Abstract
AIM: To evaluate the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for pancreatic solid tumors larger or smaller than 3 cm, and cystic lesions.
METHODS: From January/1997 to December/2006, 611 patients with pancreatic tumors were subjected to EUS-FNA. The final diagnosis was obtained either by surgery (356 cases) or after a mean clinical follow-up of 11.8 mo in the remaining patients.
RESULTS: There were 405 solid tumors, 189 cystic lesions and 17 mixed. Pancreatic specimens for cytological assessment were successfully obtained by EUS-FNA in 595 (97.4%) cases. There were 352 (57.6%) malignancies and 259 (42.4%) benign tumors. Among the malignancies, pancreatic adenocarcinomas accounted for 67% of the lesions. Overall, the sensitivity, specificity, positive and negative predictive values, and accuracy of EUS-FNA were, respectively, 78.4%, 99.2%, 99.3%, 77.2% and 87.2%. Specifically for solid tumors, the same parameters for neoplasms larger and smaller than 3 cm were, respectively, 78.8% vs 82.4%, 100% vs 98.4%, 100% vs 99%, 54.8% vs 74.1% and 83.1% vs 87.8%. For cystic lesions, the values were, respectively, 72.2%, 99.3%, 97.5%, 91% and 92.2%.
CONCLUSION: EUS-FNA can be used to sample pancreatic tumors in most patients. Even though the negative predictive value is inadequate for large solid tumors, the results are rather good for small solid tumors, especially concerning the sensitivity, negative predictive value and diagnostic accuracy. Among all pancreatic lesions, EUS-FNA for cystic lesions can reveal the best negative predictive value and diagnostic accuracy, both higher than 90%.
Keywords: Diagnosis, Endoscopic ultrasound, Fine needle-aspiration biopsy, Pancreas cancer, Pancreatic disease, Sampling
INTRODUCTION
Endoscopic ultrasound (EUS) is the best diagnostic tool for locoregional staging of pancreatic tumors[1-3]. However, similar to other imaging methods, EUS cannot differentiate easily a pancreatic malignancy from an inflammatory process[3-5]. Cytological brushes from the main pancreatic duct performed during endoscopic retrograde pancreatography, as well as surgical biopsies are additional procedures to improve the diagnostic yield, although both methods have neither a good sensitivity nor a better diagnostic accuracy for pancreatic neoplasms[6-9]. To overcome these drawbacks, EUS-guided fine needle aspiration (EUS-FNA) of pancreatic tumors is a very good choice, with its safety, feasibility and high diagnostic accuracy confirmed by many studies[10,11].
We conducted this study to evaluate the diagnostic accuracy of EUS-FNA for pancreatic solid tumors larger or smaller than 3 cm, and cystic lesions.
MATERIALS AND METHODS
From January/1997 to December/2006, 1043 patients with pancreatic tumors were subjected to EUS-FNA in a single referral center. Four hundred thirty-two cases were lost to follow-up. Six hundred eleven (58.6%) patients were available for retrospective review. The final diagnosis was based on surgical findings (n = 356) or by a mean clinical follow-up of 11.8 (range: 2 to 32) mo (n = 255). Procedures were carried out by the same endosonographer (JCA) using either Pentax linear echoendoscopes FG 32-UA, FG 36-UX and FG-38UX (Pentax Precision Instruments, Inc., Orangeburg, NY) with a HITACHI 405 or 515 EUB ultrasound platform, or Olympus UCT-160 (Olympus Optical Corp. Ltd., Tokyo, Japan) with a UC-60 ultrasound platform (Suzy-Olympus Optical Corp. Ltd., Tokyo, Japan). EUS-FNA was performed by using a 22-gauge, 8-cm shot gun aspiration needle (NA-11J-KB, Olympus Optical Co., Tokyo, Japan), under conscious sedation with propofol and cardiorespiratory monitoring. Patients were left on their left side position after overnight fast before the procedure. Antibiotic prophylaxis was given during the procedure for puncturing all cystic lesions. Passage was transduodenal for lesions in the head and uncinate process of the pancreas and transgastric through the lesser sac for lesions in the body and tail (Figure 1).
