Abstract
The relationship between recollection-mediated recognition memory and behavioral pattern separation is poorly understood. In two separate experiments, we modified a well-validated object discrimination task with previously demonstrated sensitivity to neural pattern separation with instructions to assess recollection and familiarity. In the first experiment, we included a Remember/Know (R/K) judgment, and in the second we included a source memory judgment. We found that both “Remember” and correct source judgments were higher for lures labeled “similar” (where pattern separation is engaged) but also higher on lures called “old” (where pattern separation is absent), suggesting that false alarms in pattern separation tasks are frequently mediated by recollection. As one might expect, “Remember” judgments and correct source decisions increased with greater dissimilarity for “similar” responses and increased with greater similarity for “old” responses. This suggests that recollection can occur in the presence and in the absence of pattern separation and that false alarms to similar lures are not simply driven by familiarity.
Keywords: recollection, familiarity, pattern separation, pattern completion, recognition memory
INTRODUCTION
Recognition memory is supported by the medial temporal lobe (MTL), as damage to this brain region across species impairs performance on recognition memory tasks (cf. Squire et al. 2007). Studies have consistently demonstrated a role for the perirhinal cortex in this type of memory (Brown and Aggleton, 2001; Winters and Bussey, 2005; Winters et al., 2007, 2008); however the role of the hippocampus has been subject to much debate. Numerous studies have reported impairments in recognition memory with hippocampal lesions (Clark et al., 2000; Zola et al., 2000; Stark et al., 2002; Broadbent et al., 2004, 2010), yet others have reported no impairments (Winters et al. 2004; Good et al. 2007) or even enhancements with reversible lesions (Oliveira et al., 2010).
One potential reason for this discrepancy is that recognition memory has typically been thought of as a combination of two distinct processes, recollection and familiarity (Yonelinas, 2002; Aggleton and Brown, 2006; Diana et al., 2007). According to the Dual-Process Signal Detection model (Yonelinas, 2002), recollection is represented by a threshold process, while familiarity is represented by a standard signal detection model based on a Gaussian distribution of memory strength. Such models have typically ascribed the recollective process to the hippocampus and the familiarity process to regions outside of the hippocampus such as the perirhinal cortex (Yonelinas et al., 2010). The role of the hippocampus in recollection and familiarity has been subject to much investigation in patients with MTL damage. Evidence for impaired recollection and relatively spared familiarity has been reported in fornix lesion patients (Tsivilis et al., 2008) and mammillary body lesion patients (Vann et al., 2009) as well as patients with hippocampal damage (Yonelinas, 2002; Holdstock et al., 2005; Turriziani et al., 2008).
From a computational perspective, the hippocampus is thought to be involved in pattern separation, the process by which similar memories are stored using distinct orthogonal neural codes such that interference can be minimized. The role of the hippocampus in this process has been hypothesized since Marr (1971) and was one of the central tenets of the widely influential Complementary Learning Systems approach (McClelland et al., 1995). Given its architecture, the hippocampus is exceptionally well suited for processing sparsely coded pattern separated representations. It is this ability that allows it to support detailed contextual encoding, such as that required for recollection. In contrast, the perirhinal cortex is thought to encode information in a much more distributed and overlapping fashion which would be very poor at supporting pattern separation, but might rather be particularly good at extracting statistical generalities across experiences and computing a global match (Norman et al., 2010; O’Reilly et al., 2011).
Recent data across species and methods have provided remarkably convergent evidence for the role of the hippocampus in pattern separation (cf. review by Yassa and Stark, 2011). In situations where the level of interference is parametrically manipulated, studies in animals (Leutgeb et al., 2004, 2007) and in humans (Lacy et al., 2011) have shown that the hippocampal dentate and CA3 regions respond in a discontinuous threshold-like fashion, sharply changing their representations in response to small changes in the input, compared to signals in CA1, which change much more gradually. This threshold-like process is the hallmark of pattern separation, and is thought to be the result of attractor dynamics in CA3, which force representations into stable attractor states.
Given its remarkable similarity to the threshold process thought to underlie recollection, this mode of hippocampal response has been taken as suggestive evidence that recollection is dependent on hippocampal pattern separation (Yonelinas et al., 2010). However, Norman (2010) as well as Yassa and Stark (2011) discuss the circumstances under which this relationship may or may not hold true. For example, one can have a recollective experience in the absence of pattern separation if there is minimal interference with other similar experiences (Yassa and Stark, 2011). Nevertheless, this relationship has never been directly tested.
