Abstract
Objective
To evaluate prospectively the associations of folate with assisted reproductive technology outcomes within a U.S. population.
Methods
This analysis included women (n=232) in a prospective cohort study at the Massachusetts General Hospital Fertility Center. Diet was assessed before assisted reproductive technology treatment using a validated food frequency questionnaire. Intermediate and clinical endpoints of ART were abstracted from medical records. Generalized linear mixed models with random intercepts to account for multiple cycles per woman were used to evaluate the association of folate intake with ART outcomes adjusting for calorie intake, age, BMI, race, smoking status, infertility diagnosis, and protocol type.
Results
Among the 232 women (median age=35.2 years, median folate intake=1,778 μg/day), higher folate intake was associated with higher rates of implantation, clinical pregnancy, and live birth. The adjusted percentage (95% CI) of initiated assisted reproductive technology cycles resulting in a live birth for women in increasing quartiles of folate intake were 30% (21, 42%), 47% (35, 59%), 42% (30, 35%) and 56% (43, 67%)(P-trend=0.01). Live birth rates were 20% (8, 31%) higher among women in the highest quartile of supplemental folate intake (>800μg/day) than among women in the lowest quartile (<400μg/day). Higher supplemental folate intake was associated with higher fertilization rates and lower cycle failure rates before embryo transfer (P-trend=0.03 and 0.02).
Conclusions
Higher intake of supplemental folate was associated with higher live birth rates after assisted reproductive technology treatment.
Introduction
Approximately 15% of couples in the US are infertile (1). Research on the role of diet in human fertility is limited but suggests that some nutrients, particularly folate, may improve fertility (2–4). Folate is necessary for the synthesis of DNA, transfer RNA, cysteine and methionine (5). Therefore, during periods of rapid cell growth, such as the peri-conceptional period, requirements for folate are particularly enhanced (6). While reproductive age women are currently recommended to take a folic acid supplement pre-conceptionally to prevent neural tube defects, there is growing evidence that folic acid could also improve other reproductive outcomes.
Studies among couples undergoing infertility treatment in Europe suggest that folate may improve reproductive success (7, 8). Specifically, follicular fluid folate levels were associated with 3-fold greater odds of becoming pregnant among women undergoing assisted reproduction in the Netherlands (7) and among Polish women, those who received a folic acid supplement had better quality oocytes and a higher mature oocyte yield than women who did not receive folic acid (8).However, findings have not been entirely consistent across studies, as a study among UK women undergoing assisted reproduction found no association between pre-pregnancy folate and the likelihood of a successful pregnancy (9). No studies have examined the relation between folate and assisted reproductive technology outcomes in the US, where intake is substantially higher than in Europe due to mandatory food fortification and greater use of over-the-counter dietary supplements (10, 11). To address this gap, we evaluated the relation between pre-treatment folate intake and infertility treatment outcomes among women undergoing assisted reproductive technology at an academic medical center in the US.
Materials and Methods
Participants were women enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort started in 2006 aimed at identifying determinants of fertility among couples presenting to the Massachusetts General Hospital Fertility Center (Boston, MA, USA). All women who meet eligibility requirements (age 18–46 years and no planned use of donor gametes at enrollment) are invited to participate in the study. Approximately 60% of those invited participated in the study. Dietary assessment was introduced in 2007. For this analysis, women were eligible if they had completed at least 1 assisted reproductive technology cycle between February 2007 and May 2013 (n=316). Of these, 76 women (24%) were excluded do to missing diet and 8 women (3%) were excluded because they had started their assisted reproductive technology cycle prior to diet assessment. Women missing diet were more likely to be diagnosed with diminished ovarian reserve (15.8% vs. 7.5%) or endometriosis (14.5% vs. 4.2%) and more likely to have assisted reproductive technology cycles that failed prior to embryo transfer (16.9% vs. 8.4%). All other characteristics were similar to the women included in our analysis. The study was approved by the Institutional Review Boards of the Massachusetts General Hospital and the Harvard School of Public Health. All participants provided written informed consent after study procedures were explained by a research nurse.
Diet was assessed before assisted reproductive technology treatment using a validated food frequency questionnaire (12). Participants were asked to report how often, on average, they consumed specified amounts of 131 food items during the previous year. Multivitamin and supplement users were asked to specify the brand of the multivitamin or supplement, the dose, and frequency of use. Upon return of the food frequency questionnaires, the questionnaires undergo rigorous quality checks before they are scanned to capture the data and images of each form. Nutrient intakes were estimated by summing the nutrient contribution of all food and supplement items. Nutrient contents were obtained from the nutrient database of the US Department of Agriculture with additional information from manufacturers (13). Dietary folate equivalents (DFE) were calculated to account for differences in absorption between natural and synthetic folate (14). To reduce extraneous variation in intake, folate was adjusted for total energy intake using the nutrient residual method (15). Folate intake with this questionnaire has been validated against prospectively collected diet records (r=0.71)(12) and red blood cell (r=0.51)(16) and plasma folate levels (r=0.63) (17). Foods known to be high in folate include ready to eat breakfast cereal, fortified grains, leafy green vegetables, and lentils.