Figure 1.
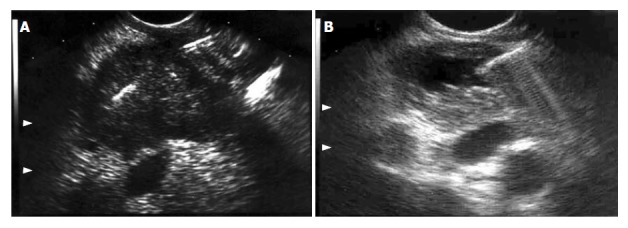
EUS images during FNA of (A) solid pancreatic tumor (colon carcinoma metastasis), and (B) cystic lesion (cystadenocarcinoma), both confirmed by the cytopathological assessment. Note the needle inside both lesions.
Cytopathological assessment
Aspirated samples were evaluated either by means of cytological smears or cell blocks. All cytological samples were interpreted by one of the two experienced cytopathologists (FV and GCS). The number of passes of the needle until satisfactory specimens were obtained was documented in each case. Briefly, once aspirated, the material was expelled onto slides, and two smears were made, followed by fixation in buffered formalin, and staining with Papanicolaou and Wright-Giemsa stains. Specimens for cell blocking were fixed in buffered formalin, submitted to centrifugation, and immersed in liquid agarose. Once solidified, the agar cone with the cells in the top was embedded in paraffin to be handled as a routine tissue block. Thin 3-mm sections from paraffin-embedded cell blocks were cut, mounted on glass slides, and stained with haematoxylin and eosin. Immunocytochemical stains were carried out by the avidin-biotin peroxidase method. On review of the slides, cellularity, presence of loosely cohesive aggregates or single tumor cells, quality and quantity of cytoplasm, nuclear pleomorphism, chromatin patterns, nucleus to cytoplasm ratio and necrosis were systematically analysed in each case.
Statistical analysis
The significance level was 5% for all statistical procedures. Numerical variables were expressed as mean ± SD and comparative analysis between them was performed by Student’s t-test. All categorical data were analysed by chi-square test with Yates correction and Fischer’s exact test. Concerning the diagnosis obtained by EUS-FNA, sensitivity, specificity, positive and negative predictive values, and accuracy were calculated with a 2 × 2 table. For statistical procedures, lesions in the head, uncinate process and pancreatic neck were grouped together; and the same was done for masses invading both body and tail, which were grouped as body lesions.
RESULTS
Three hundred fourteen (51.4%) patients were females, and their mean age was 57.8 (range: 11-89) years.
The main reasons to perform EUS-FNA in pancreatic tumors were suspicion of solid malignant neoplasia (44.2%), cystic collections (28.3%) and to obtain the differential diagnosis between pancreatic cancer and focal chronic pancreatitis (11.6%). Other indications are delineated on Figure 2. In addition to the endosonographic evaluation, as well as computed tomography, some patients were previously submitted to other diagnostic approaches (Table 1).
Figure 2.
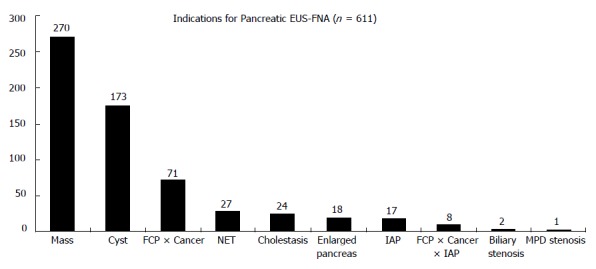
Indications for pancreatic EUS-FNA. FCP: focal chronic pancreatitis; NET: neuroendocrine tumor; IAP: idiopathic acute pancreatitis; MPD: main pancreatic duct.
