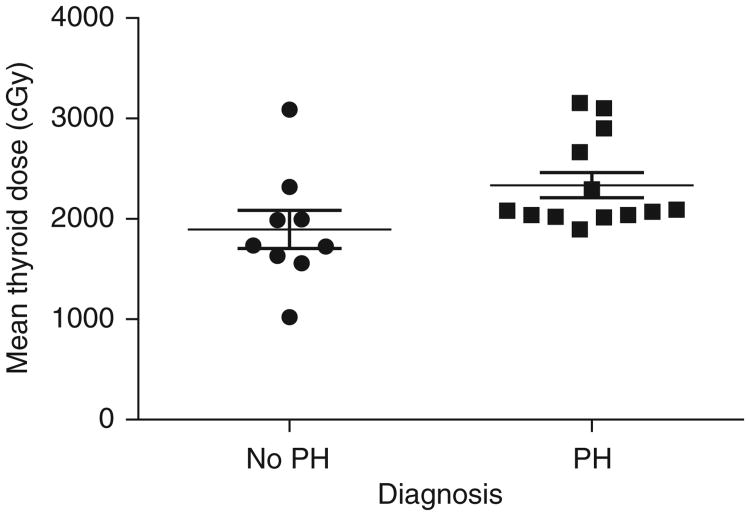
The risk factors of sex, age at end of CSI, CSI dose, minimum thyroid dose, maximum thyroid dose, and mean thyroid dose were considered. The only factor that demonstrated a trend towards significance for predicting for the development of primary hypothyroidism was mean thyroid dose (Figure 1), with a median value of 2080 cGy for patients with primary hypothyroidism compared to 1736 cGy for children without primary hypothyroidism (p=0.057). There was no association between the rate of PH on the one hand, and sex, age, CSI dose, minimum thyroid dose, and maximum thyroid dose on the other hand.
Figure 1.
Influence of mean thyroid dose on development of primary hypothyroidism in patients treated with CSI (p=0.057), PH, and primary hypothyroidism.
Discussion
Primary hypothyroidism is a known risk associated with exposure of the thyroid gland to external beam radiotherapy. The incidence of primary hypothyroidism in our patient population following CSI was quite high, with 59% of patients developing primary hypothyroidism, as compared with one-third of patients reported in most studies (1–6). The higher incidence of primary hypothyroidism in our patient cohort can potentially be explained by our institutional practice to screen thyroid function in patients who received craniospinal radiotherapy on a yearly basis, as opposed to diagnosing patients on the basis of clinical symptoms. This disorder was recognized in our patients at a median of 3.5 years after CSI, consistent with the findings of other studies (8, 9, 16).
The relationship between primary hypothyroidism and thyroid dose has been explored in other applications of radiotherapy, specifically in children undergoing neck irradiation for Hodgkin's disease and adults undergoing head and neck radiotherapy. In the adult population, the risk of developing primary hypothyroidism following neck irradiation ranges from 20% to 53% (11, 18). While higher radiation doses are suggested to increase the risk of developing hypothyroidism, the dosimetric parameters for the thyroid gland that best define this risk have yet to be clearly identified (11). Mean thyroid dose appears to better predict for the occurrence of hypothyroidism than the more complicated NTCP modeling (13). For children undergoing neck radiotherapy in the treatment of Hodgkin's disease, a threshold total radiation dose of 2600 to 3500 cGy can help predict the development of subsequent hypothyroidism (7, 10, 19). However, such data does not describe dosimetric parameters specific to the thyroid gland.
Our data suggest that the mean dose of irradiation received by the thyroid gland correlates with the development of primary hypothyroidism, with median values of 2080 cGy and 1736 cGy in children with and without primary hypothyroidism, respectively. In our patient population, no association was found between the rate of primary hypothyroidism and sex, age, CSI dose, thyroid volume, minimum thyroid dose or maximum thyroid dose.
Given the high incidence of hypothyroidism in this young population and the suggested relationship between hypothyroidism and mean thyroid dose, strategies to reduce the radiation dose to the thyroid may be warranted. One strategy employed at our institution is to establish the initial craniospinal junction as inferiorly as possible, which is immediately superior to the shoulders. In this way, the thyroid gland is maximally blocked with the leaves from the cranial field. An example of this technique is demonstrated in Figure 2. This patient undertook both the thyroid sparing technique, with an inferior craniospinal junction, and a traditional, superior junction set immediately inferior to the chin. The mean thyroid dose calculated from an example patient using the thyroidsparing technique was 1167 cGy, compared with 2902 cGy via utilizing a superior craniospinal junction (Figure 3).
Figure 2.