To test the hypothesis that recollection and pattern separation are dissociable and define the behavioral conditions under which individuals can exhibit both or one but not the other, we crossed two commonly used designs to test recollection and familiarity (the Remember/Know paradigm, and a source memory task) with a visual object recognition task that uses lures varying in similarity so that the effect of interference can be quantified.
MATERIALS AND METHODS
Participants
We tested a sample of healthy adults (Experiment 1: N = 49, mean age 28 SD 11, 33F:16M; Experiment 2: N = 30, mean age 23 SD 4, 17F:13M). Participants were recruited from the Johns Hopkins University and surrounding communities using flyer advertising and paid $10 per hour for participation in the study. All participants provided written informed consent.
Experiment 1 Procedures
Participants completed a visual object recognition memory task with a two-stage recognition test. During the first phase (incidental study), participants were shown 128 color images of common objects on a computer screen (2,000 ms, 500 ms inter-stimulus-interval [ISI]) and asked to decide whether the item was an “indoor” or an “outdoor” item. They indicated their responses using the computer keyboard. During the second phase (recognition test), participants were shown a series of 192 images on the screen (2,000 ms, 500 ms ISI), 64 of which were old targets (previously seen images), 64 were similar lures (images that were similar but not identical to ones they’ve previously seen), and 64 were dissimilar foils (never before seen images). See Figure 1A for a task schematic.
FIGURE 1.
(A) Experiment 1 task schematic. (B) Experiment 2 task schematic. In both tasks, participants incidentally encode pictures of objects and are asked to make recognition memory judgments, followed by a second-level assessment of recollection and familiarity. This is performed using a Remember/Know procedure in Experiment 1 and a source memory judgment in Experiment 2. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For each image, participants were instructed to indicate if the image was “old,” “similar” or “new.” Following this response, participants were asked to make a secondary remember/ know judgment. If participants responded “old,” they were asked to report whether they “remember” (R) seeing the same image in the study session or if they just “know” (K) that they have seen the same image without any conscious recollection of its original presentation. If participants responded “similar,” they were asked to report whether they “remember” (R) seeing the original image in the study session or if they just “know” (K) that they have seen a similar image without any conscious recollection of its original presentation. If participants responded “new,” they were asked to report whether they were “sure” or “unsure” of their response. Explicit and detailed instructions as to how to use these responses were given before the task was administered with examples of the contextual details that are required for a R response (see Supporting Information S1 for complete participant instructions.)
Participants were given up to 2,000 ms to respond “old,” “similar,” or “new” to each object. Following their response, the screen prompted them to indicate whether they “remember” or “know” (if an “old” or “similar” response was made) or to indicate whether they are “sure” or “unsure” (if a “new” response was made). Here, they were given up to 4,000 ms to respond to allow enough time for conscious recollection of details.
Experiment 2 Procedures
Participants completed a similar object recognition memory task with a two-stage recognition test (Fig. 1B). During the first phase (study), participants were shown 128 color images of common objects on a computer screen (3,000 ms, 500 ms ISI), each of which were randomly displayed in one of four quadrants on the screen. Similar to Experiment 1, participants were asked to decide whether the item was an “indoor” or an “outdoor” item and to indicate their responses using the computer mouse by clicking buttons on the screen. During the second phase (test), participants were shown a series of 192 images on the screen (3,000 ms, 500 ms ISI), 64 of which were old targets (previously seen images), 64 were similar lures (images that were similar but not identical to ones they have seen before), and 64 were dissimilar foils (never before seen images).
For each image, participants were instructed to indicate if the image was “old,” “similar” or “new.” Participants were given up to 3,000 ms for this judgment. Following their response, participants were asked to make a secondary source memory judgment. If participants responded “old,” they were asked to use the mouse to select the quadrant in which the same image was presented during the study phase. If participants responded “similar,” they were asked to select the quadrant in which the original image was presented during the study phase. If participants responded “new,” they were not asked to click any quadrants, but instead to report whether they were “sure” or “unsure” of their response. Participants were given up to 4,000 ms for this second-level judgment.