Table 1.
Diagnostic evaluation before EUS
| Imaging modalities | n | % |
| Only CT | 289 | 47.3 |
| CT + MRI | 190 | 31.1 |
| CT + ERCP | 120 | 19.6 |
| CT + MRI + ERCP | 12 | 2.0 |
CT: Computed tomography; MRI: Magnetic resonance imaging; ERCP: Endoscopic retrograde colangiopancreatography.
Four hundred ten (67.1%) tumors were located in the pancreatic head, 161 (26.3%) in the body, 40 (6.6%) in the tail (Table 2). Passage was transduodenal for 410 (67%) tumors, and transgastric for 201 (33%) cases. The general mean size of the tumors was 3.4 cm (range: 0.4-14.4), and lesions less than 3 cm accounted for 43% of the cases. The average size of solid tumors larger and smaller than 3 cm, was, respectively, 4.3 cm (3-10.3 cm) and 1.8 cm (0.4-2.9 cm). The mean size of cystic lesions was 3.7 cm (0.4-14.4).
Table 2.
Location of the pancreatic tumors in patients submitted to EUS-FNA
| Head1 | Body2 | Tail | Total | |
| Solid tumors ≥ 3 cm | 169 | 42 | 14 | 225 |
| Solid tumors < 3 cm | 119 | 52 | 9 | 180 |
| Cystic lesions | 122 | 67 | 17 | 206 |
| Total | 410 | 161 | 40 | 611 |
Lesions in the head, uncinate process and pancreatic neck were grouped together.
Tumors invading body and tail were grouped as body lesions.
EUS diagnosed 405 (66.3%) solid tumors, 189 (30.9%) cystic collections and 17 (2.8%) mixed pattern lesions. Malignant or pre-malignant disease was detected in 352 (57.6%) cases. Adenocarcinoma was diagnosed in 236 (38.6%) cases. The remaining diagnoses are depicted in Table 3. Aspiration samples were successfully collected in 595 (97.4%) patients after an average of 2.2 (range:1-4) passes. The puncture of the tumor was not possible in 2 cases, and in 14 cases the amount of the aspirated specimens was not adequate for cytological assessment, even after multiple passes of the needle. All these cases were diagnosed after surgical resection.
Table 3.
Diagnoses of pancreatic lesions obtained by surgery and clinical follow-up (n = 611)
| Tumour | Type | n |
| Solid (405) | Adenocarcinoma | 233 |
| Focal Chronic Pancreatitis | 87 | |
| Neuroendocrine tumo | 46 | |
| Metastasis | 13 | |
| Lymphnode | 9 | |
| Splenosis | 4 | |
| Lymphoma | 4 | |
| Autoimmune pancreatitis | 4 | |
| Adenoma | 2 | |
| Sarcoma | 2 | |
| Blastomycosis | 1 | |
| Cystic (189) | Pseudocyst | 84 |
| Serous cystadenoma | 42 | |
| Mucinous cystadenoma | 18 | |
| IPMT | 18 | |
| Abscess | 12 | |
| PanIN | 8 | |
| Chronic Pancreatitis | 4 | |
| Tuberculosis | 2 | |
| Neuroendocrine tumor | 1 | |
| Mixed (17) | Cystadenocarcinoma | 8 |
| Adenocarcinoma | 3 | |
| Frantz tumor | 3 | |
| IPMT | 1 | |
| Metastasis | 1 | |
| Neuroendocrine tumor | 1 |
IPMT: Intraductal papillary mucinous tumor; PanIN: Pancreatic intraepithelial neoplasia.
In total, there was agreement between the cytological diagnoses of malignancy with those from surgery or clinical follow-up in 269 of 352 (76.4%) patients. On the other hand, the cytopathology correctly classified 257 of 259 (99.2%) non-neoplastic cases as a benign condition.