At enrollment, height and weight were measured by a trained research nurse to calculate body mass index (BMI) (kg/m2) and a brief, nurse-administered questionnaire was used to collect data on demographics, medical history, and lifestyle. Participants also completed a detailed take-home questionnaire with additional questions on lifestyle factors, reproductive health, and medical history. Clinical information including infertility diagnosis and protocol type was abstracted from electronic medical records. All clinical information and questionnaire data are double-entered into our research database by research nurses to ensure accuracy and reliability.
Patients underwent one of three stimulation protocols as clinically indicated: 1) luteal-phase GnRH agonist protocol; 2) follicular-phase GnRH-agonist/Flare protocol; or 3) GnRH-antagonist protocol. Patients were monitored during gonadotropin stimulation for serum estradiol, follicle size measurements and counts, and endometrial thickness through 2 days before egg retrieval. Human chorionic gonadotropin (hCG) was administered approximately 36 hr before the scheduled egg-retrieval procedure to induce ovulation. Details of egg retrieval have been previously described (18).
Couples underwent assisted reproductive technology with conventional in vitro fertilization (IVF) or intra-cytoplasmatic sperm injection (ICSI) as clinically indicated. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II (MII) or degenerated. Embryologists determined fertilization rate 17–20 hours after insemination as the number of oocytes with two pronuclei divided by the number of MII oocytes inseminated. The resulting embryos were monitored for cell number and morphological quality (1 (best) to 5 (worst)) on day 2 and 3. For analysis we classified embryos as best quality if they had 4 cells on day 2, 8 cells on day 3, and a morphologic quality score of 1 or 2 on days 2 and 3. We defined implantation as a serum β-hCG level > 6 mIU/mL typically measured 17 days (range 15–20 days) after egg retrieval, clinical pregnancy as the presence of an intrauterine pregnancy confirmed by ultrasound, and live birth as the birth of a neonate on or after 24 weeks gestation.
Women were classified into quartiles based on total folate (in dietary folate equivalents) and separately by supplemental and food folate. Descriptive statistics were calculated for demographic characteristics, dietary nutrients, and stimulation protocol types according to quartile of folate intake. Multivariate generalized linear mixed models with random intercepts were used to evaluate the association between folate intake and assisted reproductive technology outcomes while accounting for within-person correlations in outcomes. These models also generate unbiased estimates in the presence of an unbalanced design even when data is not missing completely at random. Poisson distribution and log link function were specified for oocyte counts and binomial distribution and logit link function were specified for fertilization, embryo quality, and clinical outcomes. Tests for trend across quartiles were conducted using a variable with the median dietary folate intake in each quartile as a continuous variable. Folate was also evaluated as continuous linear and quadratic variable. All results are presented as population marginal means, adjusted for covariates (19).
Confounding was evaluated using prior knowledge and descriptive statistics from our cohort through the use of directed acyclic graphs. Variables retained in the final multivariable models were calorie intake, age, BMI, race, smoking status, infertility diagnosis, and protocol type.
Nutrients highly correlated with folate were analyzed as collinear variables and potential confounders by adding the nutrients to the fully adjusted model separately and then in combination to see if it affected the magnitude or significance of the effect estimate for folate. Effect modification by demographic and cycle characteristics were tested using cross-product terms in the final multivariate models. SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
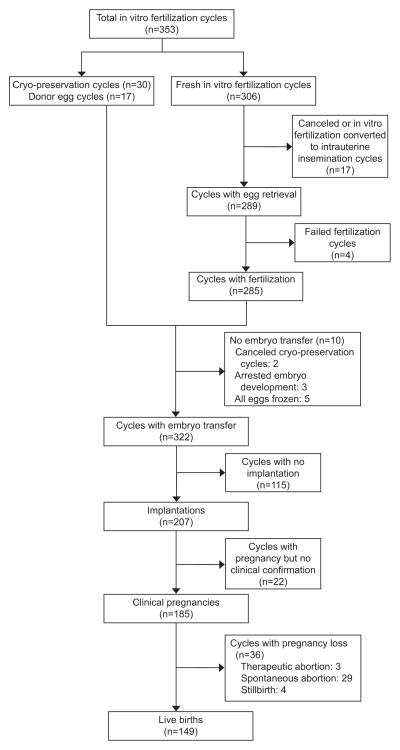
Participant’s had a median age of 35.2 years (range 27–46) and a mean BMI of 24.2 kg/m2 (range 16.1–42.4). Most women had never smoked (72%), were Caucasian (81%), and nulliparous at study entry (84%). Women with lower folate intake tended to be younger and to have lower consumption of carbohydrates and higher consumption of fat (Table 1). The energy-adjusted median intake of folate (in DFE) was 1778 μg/day (range 277–5724 μg/day) (Appendix 1, available online at http://links.lww.com/xxx). Approximately 57% of women’s total folate intake came from supplements and 43% from foods (both natural and fortified). 78% of women took at least 400 μg/day of supplemental folate and 19% of women took at least 1000 μg/day of supplemental folate. Intake of supplemental and food folate were not correlated (spearman correlation coefficient= −0.08). Figure 1 presents an overview of the 353 IVF cycles. Women were followed for 1(65%), 2(24%), or 3+(11%) assisted reproductive technology cycles.