RESULTS
Experiment 1. Remember versus Know
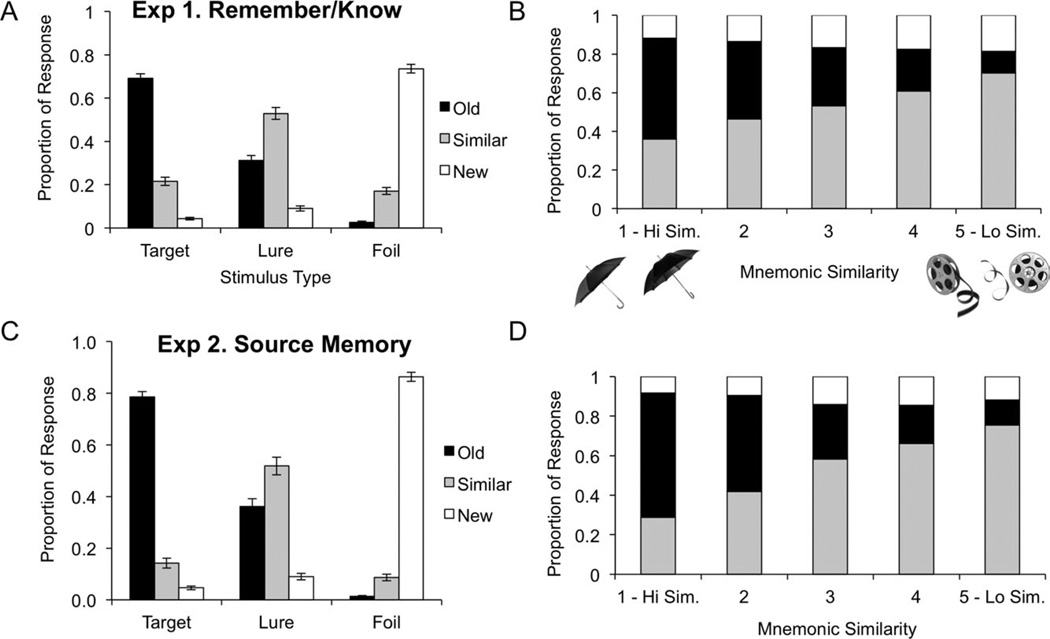
The average frequency of “old,” “similar,” and “new” responses for each stimulus type (target, lure, and foil) are shown in Figure 2A. Overall, subjects were able to correctly identify targets as “old” (70%) and foils as “new” (74%). Their responses were more varied, however, on the lure items where a significant proportion of the items were incorrectly identified as “old” (31%) instead of “similar” (53%). This pattern of behavioral performance replicates our previous results (Yassa et al., 2010) with the same task and indicates that the addition of the R/K procedure did not significantly alter recognition performance.
FIGURE 2.
(A) Recognition performance in Experiment 1. (B) Categorizing lure stimuli by mnemonic similarity in Experiment 1 shows frequency of “old” responses (false alarms) increases as similarity increases. (C) Recognition performance in Experiment 2. (D) Similar to (B), in Experiment 2, frequency of false alarms increases as similarity increases.
The lures shown in this experiment have been previously categorized according to their similarity based on a large orthogonal sample of young healthy adults (see Yassa et al., 2010 for a description of that sample). Briefly, the probability of false alarms on a stimulus-by-stimulus basis was used to create a continuous parametric distribution of mnemonic similarity. We then derived five similarity bins (L1–L5) by splitting this distribution into quintiles, with stimulus pairs in L1 being the most similar and those in L5 being the least similar. Here, we also replicated our prior findings (Yassa et al., 2010) that the probability of calling a lure item “similar” increases monotonically as a function of decreasing similarity and the opposite pattern is true for calling lure items “old” (Fig. 2B). Simple regression showed that the capacity for rejecting lures (i.e., discrimination) was linearly correlated with mnemonic similarity (R2 = 0.99).
Next, we examined the second-stage R/K responses to target and lure items called “similar” or “old.” On targets called “old,” participants were biased toward “remember” responses rather than “know” (54% vs. 14% respectively), suggesting that memory for previously seen items was strong and predominantly driven by recollection (Fig. 3B). On foils called “new,” participants were biased toward “sure” responses rather than “unsure” (57% vs. 15%, respectively), suggesting that their confidence in rejecting foils was also high (Fig. 3C).
FIGURE 3.