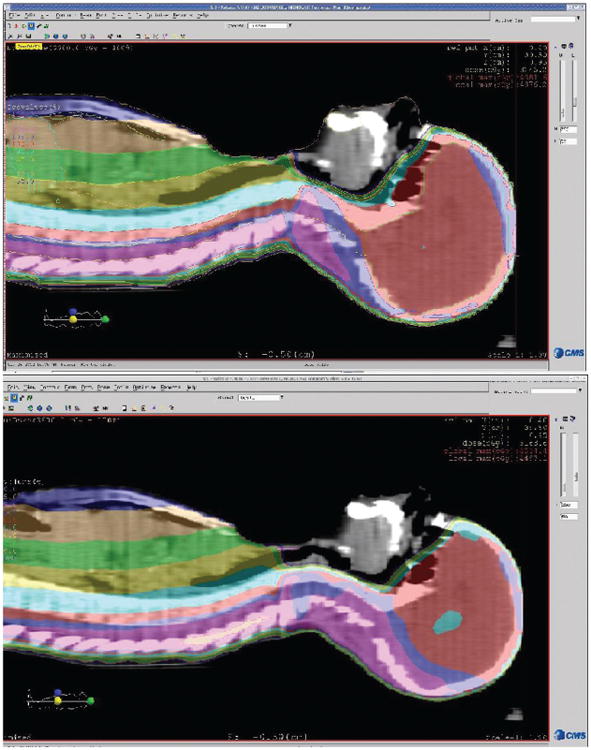
Comparison of superior craniospinal junction versus inferior craniospinal junction for an example patient undergoing craniospinal irradiation and sagittal images. The top panel represents the plan obtained with a superior match line, whereas the bottom panel represents the plan from an inferior match line. The mean thyroid dose with the superior junction was 2902 cGy, as compared with 1167 cGy for the inferior junction. Colorwash range: 50%–120%.
Figure 3.

Dose-volume histogram comparison of thyroid dose for superior and inferior craniospinal match line plans. Solid line represents superior craniospinal junction plan; dashed line represents inferior craniospinal junction plan.
Radiation treatment planning strategies have been explored to reduce the risk of primary hypothyroidism subsequent to neck irradiation. The utilization of volumetric modulated arc therapy in the delivery of CSI can help achieve high target conformality (20). A previous study reported a high degree of thyroid sparing with the use of protons as compared with photon 3D conformal radiation therapy (21). At CSI doses ≥ 2000 cGy, proton plans were reported to deliver 0% thyroid irradiation as compared with 47% for photon plans.
Another strategy reported to reduce the risk of iatrogenic primary hypothyroidism is thyroid-stimulating hormone suppression. An Italian group induced TSH suppression in 14 euthyroid pediatric Hodgkin's disease patients by administration of L-thyroxine beginning 2 weeks prior to neck radiotherapy, with a rapid taper following the completion of radiotherapy (22). TSH suppression was sufficiently obtained in 8 patients, and was inadequate in 6 patients. A statistically significant improvement in hypothyroidism-free survival was obtained for patients with adequate thyroid suppression compared with their inadequately suppressed peers, with an 8-year hypothyroidism-free survival of 75% vs. 0%, respectively (p=0.009).
These results suggest that the thyroid has a radiation dose response. However, there are a number of limitations to our study. This is a relatively small, retrospective study with variable intervals at which thyroid function tests have been obtained. Furthermore, there is a significantly longer follow-up for patients diagnosed with primary hypothyroidism compared with patients who have yet to be diagnosed, with some in the non-hypothyroidism group potentially becoming hypothyroid in the future. These results should be validated in a larger cohort of patients. The development of new strategies to reduce the radiation dose to the thyroid gland without compromising treatment efficacy may be warranted.
Contributor Information
Christine Lauro, University of Colorado Denver-Radiation Oncology, Aurora, CO, USA.
Margaret E. Macy, Children's Hospital Colorado-Pediatric Neuro-Oncology, CO, USA
Philip Zeitler, Children's Hospital Colorado-Pediatric Endocrinology, CO, USA.
Jennifer Backus, University of Colorado Denver-Radiation Oncology, Aurora, CO, USA.
Pamela Mettler, University of Colorado Denver-Biostatistics and Informatics, Aurora, CO, USA.
Nicholas Foreman, Children's Hospital Colorado-Pediatric Neuro-Oncology, CO, USA.