In an intention-to-treat analysis, EUS-FNA confirmed the final diagnosis in 526 of 611 (86%) cases. Overall, the sensitivity, specificity, positive and negative predi-ctive values, and diagnostic accuracy of EUS-FNA were, respectively, 78.4%, 99.2%, 99.3%, 77.2% and 87.2% (Table 4).
Table 4.
Sensitivity, specificity, positive and negative predictive values and accuracy of EUS-FNA in the diagnosis of pancreatic tumors
| General (n = 611) | Solid tumors ≥ 3 cm (n = 225) | Solid tumors < 3 cm (n = 180) | Cystic lesions (n = 206) | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Sensitivity | 78.4 | 74.1-82.8 | 78.8 | 72.8-84.8 | 82.4 | 75.5-89.2 | 72.2 | 60.3-84.2 |
| Specificity | 99.2 | 98.1-100 | 100 | 100-100 | 98.4 | 95.2-100 | 99.3 | 98.1-100 |
| PPV | 99.3 | 98.2-100 | 100 | 100-100 | 99.0 | 97-100 | 97.5 | 92.7-100 |
| NPV | 77.2 | 72.6-81.7 | 54.8 | 44.1-65.4 | 74.1 | 64.5-83.6 | 91.0 | 86.6-95.3 |
| Accuracy | 87.2 | 84.5-89.9 | 83.1 | 78.2-88 | 87.8 | 83-92.6 | 92.2 | 88.6-95.9 |
In regard to the value of the method for solid lesions smaller or larger than 3 cm, and cystic lesions, our results were rather good for small solid tumors, especially concerning the sensitivity, negative predictive value and diagnostic accuracy. For large solid tumors, although the positive predictive value was 100%, the negative predictive value was somewhat disappointing, reaching a rate lower than 55%. Furthermore, among all pancreatic lesions, EUS-FNA for cystic lesions revealed the best negative predictive value and diagnostic accuracy of this series, both higher than 90% (Table 4).
Five patients developed FNA-related minor com-plications (fever in 2 after puncturing of cystic lesions, acute pancreatitis in 2 and haemorrhage in 1) managed clinically for 48 h. One case developed a severe abdominal pain due to bile peritoneum after puncturing a serous cystadenoma. Only conservative management in an intensive care unit was offered, with no surgery, and the patient was discharged after 20 d.
DISCUSSION
Histological diagnosis of pancreatic tumors can influence the choice of the best therapeutic approach[10,12]. CT- or US-guided percutaneous puncture of the pancreatic tumors is usually more difficult to undertake due to the retroperitoneal situation of the pancreas[13-15]. Specimens from pancreatic cancer can also be collected by means of laparoscopy[2,6], brushing or forceps and needle aspiration biopsies during ERCP[7], as well as directly from the pancreatic juice. The sensitivity of the biopsy from the pancreatic duct is similar to that obtained by needle aspiration biopsy and intraductal brushing during ERCP, ranging from 40% to 67%[7-9].
The low sensitivity of all these sampling methods, in addition to more complex and laborious techniques, has increased the interest in the use of EUS for pancreatic tumors. However, the images obtained by endoscopic ultrasonography are not able to differentiate precisely malignancy from inflammation[4]. Wiersema et al[5] reported a sensitivity of 76% to detect pancreatic cancer, and Palazzo et al[4] demonstrated a specificity of only 73% for diagnosing inflammatory processes. In fact, in an attempt to improve the diagnostic yield of pancreatic lesions by means of cytopathological assessment[10], EUS-FNA has been used more frequently, and constitutes a very useful method for locoregional staging of pancreatic cancer, making EUS possible to sample almost everything detected during the diagnostic procedure[1,16].