(A–E) Behavioral characterization of Remember/ Know responses (Exp. 1) (A) Remember/Know and Sure/Unsure response distributions for lure stimuli, (B) target stimuli and (C) foil stimuli. Categorizing lure stimuli by mnemonic similarity shows frequency of R responses decreases with decreasing similarity in false alarms (D) and increases with decreasing similarity in lure correct rejections (E). (F–J) Behavioral characterization of source memory judgments (Exp. 2) (F) Source correct and incorrect and Sure/Unsure response distributions for lure stimuli, (G) target stimuli and (H) foil stimuli. Similar to Exp. 1, frequency of source correct responses decreases with decreasing similarity in false alarms (I) and increases with decreasing similarity in lure correct rejections (J). Overall, the data from both experimental manipulations are very similar.
Performance on the lure items (Fig. 3A) was used as the critical test of our hypothesis. On lures called “similar,” participants were biased toward “remember” responses rather than “know” (32% vs. 20% respectively), which is consistent with the notion that trials requiring pattern separation do preferentially involve recollection. Surprisingly, however, we also found that participants were also biased toward “remember” responses rather than “know” on lures called “old” (20% vs. 10%). Lures called “old” are instances of false memories where the suspected cause is a failure of pattern separation and an increased propensity towards pattern completion instead.
We examined R/K response frequencies across mnemonic similarity bins to elucidate the relationship between recollection and varying levels of interference. Here, we found that the R responses decreased monotonically as a function of decreasing similarity for “old” responses (slope of regression = −0.07, R2 = 0.98; Fig. 3D) and increased monotonically as a function of decreasing similarity for “similar” responses (slope of regression = 0.06, R2 = 0.98; Fig. 3E). K responses were also modulated by level of similarity during lure correct rejections, although the slope was much less steep for both lures called “similar” (slope of regression = 0.02, R2 = 0.86; Fig. 3E) and false alarms (slope of regression = −0.02, R2 = 0.98; Fig. 3D). This pattern suggests that accurate recollection is more likely in situations where there is low interference (i.e., items are not too similar), while false recollection is more likely in situations where there is high interference (i.e., items are very similar).
Experiment 2. Source Memory Judgment
The average frequency of “old,” “similar,” and “new” responses for each stimulus type (target, lure, and foil) are shown in Figure 2C. Overall, subjects were able to correctly identify targets as “old” (79%) and foils as “new” (88%). Their responses were more varied, however, on the lure items where a significant proportion of the items were incorrectly identified as “old” (35%) instead of “similar” (54%). This pattern was very similar to results from Experiment 1 and suggests that the two experimental manipulations were largely comparable.
Next, we examined the second-stage source memory judgments. On foils called “new,” participants had a higher number of “sure” responses rather than “unsure” (77% vs. 8% respectively; Fig. 3H). On targets called “old”, participants had a higher number of correct than incorrect source judgments (58.9% vs. 19.5% respectively; Fig. 3G). On lures called “similar” (Fig. 3F), participants had a higher number of correct than incorrect source judgments (38% vs. 14%, respectively). Participants also had a higher number of correct than incorrect source judgments on lures called “old” (false alarms) (25% vs. 11%). This pattern is similar to the pattern observed in Experiment 1.
We further examined source memory judgments across mnemonic similarity bins and found that much like Experiment 1, correct source responses decreased monotonically as a function of decreasing similarity for “old” responses (slope of regression = −0.096, R2 = 0.96; Fig. 3I) and increased monotonically as a function of decreasing similarity for “similar” responses (slope of regression = 0.086, R2= .99; Fig. 3J). Incorrect source responses were also modulated by levels of similarity during lure correct rejections, although the slope was much less steep for both lures called “similar” (slope of regression = 0.025, R2 = 0.80; Fig. 3J) and false alarms (slope of regression = −0.028, R2 = 0.95; Fig. 3I). Consistent with Experiment 1, this pattern further suggests that accurate recollection (here, as indexed by source memory) is more likely when interference is low, and false recollection is more likely when interference is high.