Table 1.
Baseline characteristics of 232 women in Environment and Reproductive Health Study by quartile of total folate (in DFE) and supplemental folate intake.
| Total Cohort | Total Folate (in DFE) | Supplemental Folate | |||
|---|---|---|---|---|---|
| Quartile (Range of Intake, μg/day) | Q1 (<1262) | Q4 (>2352) | Q1 (<400) | Q4 (>800) | |
| N | 232 | 58 | 58 | 51 | 60 |
| Personal Characteristics | |||||
| Age, years | 35.2 (4.5) | 33.8 (6.0) | 35.0 (4.2)* | 33.7 (6.2) | 35.3 (4.1) |
| BMI, kg/m2 | 24.2 (4.2) | 24.0 (3.8) | 24.4 (4.3) | 24.2 (4.1) | 24.7 (4.4) |
| Ever smoker, n (%) | 66 (28.5) | 17 (29.3) | 18 (31.0) | 14 (27.5) | 19 (31.7) |
| White/Caucasian, n (%) | 187 (80.6) | 45 (77.6) | 48 (82.8) | 40 (78.4) | 51 (85.0) |
| Baseline Reproductive Characteristics | |||||
| Infertility diagnosis, n (%) | |||||
| Female factor | 65 (28.0) | 13 (22.4) | 24 (41.4) | 12 (23.5) | 24 (40.0) |
| Ovulation Disorders | 20 (8.6) | 1 (1.7) | 9 (15.5) | 1 (2.0) | 9 (15.0) |
| Diminished Ovarian Reserve | 17 (7.3) | 1 (1.7) | 8 (13.8) | 1 (2.0) | 8 (13.3) |
| Tubal | 17 (7.3) | 7 (12.1) | 4 (6.9) | 6 (11.8) | 4 (6.7) |
| Endometriosis | 9 (3.9) | 2 (3.5) | 3 (5.2) | 3 (5.9) | 3 (5.0) |
| Uterine | 2 (0.9) | 2 (3.5) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Male factor | 82 (35.3) | 24 (41.4) | 17 (29.3) | 22 (43.1) | 17 (28.33) |
| Unexplained | 85 (36.6) | 21 (36.2) | 17 (29.3) | 17 (33.3) | 19 (31.7) |
| Treatment protocol, n (%) | |||||
| Antagonist | 23 (9.9) | 5 (8.6) | 5 (8.6) | 6 (11.8) | 6 (10.0) |
| Flare | 28 (12.1) | 7 (12.1) | 8 (13.8) | 6 (11.8) | 8 (13.3) |
| Luteal phase agonist | 181 (78.0) | 46 (79.3) | 45 (77.6) | 39 (76.5) | 46 (76.7) |
| Day 3 FSH, IU/L | 7.0 (2.0) | 7.0 (1.9) | 7.0 (2.3) | 6.9 (1.6) | 6.9 (2.4) |
| Embryo Transfer Day, n (%) | |||||
| No embryos transferred | 21 (9.1) | 12 (20.7) | 1 (1.7)* | 10 (19.6) | 2 (3.3) |
| Day 2 | 11 (4.7) | 2 (3.5) | 3 (5.2) | 3 (5.9) | 3 (5.0) |
| Day 3 | 111 (47.8) | 22 (37.9) | 24 (41.4) | 20 (39.2) | 27 (45.0) |
| Day 5 | 72 (31.0) | 19 (32.8) | 25 (43.1) | 16 (31.4) | 23 (38.3) |
| Egg Donor or Cryo Cycle | 17 (7.3) | 3 (5.2) | 5 (8.6) | 2 (3.9) | 5 (8.3) |
| Number of Embryos Transferred, n (%) | |||||
| No embryos transferred | 21 (9.1) | 12 (20.7) | 1 (1.7)* | 10 (19.6) | 2 (3.3) |
| 1 embryo | 25 (10.8) | 3 (5.2) | 5 (8.6) | 2 (3.9) | 4 (6.7) |
| 2 embryos | 133 (57.3) | 38 (65.5) | 34 (58.6) | 33 (64.7) | 35 (58.3) |
| 3+ embryos | 36 (15.5) | 2 (3.4) | 13 (22.4) | 4 (7.8) | 14 (23.0) |
| Egg Donor or Cryo Cycle | 17 (7.3) | 3 (5.2) | 5 (8.6) | 2 (3.9) | 5 (8.3) |
| Dietary Characteristics | |||||
| Total Calories, kcal/day | 1815 (583) | 1762 (489) | 1766 (635) | 1750 (525) | 1760 (577) |
| Carbohydrates, % of kcal/day | 49.8 (7.6) | 47.4 (9.2) | 50.3 (7.7)* | 49.2 (8.9) | 49.7 (7.2) |