Our diagnostic accuracy by EUS-FNA was 86.6%, which is in accordance with the reported experience, ranging from 84% to 95%[1,10,11,17]. Sampling was successful in 98.4% of the cases, with a mean number of punctures for every pancreatic lesion lower than that reported by other authors without an on-site cytopathologist[10,18]. It was not possible to obtain cytological specimens in 16 cases, either due to extremely hard lesions, in which insertion of the needle into the mass was not possible for more than 1 cm (1 pancreatic carcinoma and 1 nodule of focal chronic pancreatitis), or due to scarce material obtained even after multiple passes of the needle, half of them for lesions further than 3 cm from the probe. Although a subject of much discussion, in this particular group of patients we believe on-site cytopathologist might enhance the diagnostic yield, as proposed by Chang et al[10]. In addition, another way to improve the sampling might be the use of larger needles. Nevertheless, 19-gauge needles could also increase the occurrence of complications, which should be evaluated in randomized clinical trials[19].
In an attempt to improve the diagnostic yield, new methods are available to guide EUS-FNA of the pancreas, such as elastography and contrast-enhanced ultrasound. Giovannini et al[20] evaluated the tissue elasticity of pancreatic malignancies during ultrasound examination, and reported that its sensitivity is 100% and specificity is 67%, respectively. In addition, echo-enhanced ultrasound is a newly available imaging modality for the evaluation of pancreatic lesions. Based on the characteristic vascularization patterns of different tumors, experience has demonstrated that the sensitivity and specificity of the method in diagnosing pancreatic masses are greater than 85% and 90%, respectively[21]. However, even with these recent advances, histology is still the standard of reference. At this point, a combined evaluation of cytological smears and cell blocks can guarantee the maximum utilization of the material aspirated from pancreatic tumors, thus providing more accurate and clinically useful findings[22,23].
In our referral center, the general sensitivity for detection of pancreatic neoplasms by CT or US is respectively, 89% and 76% (data not shown). The literature reports a sensitivity of 100% to detect pancreatic tumors bigger than 3 cm, higher than that obtained by CT or US, and similar to the findings from ERCP[2,3,10,24]. Nonetheless, for small tumors, EUS-FNA presents a better sensitivity in relation to CT or ERCP[4,10,25], which might be a great advantage, as tumors less than 3 cm accounted for 43% of our patients. Regarding the value of EUS-FNA for solid tumors, our results were rather good for tumors smaller than 3 cm, as reported in the literature[26-28]. For larger tumors, even with an excellent positive predictive value, the negative predictive value was lower than 55%. Furthermore, the negative predictive value and diagnostic accuracy of EUS-FNA for cystic lesions were higher than 90%, which are consistent with other studies[29,30].
EUS-FNA has the following advantages over CT- or US-guided FNA: the shorter distance between the gut wall and tumor, the real time visualization of the needle, and the Doppler scanning to avoid punctures of the adjacent blood vessels[10,11]. Potential disadvantages could be the sedation and seeding of the needle tract by malignant cells, which have been reported with the percutaneous approach as well[31,32]. However, except for body-tail lesions[10,11], tumors located in the pancreatic head should be punctured through the duodenum, which is resected with the probable site of malignant seeding in case of surgery. Besides, the sensitivity of percutaneous CT- or US-guided FNA for pancreatic tumors ranges from 45% to 100%, with a specificity being close to 100%[13,14]. However, a pitfall of this technique is the difficulty for identifying and positioning the lesion properly to be punctured[13-15]. In our experience, EUS could identify all lesions previously detected by other imaging methods and insertion of the needle was possible in 99.6% of the cases.
FNA-related complications occurred in 1.1% of the patients, with the minor complications managed clinically. Most likely our lower number of passes of the needle could explain our low rate of complications. In fact, two factors have led to the occurrence of complications: the puncture of cystic lesions and the passage of the needle through large areas of normal parenchyma to reach tumors less than 2 cm. On the other hand, only one case developed a life-threatening complication, a bile peritoneum which could be successfully treated with intensive care support. None of these cases required surgical intervention. Our experience is in line with the literature about this issue, in which the complication rate ranges from 0.5% to 5%[17,33]. Besides, CT-guided FNA presents a complication rate close to 1%[14,15], similar to that obtained in our series.