DISCUSSION
A large prior literature has consistently suggested that false memories are based on gist of familiarity (Johnson and Raye, 1981; Roediger and McDermott, 1995; Mather et al., 1997; Norman and Schacter, 1997; Miller and Wolford, 1999; Fabiani et al., 2000). However, false memories based on recollection such as what we observed in our study have also been reported in several previous studies (Gallo et al., 2001; Roediger et al., 2004; Kensinger and Schacter, 2007; Stahl and Klauer, 2008). Our results suggest that the frequency with which these false recollections occur is similarly distributed across experiences where pattern separation occurs and does not occur. These results can be taken as evidence against the notion that recollection is in any way selective for pattern separation, since it can clearly occur in its absence. Furthermore, they suggest that the amount of recollection varies as a function of interference level during both pattern separation and pattern completion. This sheds new light on the computational processes that underlie recollection and familiarity.
One important point to make is that many situations that are often interpreted as evidence for recollection or recollection impairments in amnesic patients can be thought of as instances where demands on hippocampal pattern separation are varied. For example, Patient Y.R., who has damage limited to the hippocampus, showed relatively spared item yes/no recognition memory performance on a number of tests (Mayes et al., 2002). However, not all recognition tests were spared. When targets and lures were similar, Y.R.’s yes/no recognition memory performance was impaired relative to controls. This precisely describes situations in which pattern separation is required. When pattern separation demands were reduced, that is, by presenting targets and related foils in a forced-choice format or by presenting targets and unrelated foils, Y.R.’s performance was unimpaired relative to controls (Holdstock et al., 2002).
A recent study by Migo et al. (2009) used the same test procedures used with Y.R. in healthy adults. Results suggest that recollection mediates yes/no recognition by reducing the frequency of false alarms using a “recall-to-reject” strategy. The study had the distinct advantage of using a “justified RF” procedure where participants were asked to verbalize their justification for recollection by explaining what they recollected at the time, which allowed inferences about recall-to-reject to be made. This process was assessed in our study on trials where participants made “similar” responses to lures and designated them as “remembered.” On these trials, participants were instructed to use this designation if they can recall details about the original presentation (i.e., “recall-to-reject” occurs). Both studies suggest that correct performance on these trials is mediated by recollection. Additionally, our study demonstrates that false alarms can also be mediated by recollection, especially under conditions of high similarity. This is somewhat at odds with the findings of Migo et al., who conclude that false alarms are primarily driven by familiarity; however, the two studies are not directly comparable since the study by Migo et al. did not manipulate stimulus similarity or assess the interaction between recollection and mnemonic interference. Our results, based on both an R/K procedure as well as a source memory test suggest that false recollection mediates false alarms as stimulus similarity is increased, which is consistent with a failure of pattern separation at high levels of interference (Norman et al., 2010).
Both Yonelinas et al. (2010) and Norman (2010) discuss situations where similarity is so high that pattern separation fails. We believe that our Lure bin 1 (most similar) is an instance where pattern separation will often fail. The probability of calling a lure “old” in Lure bin 1 (i.e., false alarm) is around 55%. Key to the current investigation, this is precisely the bin that has the largest difference between recollection and familiarity-mediated responses with recollection being much higher. Thus, despite the failure of pattern separation, recollection still manifests, suggesting that the two processes are dissociable.
Although the R/K procedure has been widely used and accepted as a tool for assessing recognition memory in the past, recent literature argues that it is an unreliable measure for recollection and familiarity due to inadequate R/K explanation, poor administration of the task, and flawed overall design (Eldridge et al., 2002; Mayes et al., 2007; Dunn, 2008; Wais et al., 2008; Wixted, 2009). We took considerable measures to ensure participants recognized this distinction using extensive descriptions and examples, as shown in the participant instructions (see Supporting Information S1). Another potential limitation of the R/ K procedure, unrelated to its administration, is the subjective nature of the decisions, which makes it difficult to map onto a specific neural process. This concern is ameliorated by our second experiment, which uses a source memory judgment as an objective measure of recollection. Our results demonstrate that there is no one-to-one mapping between pattern separation and either subjective or objective measures of recollection.
Although source memory judgments offer a more objective index of recollection than R/K, It should be noted that source memory judgments could also be driven by associative familiarity (Migo et al., 2012). With only four position choices to choose from in the source memory task, relative familiarity strength can potentially contaminate behavioral performance traditionally thought to be mediated by recollection. However, since results from both the source memory experiment and the R/K experiment are convergent, this likely does not explain the results. Another facet of behavioral performance that is relevant but not emphasized here is the extent to which task instructions may bias participants to use more or less stringent criteria for rejections. If participants are instructed that they will encounter highly similar items and that their tasks is really to discriminate the two, they may be more tuned to details and behavioral performance may demonstrate fewer false recollections. This is an important possibility to test in future experiments.