| Protein, % of kcal/day | 16.5 (2.6) | 16.7 (2.9) | 16.9 (2.7) | 16.7 (2.9) | 17.0 (2.8) |
| Fat, % of kcal/day | 32.5 (2.6) | 33.8 (7.4) | 32.3 (5.3)* | 32.4 (7.5) | 32.8 (4.9) |
| MUFA, % of kcal/day | 13.0 (3.6) | 13.8 (4.6) | 12.4 (2.6) | 13.4 (4.8) | 12.6 (2.4) |
| PUFA, % of kcal/day | 6.3 (1.8) | 6.3 (1.7) | 6.2 (2.0) | 6.0 (1.7) | 6.4 (2.0) |
| SFA, % of kcal/day | 10.5 (2.5) | 10.8 (2.7) | 10.9 (2.6)* | 10.3 (2.9) | 10.9 (2.4) |
| Multivitamin User, n (%) | 204 (89.1) | 41 (70.7) | 57 (100)* | 34 (66.7) | 59 (100)* |
| Folic Acid Supplement User, n (%) | 38 (16.4) | 4 (6.9) | 9 (15.5) | 4 (7.8) | 11 (18.3) |
| Alcohol, g/day | 8.8 (11.1) | 10.9 (15.4) | 6.8 (6.6) | 9.5 (13.3) | 7.4 (7.3) |
| Caffeine, mg/day | 119.4 (107.3) | 118.9 (113.0) | 121.0 (119.6) | 123.5 (117.0) | 126.4 (116.4) |
| Male Folate Intake, μg/day | 672.8 (313.5) | 628.7 (275.6) | 800.4 (445.6) | 613.0 (273.5) | 767.8 (446.0) |
Abbreviations: BMI, body mass index; DFE, dietary folate equivalents; FSH, follicle stimulating hormone; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; Q1, quartile 1; Q4, quartile 4.
Data are mean (standard deviation) unless otherwise specified.
Indicates a p-value < 0.05 across all quartiles.
Figure 1.
Overview of assisted reproductive technology outcomes of 232 women (353 cycles) in the Environment and Reproductive Health Study.
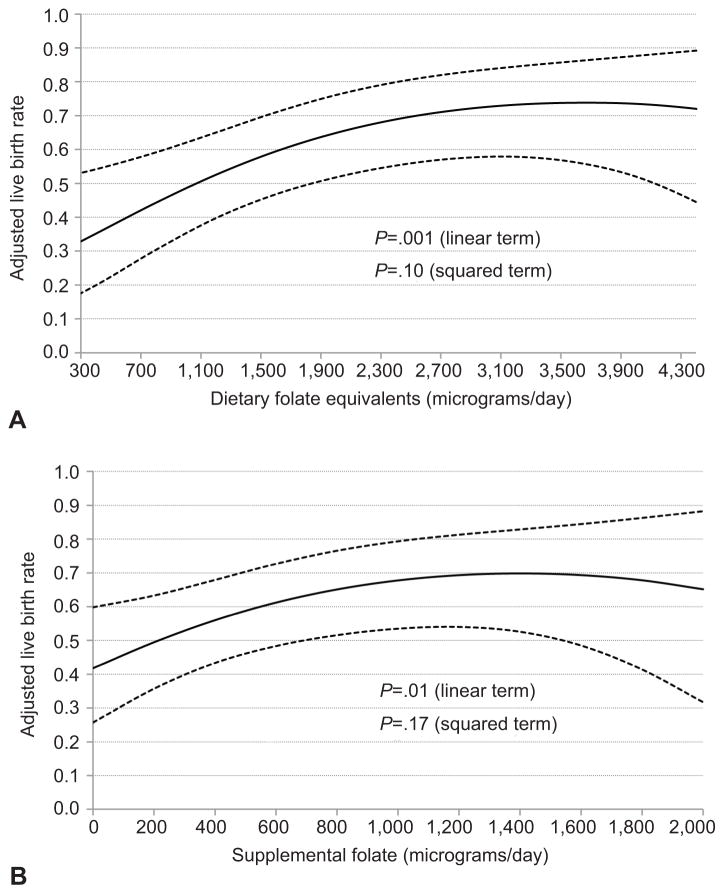
Total folate intake was positively associated with implantation, clinical pregnancy, and live birth rates per initiated cycle (Table 2). The adjusted difference (95% CI) in implantation, clinical pregnancy, and live birth rates between women in the highest (>2352 μg/day) versus lowest (<1262 μg/day) quartile of dietary folate equivalents was 0.22 (0.11, 0.32), 0.24 (0.12, 0.35), and 0.26 (0.13, 0.37), respectively. Live birth rates were 20% (8, 31%) higher among women in the highest quartile of supplemental folate intake (>800μg/day) than among women in the lowest quartile (<400μg/day). When the association between folate and live birth rate was modeled continuously, there was evidence of a non-linear relationship; there was a positive linear relationship up to 3200 μg/day of dietary folate equivalents and up to 1200 μg/day of supplemental folate without evidence of additional benefit with higher intakes (Figure 2). The association between folate and live birth rate was slightly attenuated when analyses were restricted to cycles with embryo transfer (Appendix 2, available online at http://links.lww.com/xxx). For example, the adjusted difference in live birth rates between women consuming 1200 μg/day vs. 200 μg/day of supplemental folate was 0.20 per initiated cycle and 0.14 per embryo transfer. We did not observe an association between folate intake and multiple birth rate. Similar results were seen after restricting the analyses to first cycles only.