EUS-FNA proved to be a safe and successful procedure for sampling pancreatic lesions in most patients. The cytopathological assessment confirmed the final diagnosis in almost 90% of the cases. As a result of an impressive specificity and positive predictive value, a malignant cytopathology guarantees the presence of cancer. The sensitivity, negative predictive value and diagnostic accuracy are pretty good for small solid tumors. EUS-FNA for cystic lesions can reveal the best negative predictive value and diagnostic accuracy.
Footnotes
S- Editor Liu Y L- Editor Wang XL E- Editor Che YB
References
- 1.Akahoshi K, Chijiiwa Y, Nakano I, Nawata H, Ogawa Y, Tanaka M, Nagai E, Tsuneyoshi M. Diagnosis and staging of pancreatic cancer by endoscopic ultrasound. Br J Radiol. 1998;71:492–496. doi: 10.1259/bjr.71.845.9691893. [DOI] [PubMed] [Google Scholar]
- 2.Al-Kaisi N, Siegler EE. Fine needle aspiration cytology of the pancreas. Acta Cytol. 1989;33:145–152. [PubMed] [Google Scholar]
- 3.Rösch T, Lorenz R, Braig C, Classen M. Endoscopic ultrasonography in diagnosis and staging of pancreatic and biliary tumors. Endoscopy. 1992;24 Suppl 1:304–308. doi: 10.1055/s-2007-1010488. [DOI] [PubMed] [Google Scholar]
- 4.Palazzo L, Roseau G, Gayet B, Vilgrain V, Belghiti J, Fékéte F, Paolaggi JA. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy. 1993;25:143–150. doi: 10.1055/s-2007-1010273. [DOI] [PubMed] [Google Scholar]
- 5.Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555–564. doi: 10.1055/s-2007-1010405. [DOI] [PubMed] [Google Scholar]
- 6.Edoute Y, Lemberg S, Malberger E. Preoperative and intraoperative fine needle aspiration cytology of pancreatic lesions. Am J Gastroenterol. 1991;86:1015–1019. [PubMed] [Google Scholar]
- 7.Ferrari Júnior AP, Lichtenstein DR, Slivka A, Chang C, Carr-Locke DL. Brush cytology during ERCP for the diagnosis of biliary and pancreatic malignancies. Gastrointest Endosc. 1994;40:140–145. doi: 10.1016/s0016-5107(94)70155-5. [DOI] [PubMed] [Google Scholar]
- 8.Ryan ME. Cytologic brushings of ductal lesions during ERCP. Gastrointest Endosc. 1991;37:139–142. doi: 10.1016/s0016-5107(91)70671-8. [DOI] [PubMed] [Google Scholar]
- 9.Scudera PL, Koizumi J, Jacobson IM. Brush cytology evaluation of lesions encountered during ERCP. Gastrointest Endosc. 1990;36:281–284. doi: 10.1016/s0016-5107(90)71024-3. [DOI] [PubMed] [Google Scholar]
- 10.Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–393. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 11.Vilmann P, Hancke S, Henriksen FW, Jacobsen GK. Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy. 1993;25:523–527. doi: 10.1055/s-2007-1010389. [DOI] [PubMed] [Google Scholar]
- 12.Hünerbein M, Dohmoto M, Haensch W, Schlag PM. Endosonography-guided biopsy of mediastinal and pancreatic tumors. Endoscopy. 1998;30:32–36. doi: 10.1055/s-2007-993725. [DOI] [PubMed] [Google Scholar]
- 13.Pinto MM, Avila NA, Criscuolo EM. Fine needle aspiration of the pancreas. A five-year experience. Acta Cytol. 1988;32:39–42. [PubMed] [Google Scholar]
- 14.Rodriguez J, Kasberg C, Nipper M, Schoolar J, Riggs MW, Dyck WP. CT-guided needle biopsy of the pancreas: a retrospective analysis of diagnostic accuracy. Am J Gastroenterol. 1992;87:1610–1613. [PubMed] [Google Scholar]