Recollection is thought to reflect a neural process that involves associating source (where and when) information with the experience to create a detailed contextual representation. Pattern separation, at face value, offers a suitable mechanism for this type of detailed encoding. However, our results demonstrate that recollection can occur in the absence of pattern separation. It is possible that accurate recollection requires pattern separation, while false recollection does not. The mechanism underlying false memories and the extent to which they are different from true memories has been the subject of several neuroimaging investigations. In general, studies have found that much of the neural representation is overlapping across both true and false memories with some differences in early sensory cortical regions (Slotnick and Schacter, 2004; Garoff-Eaton et al., 2006; Stark et al., 2010a,b; Dennis et al., 2012). Differences in the MTL have generally not been found except by one study (Dennis et al., 2012).
Several behavioral and neuroimaging studies have shown that false memories can result from a failure of pattern separation and an increased propensity towards pattern completion (Stark et al., 2010a,b; Yassa et al., 2010, 2011). The hippocampus is capable of performing both separation and completion, albeit in different subfields that possess the appropriate circuitry for each computation. The hippocampal dentate gyrus is capable of performing pattern separation due to its sparse firing pattern and is thought to project this powerful signal onto the downstream CA3 region, which contains a massive recurrent collateral network capable of autoassociation (i.e., pattern completion) (Marr, 1971; O’Reilly and McClelland, 1994; Treves and Rolls, 1994; McClelland et al., 1995; Norman and O’Reilly, 2003; Yassa and Stark, 2011). The fact that true recollections (likely dependent on a mixture of pattern separation and pattern completion) and false recollections (likely dependent on pattern completion alone) both seem to engage the hippocampus is perhaps not surprising in light of the fact that neuroimaging studies have typically averaged activity across hippocampal subfields. Thus, one hypothesis that can be tested in future high-resolution neuroimaging studies is whether false recollection engages the CA3 network alone while accurate recollection engages both the CA3 and the dentate gyrus. This may prove difficult, however, since current high-resolution imaging protocols do not distinguish between dentate and CA3 subfields (i.e., spatial resolution is still not high enough to separate them reliably).
In general, our results suggest that the process of recollection can be dissociated from pattern separation, since it can clearly occur in its absence. This has implications that are quite far reaching. For example, there is a large literature on the hippocampus’ disproportionate involvement in recollection versus familiarity (Yonelinas et al., 2010), although these results are still subject to debate (Wixted et al., 2010), and there is also an amassing literature on the role of the hippocampus in pattern separation (cf. Yassa and Stark, 2011). Our paradigms and results suggest that there are situations in which pattern separation and recollection can be assessed independently and may serve as a future platform for imaging studies that will dissociate the contribution of the hippocampus to these two phenomena. We can conclude that recollection and pattern separation are behaviorally dissociable and that false alarms to similar lures are not merely driven by gist or familiarity. The crossed paradigms we used are novel tools by which the behavioral parameters of pattern separation/completion and recollection/familiarity can be measured, and the neural contributions of different MTL regions to these processes can be examined.
Acknowledgments
The authors thank Kristen Thompson and Elizabeth Murray for their assistance in data collection. They also thank Mick Rugg and Andy Yonelinas for helpful discussions regarding this manuscript.