Table 2.
Associations between folate intake and clinical outcomes in 232 women (353 initiated cycles) from the Environment and Reproductive Health Study.
| Implantation Rate | Clinical Pregnancy Rate | Live Birth Rate | |
|---|---|---|---|
| Quartile (range, μg/day) | |||
| Adjusted Mean (95% CI) | |||
|
| |||
| Total Folate (in DFE) | |||
| Q1 ((<1262) | 0.46 (0.35, 0.57) | 0.39 (0.29, 0.51) | 0.30 (0.21, 0.42) |
| Q2 (1262–1778) | 0.62 (0.50, 0.72) | 0.56 (0.44, 0.67)* | 0.47 (0.35, 0.59)* |
| Q3 ((1779–2352) | 0.64 (0.51, 0.74)* | 0.58 (0.46, 0.70)* | 0.42 (0.30, 0.55) |
| Q4 (>2352) | 0.68 (0.57, 0.78)* | 0.63 (0.52, 0.74)* | 0.56 (0.43, 0.67)* |
| P trend | 0.01 | 0.007 | 0.01 |
| Supplemental Folate | |||
| Q1 ((<400) | 0.43 (0.31, 0.55) | 0.41 (0.29, 0.53) | 0.35 (0.24, 0.48) |
| Q2 (400–543) | 0.66 (0.55, 0.75)* | 0.55 (0.44, 0.65) | 0.43 (0.32, 0.54) |
| Q3 (544–800) | 0.58 (0.46, 0.70) | 0.55 (0.42, 0.66) | 0.39 (0.28, 0.52) |
| Q4 ((>800) | 0.67 (0.56, 0.77)* | 0.62 (0.51, 0.73)* | 0.55 (0.43, 0.66)* |
| P trend | 0.03 | 0.03 | 0.07 |
| Food Folate | |||
| Q1 (<371) | 0.58 (0.47, 0.69) | 0.47 (0.36, 0.58) | 0.35 (0.25, 0.47) |
| Q2 (372–436) | 0.55 (0.44, 0.66) | 0.51 (0.40, 0.63) | 0.41 (0.30, 0.52) |
| Q3 (437–534) | 0.61 (0.49, 0.71) | 0.56 (0.45, 0.67) | 0.49 (0.37, 0.60) |
| Q4 (>534) | 0.64 (0.52, 0.75) | 0.60 (0.48, 0.71) | 0.49 (0.37, 0.61) |
| P trend | 0.35 | 0.10 | 0.08 |
All analyses were run using generalized linear mixed models with random intercepts, binomial distribution, and logit link function.
Indicates a p-value < 0.05 comparing that quartile vs. first quartile.
Data are predicted marginal means adjusted for total calorie intake, age, BMI, race, smoking status, infertility diagnosis, and protocol type, unless otherwise specified.
Figure 2.
Associations between folate intake and live birth rates in 232 women (353 initiated cycles) from the Environment and Reproductive Health Study. Shown are the associations between total folate (in dietary folate equivalents) (A) and supplemental folate (B) and live birth rate per initiated cycle after assisted reproductive technology. All analyses were run using a generalized linear mixed model with random intercepts, binomial distribution, and logit link function. The solid line represents the adjusted mean live birth rate by level of folate intake and the dotted lines are the upper and lower 95% confidence intervals for these adjusted means. Adjusted means presented for average total calorie intake (1797 kcals/day), age (35 years), body mass index (24.3 kg/m2), race (white), smoking status (never smoker), infertility diagnosis (female factor), and protocol type (luteal phase agonist).
Next we investigated whether folate was associated with intermediate assisted reproductive technology endpoints. Women in the 2nd, 3rd, and 4th quartiles of supplemental folate intake had fewer mature oocytes retrieved (Table 3). However, women with higher intake of supplemental folate had higher fertilization rates (p-trend=0.03). Upon further examination, the positive association between supplemental folate and fertilization was only in conventional IVF cycles (adjusted difference Q4 v. Q1= 0.15 [0.07, 0.22]) and not in ICSI cycles (adjusted difference Q4 v. Q1= 0.01 [−0.05, 0.09])(p-interaction=0.06)(Appendix 3, available online at http://links.lww.com/xxx). Folate intake was unrelated to embryo quality. Treatment failure prior to embryo transfer was higher among women with low folate intake. The adjusted percentages of cycles failing prior to embryo transfer in increasing quartiles of supplemental folate intake were 14%, 6%, 7%, and 2% (p-trend=0.02).
Table 3.