- 15.Welch TJ, Sheedy PF, Johnson CD, Johnson CM, Stephens DH. CT-guided biopsy: prospective analysis of 1,000 procedures. Radiology. 1989;171:493–496. doi: 10.1148/radiology.171.2.2704815. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad NA, Lewis JD, Ginsberg GG, Rosato EF, Morris JB, Kochman ML. EUS in preoperative staging of pancreatic cancer. Gastrointest Endosc. 2000;52:463–468. doi: 10.1067/mge.2000.107725. [DOI] [PubMed] [Google Scholar]
- 17.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 18.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 19.Itoi T, Itokawa F, Sofuni A, Nakamura K, Tsuchida A, Yamao K, Kawai T, Moriyasu F. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: a pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362–366. doi: 10.1055/s-2004-826156. [DOI] [PubMed] [Google Scholar]
- 20.Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344–348. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 21.Rickes S, Mönkemüller K, Malfertheiner P. Contrast-enhanced ultrasound in the diagnosis of pancreatic tumors. JOP. 2006;7:584–592. [PubMed] [Google Scholar]
- 22.Mitsuhashi T, Ghafari S, Chang CY, Gu M. Endoscopic ultrasound-guided fine needle aspiration of the pancreas: cytomorphological evaluation with emphasis on adequacy assessment, diagnostic criteria and contamination from the gastrointestinal tract. Cytopathology. 2006;17:34–41. doi: 10.1111/j.1365-2303.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Dodge R, Glasgow BJ, Layfield LJ. Fine-needle aspiration: comparison of smear, cytospin, and cell block preparations in diagnostic and cost effectiveness. Diagn Cytopathol. 1998;19:70–74. doi: 10.1002/(sici)1097-0339(199807)19:1<70::aid-dc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Snady H, Cooperman A, Siegel J. Endoscopic ultrasonography compared with computed tomography with ERCP in patients with obstructive jaundice or small peri-pancreatic mass. Gastrointest Endosc. 1992;38:27–34. doi: 10.1016/s0016-5107(92)70326-5. [DOI] [PubMed] [Google Scholar]
- 25.Kahl S, Malfertheiner P. Role of endoscopic ultrasound in the diagnosis of patients with solid pancreatic masses. Dig Dis. 2004;22:26–31. doi: 10.1159/000078732. [DOI] [PubMed] [Google Scholar]
- 26.Nakaizumi A, Uehara H, Iishi H, Tatsuta M, Kitamura T, Kuroda C, Ohigashi H, Ishikawa O, Okuda S. Endoscopic ultrasonography in diagnosis and staging of pancreatic cancer. Dig Dis Sci. 1995;40:696–700. doi: 10.1007/BF02064392. [DOI] [PubMed] [Google Scholar]
- 27.Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188–199. doi: 10.1016/0016-5085(92)91800-j. [DOI] [PubMed] [Google Scholar]
- 28.Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: a review. Gastrointest Endosc. 2002;55:232–237. doi: 10.1067/mge.2002.121342. [DOI] [PubMed] [Google Scholar]
- 29.Sedlack R, Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543–547. doi: 10.1067/mge.2002.128106. [DOI] [PubMed] [Google Scholar]
- 30.Bhutani MS. Role of endoscopic ultrasonography in the diagnosis and treatment of cystic tumors of the pancreas. JOP. 2004;5:266–272. [PubMed] [Google Scholar]
- 31.Ferrucci JT, Wittenberg J, Margolies MN, Carey RW. Malignant seeding of the tract after thin-needle aspiration biopsy. Radiology. 1979;130:345–346. doi: 10.1148/130.2.345. [DOI] [PubMed] [Google Scholar]
- 32.Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610–611. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 33.Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O'Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–249. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]