Grant sponsor: NIA; Grant numbers: P50 AG05146 and R01 AG034613; Grant sponsor: Cornell University Summer Internship.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–1420. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Bowman CR, Vandekar SN. True and phantom recollection: An fMRI investigation of similar and distinct neural correlates and connectivity. Neuroimage. 2012;59:2982–2993. doi: 10.1016/j.neuroimage.2011.09.079. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dunn JC. The dimensionality of the remember-know task: A state-trace analysis. Psychol Rev. 2008;115:426–446. doi: 10.1037/0033-295X.115.2.426. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Sarfatti S, Knowlton BJ. The effect of testing procedure on remember-know judgments. Psychon Bull Rev. 2002;9:139–145. doi: 10.3758/bf03196270. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Stadler MA, Wessels PM. True but not false memories produce a sensory signature in human lateralized brain potentials. J Cogn Neurosci. 2000;12:941–949. doi: 10.1162/08989290051137486. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Roediger HL, 3rd, McDermott KB. Associative false recognition occurs without strategic criterion shifts. Psychon Bull Rev. 2001;8:579–586. doi: 10.3758/bf03196194. [DOI] [PubMed] [Google Scholar]
- Garoff-Eaton RJ, Slotnick SD, Schacter DL. Not all false memories are created equal: The neural basis of false recognition. Cereb Cortex. 2006:161645–161652. doi: 10.1093/cercor/bhj101. [DOI] [PubMed] [Google Scholar]
- Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci. 2007;121:218–223. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Cezayirli E, Isaac CL, Roberts N, O’Reilly R, Norman K. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL. Reality monitoring. Psychological Review. 1981;88:67–85. [Google Scholar]
- Kensinger EA, Schacter DL. Remembering the specific visual details of presented objects: Neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45:2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human Ca3/dentate and Ca1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and Ca3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas Ca3 and Ca1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Mather M, Henkel LA, Johnson MK. Evaluating characteristics of false memories: Remember/know judgments and memory characteristics questionnaire compared. Mem Cognit. 1997;25:826–837. doi: 10.3758/bf03211327. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cognit Neurosci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with damage limited to the hippocampus. Hippocampus. 2002;12:325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Migo E, Mayes A, Montaldi D. Measuring recollection and familiarity: Improving the remember/know procedure. Conscious Cogn. 2012;21:1435–1455. doi: 10.1016/j.concog.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Migo E, Montaldi D, Norman KA, Quamme J, Mayes A. The contribution of familiarity to recognition memory is a function of test format when using similar foils. Q J Exp Psychol (Hove) 2009;62:1198–1215. doi: 10.1080/17470210802391599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Wolford GL. Theoretical commentary: the role of criterion shift in false memory. Psychological Review. 1999;106:398–405. [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: Exploring the characteristics of illusory memories. Mem Cognit. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: Revisiting the complementary learning systems model. Hippocampus. 2010;20:1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Bhattacharyya R, Howard MD, Ketz N. Complementary Learning Systems. Cogn Sci. 2011 doi: 10.1111/j.1551-6709.2011.01214.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: remembering words not presented in lists. J Exp Psychol Learn Mem Cognit. 1995;21:803–814. [Google Scholar]
- Roediger HL, 3rd, McDermott KB, Pisoni DB, Gallo DA. Illusory recollection of voices. Memory. 2004;12:586–602. doi: 10.1080/09658210344000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A New perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C, Klauer KC. A simplified conjoint recognition paradigm for the measurement of gist and verbatim memory. J Exp Psychol Learn Mem Cognit. 2008;34:570–586. doi: 10.1037/0278-7393.34.3.570. [DOI] [PubMed] [Google Scholar]
- Stark CE, Bayley PJ, Squire LR. Recognition memory for single items and for associations is similarly impaired following damage to the hippocampal region. Learn Mem. 2002;9:238–242. doi: 10.1101/lm.51802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Okado Y, Loftus EF. Imaging the reconstruction of true and false memories using sensory reactivation and the misinformation paradigms. Learn Mem. 2010a;17:485–488. doi: 10.1101/lm.1845710. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010b;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Turriziani P, Serra L, Fadda L, Caltagirone C, Carlesimo GA. Recollection and familiarity in hippocampal amnesia. Hippocampus. 2008;18:469–480. doi: 10.1002/hipo.20412. [DOI] [PubMed] [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, Quamme JR, Yonelinas AP, Aggleton JP, Montaldi D, Mayes AR. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA. 2009;106:5442–5447. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Mickes L, Wixted JT. Remember/know judgments probe degrees of recollection. J Cognit Neurosci. 2008;20:400–405. doi: 10.1162/jocn.2008.20041. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, Bussey TJ. Scopolamine infused into perirhinal cortex improves object recognition memory by blocking the acquisition of interfering object information. Learn Mem. 2007;14:590–596. doi: 10.1101/lm.634607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT. Remember/Know judgments in cognitive neuroscience: An illustration of the underrepresented point of view. Learn Mem. 2009;16:406–412. doi: 10.1101/lm.1312809. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Mickes L, Squire LR. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20:1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci USA. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal Ca3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research 1. J Mem Language. 2002;46:441–517. [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]