Associations between folate intake and early ART outcomes in 222 women (289 fresh IVF cycles with egg retrieval) from the Environment and Reproductive Health Study.
| Quartile (median, μg/day) | Total Oocyte Yield | M2 Oocytes | Fertilization Rate | % With ≥1 Best Quality Embryo on Day 2 & 3 |
|---|---|---|---|---|
| Adjusted Mean (95% CI) | ||||
|
| ||||
| Total Folate (in DFE) | ||||
| Q1 ((<1262) | 11.5 (10.3, 12.9) | 10.0 (9.0, 11.1) | 0.70 (0.64, 0.75) | 62 (48, 74) |
| Q2 (1262–1778) | 9.9 (8.9, 11.1) | 8.8 (7.9, 9.9) | 0.69 (0.63, 0.74) | 62 (48, 74) |
| Q3 ((1779–2352) | 11.3 (10.1, 12.6) | 9.2 (8.2, 10.3) | 0.72 (0.67, 0.78) | 60 (46, 73) |
| Q4 (>2352) | 10.3 (9.2, 11.5) | 8.7 (7.8, 9.7) | 0.76 (0.71, 0.81) | 68 (55, 79) |
| P trend | 0.48 | 0.16 | 0.06 | 0.54 |
| Supplemental Folate | ||||
| Q1 ((<400) | 12.1 (10.9, 13.6) | 10.7 (9.6, 11.9) | 0.68 (0.62, 0.73) | 62 (47, 74) |
| Q2 (400–543) | 10.3 (9.3, 11.5)* | 8.8 (7.9, 9.8)* | 0.70 (0.65, 0.75) | 58 (45, 70) |
| Q3 (544–800) | 10.3 (9.1, 11.5)* | 8.6 (7.7, 9.6)* | 0.73 (0.67, 0.78) | 68 (53, 79) |
| Q4 ((>800) | 10.4 (9.4, 11.6) | 8.8 (8.0, 9.8)* | 0.76 (0.71, 0.80)* | 65 (52, 77) |
| P trend | 0.08 | 0.02 | 0.03 | 0.42 |
| Food Folate | ||||
| Q1 (<371) | 11.0 (9.9, 12.3) | 9.4 (8.4, 10.5) | 0.68 (0.62, 0.74) | 56 (42, 69) |
| Q2 (372–436) | 10.4 (9.3, 11.6) | 8.9 (7.9, 9.9) | 0.74 (0.68, 0.79) | 71 (58, 81) |
| Q3 (437–534) | 9.8 (8.8, 11.0) | 8.4 (7.5, 9.4) | 0.75 (0.69, 0.80) | 68 (54, 79) |
| Q4 (>534) | 11.7 (10.5, 13.1) | 10.0 (9.0, 11.2) | 0.71 (0.65, 0.76) | 57 (43, 71) |
| P trend | 0.36 | 0.35 | 0.78 | 0.85 |
Abbreviations: CI, confidence interval; ART, assisted reproductive technology; DFE, dietary folate equivalents; M2, mature oocytes.
All analyses were run using generalized linear mixed models with random intercepts, Poisson (for oocyte counts) or binomial (for fertilization and embryo quality) distribution, log (for oocyte counts) or logit (for fertilization and embryo quality) link function.
Indicates a p-value < 0.05 comparing that quartile vs. first quartile.
Data are predicted marginal means adjusted for calorie intake, age, BMI, race, smoking status, infertility diagnosis, and protocol type, unless otherwise specified.
We also investigated whether the intake of iron or other B-vitamins, which are highly correlated with folate intake, could explain these associations (Appendix 4, available online at http://links.lww.com/xxx). After further adjustment for folate intake, only vitamin B12 intake was significantly associated with live birth rates (adjusted difference in live birth rate Q4 vs. Q1= 0.24 [0.09, 0.38]). Folate remained significantly related to higher live birth rates in all of these models and effect estimates were similar. To explore whether the observed associations could be at least partially attributable to male folate intake (correlation with female intake: r=0.21), we restricted analyses to couples with complete information on diet (n=106 couples, 154 cycles). After adjusting for male folate intake, the results were similar (results not shown).
The association between folate and live birth rates was not modified by BMI, race, smoking status, alcohol or B12 intake, number of embryos transferred, embryo transfer day, stimulation protocol, or primary infertility diagnosis.
Discussion
In a prospective cohort of women undergoing infertility treatment in the United States we found that pre-treatment supplemental folic acid above 800μg/day was related to a higher probability of live birth among women undergoing assisted reproductive technology on the background of a fortified food supply. Higher live births may result from higher fertilization rates, lower probability of cycle failure prior to embryo transfer and improved embryo survival manifested in higher implantation rates. Vitamin B12 intake was also positively associated with live birth rates after assisted reproduction.