Arthur K. Liu, University of Colorado Denver-Radiation Oncology, Aurora, CO, USA
References
- 1.Duffner PK, Cohen ME, Anderson SW, Voorhess ML, MacGillivray MH, et al. Long-term effects of treatment on endocrine function in children with brain tumors. Ann Neurol. 1983;14:528–32. doi: 10.1002/ana.410140506. [DOI] [PubMed] [Google Scholar]
- 2.Livesey EA, Brook CG. Thyroid dysfunction after radiotherapy and chemotherapy of brain tumors. Arch Dis Child. 1989;64:593–5. doi: 10.1136/adc.64.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogilvy-Stuart AL, Shalet SM, Gottamaneni HR. Thyroid function after treatment of brain tumors in children. J Pediatr. 1991;119:733–7. doi: 10.1016/s0022-3476(05)80288-4. [DOI] [PubMed] [Google Scholar]
- 4.Constine LS, Woolf PD, Cann D, Mick G, McCormick K, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 5.Chin D, Sklar C, Donahue B, Uli N, Geneiser N, et al. Thyroid dysfunction as a late effect in survivors of pediatric medulloblastoma/primitive neuroectodermal tumors: a comparison of hyperfractionated versus conventional radiotherapy. Cancer. 1997;80:798–804. doi: 10.1002/(sici)1097-0142(19970815)80:4<798::aid-cncr19>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Paulino AC. Hypothyroidism in children with medulloblastoma: a comparison of 3600 and 2340 cGy craniospinal radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:543–7. doi: 10.1016/s0360-3016(02)02744-x. [DOI] [PubMed] [Google Scholar]
- 7.Constine LS, Donaldson SS, McDougall IR, Cox RS, Link MP, et al. Thyroid dysfunction after radiotherapy in children with Hodgkin's disease. Cancer. 1984;53:878–83. doi: 10.1002/1097-0142(19840215)53:4<878::aid-cncr2820530411>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Hancock SL, McDougall IR, Constine LS. Thyroid abnormalities after therapeutic external radiation. Int J Radiat Oncol Biol Phys. 1995;31:1165–70. doi: 10.1016/0360-3016(95)00019-U. [DOI] [PubMed] [Google Scholar]
- 9.Kuten A, Lubochitski R, Fishman G, Dale J, Stein ME. Postradiotherapy hypothyroidism: radiation dose response and chemotherapeutic radiosensitization at < 40 Gy. J Surg Oncol. 1996;61:281–3. doi: 10.1002/(SICI)1096-9098(199604)61:4<281::AID-JSO10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Sklar C, Whitton J, Mertens A, Stovall M, Green D, et al. Abnormalities of the thyroid in survivors of Hodgkin's disease: date from the Child Cancer Survivor Study. J Clin Endocrinol Metab. 2000;85:3227–32. doi: 10.1210/jcem.85.9.6808. [DOI] [PubMed] [Google Scholar]
- 11.Boomsma MJ, Bijl H, Langendijk JA. Radiation-induced hypthyroidism in head and neck cancer patients: a systemic review. Radiother Oncol. 2011;99:1–5. doi: 10.1016/j.radonc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Marks LB, Yorke E, Jackson A, Ten Haken RK, Constine LS, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:s10–19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakhshandeh M, Hashemi B, Mahdavi SR, Nikoofar A, Vasheghani M, et al. Normal tissue complication probability modeling of radiation-induced hypothyroidism after head-and-neck radiation therapy. Int J Radiat Oncol Biol Phys. 2010;85:514–21. doi: 10.1016/j.ijrobp.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Emami B, Lyman J, Brown A, Coia L, Goitein M, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–8. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 16.Turkkahraman D, Alper OM, Aydin F, Yildiz A, Pehlivanoglu S, et al. Final diagnosis in children with sublinical hypothyroidism and mutation analysis of the thyroid peroxidase gene (TPO) J Pediatr Endocrinol Metab. 2011;22:767–873. doi: 10.1515/jpem.2009.22.9.845. [DOI] [PubMed] [Google Scholar]
- 17.Bonato C, Severino RF, Elnecave RH. Reduced thyroid volume and hypothyroidism in survivors of childhood cancer treted with radiotherapy. J Clin Endocrinol Metab. 2008;21:943–9. doi: 10.1515/jpem.2008.21.10.943. [DOI] [PubMed] [Google Scholar]
- 18.Tell R, Lundell G, Nilsson B, Sjödin H, Lewin F, et al. Long-term incidence of hypothyroidism after radiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;60:395–400. doi: 10.1016/j.ijrobp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia S, Ramsay NK, Bantle JP, Mertens A, Robison LL. Thyroid abnormalities after therapy for Hodgkin's disease in childhood. Oncologist. 1996;1:62–7. [PubMed] [Google Scholar]
- 20.Fogliata A, Bergström S, Cafaro I, Clivio A, Cozzi L, et al. Craniospinal irradiation with volumetric modulated arc therapy: a multi-institutional treatment experience. Radiother Oncol. 2011;99:79–85. doi: 10.1016/j.radonc.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Lee CT, Bilton SD, Famiglietti RM, Riley BA, Mahajan A, et al. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005;63:362–72. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 22.Massimino M, Gandola L, Pignoli E, Seregni E, Marchianò A, et al. TSH suppression as a possible means of protection against hypothyroidism after irradiation for childhood Hodgkins lymphoma. Pediatr Blood Cancer. 2011;57:166–8. doi: 10.1002/pbc.22915. [DOI] [PubMed] [Google Scholar]