A study of 602 women undergoing assisted reproduction in the UK found no association between pre-pregnancy folate and the likelihood of a successful pregnancy (9) but high plasma folate levels were associated with increased risk of twinning. However, the UK study included women with lower folate intake (median intake ~800 vs. 1078 μg/day) and unlike our study, excluded cycles with a gestational sac but no fetal heart, cycles ending in chemical pregnancy, ectopic pregnancy, termination, stillbirth, or neonatal death, oocyte donor cycles, and cycles without embryo transfer (n=98, 15% of total women). If folate’s main impact is on outcomes that take place before embryo transfer or the clinical recognition of a pregnancy, as our data suggest, the exclusions in the UK study would bias the associations towards the null, which we observed in our analyses restricted to cycles with an embryo transfer. In contrast to the results of the UK study, but in agreement with our findings, a prospective cohort of women undergoing assisted reproduction in the Netherlands (n=181) found that a doubling in follicular fluid folate levels was associated with 3-fold greater odds of becoming pregnant during an assisted reproductive technology cycle (7).
An association between folate and fertilization rates has been observed in in vitro models. Studies of mouse pre-implantation embryos have shown that endogenous folates are essential for embryo development due to their role in thymidine synthesis (20) (21). Further, since thymidine does not accumulate in cells, significant amounts of reduced folates are required to accumulate in the oocyte during gametogenesis, to support the exponential increase in DNA synthesis that occurs during early embryo development. This and other animal data support the observation that folate increases embryo survival (22–24) in agreement with our results on implantation. Moreover, in a Polish study, women who received a folic acid supplement had better quality oocytes and a higher degree of mature oocytes compared to women who did not receive folic acid (8).
Our data suggest that supplemental folate is preferable to food folate for reproductive benefits. Relative to folic acid (supplemental folate), natural food folate has a lower proportion of folate that is absorbed and available for metabolic reactions and storage. Several luminal factors also hinder the absorption of natural food folate (25). In addition, even with a fortified food supply, it is difficult to consume high levels of folate from diet alone. Therefore, the stronger associations we observed with supplemental folate could be driven by wider intakes and greater absorption, which combined allow for more extreme comparisons.
Our study had some limitations. Diet assessment by food frequency questionnaire is subject to measurement error. However, we used a questionnaire known to relate well to biomarker levels (16, 17). Moreover, due to the prospective nature of our study, measurement error would most likely be non-differential with respect to the outcomes and result in attenuated associations.. In addition, due to the observational nature of our study, there remains the possibility of residual confounding by lifestyle factors that were not or poorly measured. As such, our findings need to be confirmed by a randomized trial before causality can be implied. Strengths of our study include the prospective design and the ability to evaluate early endpoints that cannot be observed in couples attempting to conceive naturally. Also, demographic characteristics of study participants are comparable to those of patients presenting to fertility clinics nationwide suggesting that results may be generalizable to other couples seeking infertility treatment (31).We also benefitted from having a wide range of folate intake in our population. The standardized assessment of a wide variety of participant and dietary characteristics also increased the ability to adjust for confounding.
In summary, supplemental folate was related to a higher probability of live birth among women undergoing ART. The apparent benefits of folic acid were observed at intake levels that are much higher than those currently recommended for the prevention of neural tube defects (32–38), but substantially lower than those prescribed to some women seeking preconception care (39) and women undergoing infertility treatment in other parts of the world (40).
Supplementary Material
Acknowledgments
Supported by NIH grants R01-ES009718, R01-ES022955, P30-DK046200, T32-DK007703-16, and T32- HD060454.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331. e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013;19:640–55. doi: 10.1093/humupd/dmt041. [DOI] [PubMed] [Google Scholar]
- 3.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Use of multivitamins, intake of B vitamins, and risk of ovulatory infertility. Fertil Steril. 2008;89:668–76. doi: 10.1016/j.fertnstert.2007.03.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaskins AJ, Mumford SL, Chavarro JE, Zhang C, Pollack AZ, Wactawski-Wende J, Perkins NJ, Schisterman EF. The impact of dietary folate intake on reproductive function in premenopausal women: a prospective cohort study. PLoS ONE. 2012;7:e46276. doi: 10.1371/journal.pone.0046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–74. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- 6.Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;13:225–38. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- 7.Boxmeer JC, Macklon NS, Lindemans J, Beckers NG, Eijkemans MJ, Laven JS, Steegers EA, Steegers-Theunissen RP. IVF outcomes are associated with biomarkers of the homocysteine pathway in monofollicular fluid. Hum Reprod. 2009;24:1059–66. doi: 10.1093/humrep/dep009. [DOI] [PubMed] [Google Scholar]
- 8.Szymanski W, Kazdepka-Zieminska A. Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Ginekol Pol. 2003;74:1392–6. [PubMed] [Google Scholar]
- 9.Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G, Templeton A, Haites N, Campbell D, Bhattacharya S. Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet. 2006;367:1513–9. doi: 10.1016/S0140-6736(06)68651-0. [DOI] [PubMed] [Google Scholar]
- 10.Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, Sempos CA, Burt VL, Radimer KL, Picciano MF. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr. 2010;91:231–7. doi: 10.3945/ajcn.2009.28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JY, Nicolas G, Freisling H, Biessy C, Scalbert A, Romieu I, Chajes V, Chuang SC, Ericson U, Wallstrom P, Ros MM, Peeters PH, Mattiello A, Palli D, Maria Huerta J, Amiano P, Halkjaer J, Dahm CC, Trichopoulou A, Orfanos P, Teucher B, Feller S, Skeie G, Engeset D, Boutron-Ruault MC, Clavel-Chapelon F, Crowe F, Khaw KT, Vineis P, Slimani N. Comparison of standardised dietary folate intake across ten countries participating in the European Prospective Investigation into Cancer and Nutrition. Br J Nutr. 2012;108:552–69. doi: 10.1017/S0007114511005733. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Agriculture Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 25. 2012. [Google Scholar]
- 14.Bailey LB. Dietary reference intakes for folate: the debut of dietary folate equivalents. Nutr Rev. 1998;56:294–9. doi: 10.1111/j.1753-4887.1998.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 15.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–9. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 18.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. In J Androl. 2010;33:385–93. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34:216–221. [Google Scholar]
- 20.Kwong WY, Adamiak SJ, Gwynn A, Singh R, Sinclair KD. Endogenous folates and single-carbon metabolism in the ovarian follicle, oocyte and pre-implantation embryo. Reproduction. 2010;139:705–15. doi: 10.1530/REP-09-0517. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill C. Endogenous folic acid is essential for normal development of preimplantation embryos. Hum Reprod. 1998;13:1312–6. doi: 10.1093/humrep/13.5.1312. [DOI] [PubMed] [Google Scholar]
- 22.Matte JJ, Girard CL, Brisson GJ. Folic acid and reproductive performances of sows. J Anim Sci. 1984;59:1020–5. doi: 10.2527/jas1984.5941020x. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay GF, Matte JJ, Dufour JJ, Brisson GJ. Survival rate and development of fetuses during the first 30 days of gestation after folic acid addition to a swine diet. J Anim Sci. 1989;67:724–32. doi: 10.2527/jas1989.673724x. [DOI] [PubMed] [Google Scholar]
- 24.Habibzadeh N, Schorah CJ, Smithells RW. The effects of maternal folic acid and vitamin C nutrition in early pregnancy on reproductive performance in the guinea-pig. Br J Nutr. 1986;55:23–35. doi: 10.1079/bjn19860006. [DOI] [PubMed] [Google Scholar]
- 25.McNulty H, Pentieva K. Folate bioavailability. Proc Nutr Soc. 2004;63:529–36. doi: 10.1079/pns2004383. [DOI] [PubMed] [Google Scholar]
- 26.Girdwood RH, Eastwood MA, Finlayson ND, Graham GS. Pernicious anaemia as a cause of infertility in twins. Lancet. 1971;1:528–30. doi: 10.1016/s0140-6736(71)91128-7. [DOI] [PubMed] [Google Scholar]
- 27.Jackson IM, Doig WB, McDonald G. Pernicious anaemia as a cause of infertility. Lancet. 1967;2:1159–60. doi: 10.1016/s0140-6736(67)91887-9. [DOI] [PubMed] [Google Scholar]
- 28.Bennett M. Vitamin B12 deficiency, infertility and recurrent fetal loss. J Reprod Med. 2001;46:209–12. [PubMed] [Google Scholar]
- 29.Stabler SP. Vitamin B12.1. Washington, D.C: International Life Sciences Institute; 2006. pp. 302–313. [Google Scholar]
- 30.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–32. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 31.Stephen EH, Chandra A. Use of infertility services in the United States: 1995. Fam Plann Perspect. 2000;32:132–7. [PubMed] [Google Scholar]
- 32.United Kingdom Department of Health. Report of the Panel on Dietary Reference Values of the Committe on Medical Aspects of Food Policy. London, UK: The Stationery Office; 1991. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. [PubMed] [Google Scholar]
- 33.German Nutrition Society, Austrian Nutrition Society, Swiss Society for Nutrition Research, Swiss Nutrition Association. Reference Values for Nutrient Intake. Frankfurt/Main, Germany: Umschau Braus GmbH/German Nutrition Society; 2002. [Google Scholar]
- 34.Health Council of the Netherlands. Towards an optimal use of folic acid. Hague: 2008. [Google Scholar]
- 35.Food Safety Authority of Ireland RDA Working Group. Recommended Dietary Allowances for Ireland. Dublin, Ireland: 1999. [Google Scholar]
- 36.Nordic Council of Ministers. Nordic Nutrition Recommendations 2004: Integrating Nutrition and Physical Activity. 4. Copenhagen, Denmark: Nordic Council of Ministers; 2005. [Google Scholar]
- 37.Australian National Health and Medical Research Council, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. Canberra, Australia: National Health and Medical Research Council; 2006. [Google Scholar]
- 38.Institute of Medicine. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, D.C: National Academy Press; 1998. [PubMed] [Google Scholar]
- 39.Kennedy D, Koren G. Identifying women who might benefit from higher doses of folic acid in pregnancy. Can Fam Physician. 2012;58:394–7. [PMC free article] [PubMed] [Google Scholar]
- 40.What is the current policy on folic acid supplementation for reducing NTDs? 2013. New Zealand: Ministry of Health; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.