Abstract
Several experiments sustain healthful benefits of the flavanone naringenin (Nar) against chronic diseases including its protective effects against estrogen-related cancers. These experiments encourage Nar use in replacing estrogen treatment in post-menopausal women avoiding the serious side effects ascribed to this hormone. However, at the present, scarce data are available on the impact of Nar on E2-regulated cell functions. This study was aimed at determining the impact of Nar on the estrogen receptor (ERα and β)-dependent signals important for 17β-estradiol (E2) effect in muscle cells (rat L6 myoblasts, mouse C2C12 myoblasts, and mouse skeletal muscle satellite cells). Dietary relevant concentration of Nar delays the appearance of skeletal muscle differentiation markers (i.e., GLUT4 translocation, myogenin, and both fetal and slow MHC isoforms) and impairs E2 effects specifically hampering ERα ability to activate AKT. Intriguingly, Nar effects are specific for E2-initiating signals because IGF-I-induced AKT activation, and myoblast differentiation markers were not affected by Nar treatment. Only 7 days after Nar stimulation, early myoblast differentiation markers (i.e., myogenin, and fetal MHC) start to be accumulated in myoblasts. On the other hand, Nar stimulation activates, via ERβ, the phosphorylation of p38/MAPK involved in reducing the reactive oxygen species formation in skeletal muscle cells. As a whole, data reported here strongly sustain that although Nar action mechanisms include the impairment of ERα signals which drive muscle cells to differentiation, the effects triggered by Nar in the presence of ERβ could balance this negative effect avoiding the toxic effects produced by oxidative stress .
Electronic supplementary material
The online version of this article (doi:10.1007/s12263-014-0425-3) contains supplementary material, which is available to authorized users.
Keywords: Estrogen, Estrogen receptors, Naringenin, Signal transduction, Skeletal muscle differentiation
Introduction
In recent years, plant-derived polyphenols, in particular flavonoids, deserved particular attention being associated with healthful benefits against chronic diseases (Shulman et al. 2011; Crozier et al. 2009). Examples of such polyphenols are resveratrol in red wine and epigallocatechin in green tee (Bengmark et al. 2009). One of the most abundant bioactive flavonoids is the glycoside naringin, responsible for the bitter taste in grapefruit, which is hydrolyzed to naringenin (4,5,7-trihydroxy-flavanone-7 rhamnoglucoside, Nar) by the gut flora prior to being absorbed by enterocytes. Naringenin protective effects have been widely studied: antioxidant effects (Renugadevi and Prabu 2009), modulation of cytochrome P450 detoxification enzymes (Huong et al. 2006), modulation of plasma cholesterol levels (Jeon et al. 2007), and antihyperglycemic effects (Jung et al. 2004) have been reported. In addition, several experimental data support anticarcinogenic and pro-apoptotic effects of Nar in numerous types of cancer (Arul and Subramanian 2013; Meiyanto et al. 2012; Weng and Yen 2012). Most of these effects have been obtained by using variable Nar concentrations ranging from 1 to 100 µM. We recently reported that Nar concentrations (1–10 μM), achievable in human plasma after a meal rich in flavonoids (i.e., tomatoes, oranges, and grapefruit juice typical of Mediterranean diet), exert protective pro-apoptotic effects against estrogen-related cancers (Galluzzo and Marino 2006; Bulzomi et al. 2012, 2010). Nar binds to both estrogen receptors (i.e., ERα and ERβ) (Kuiper et al. 1998; Bulzomi et al. 2012) and acts as a mimetic of 17β-estradiol (E2), the most active among estrogens, activating a pro-apoptotic cascade in the presence of ERβ subtype (Totta et al. 2004; Galluzzo and Marino 2006; Marino et al. 2012). On the other hand, Nar selectively impairs the membrane-initiating signals of ERα subtype important for cell cycle progression (Virgili et al. 2004; Galluzzo and Marino 2006; Galluzzo et al. 2008; Marino et al. 2012).
As a whole, these data suggest that Nar could potentially replace the actions of E2. However, E2 effects in mammals include the regulation of growth and differentiation of several organs and tissues influencing the whole homeostasis maintenance in both male and female organisms (Thomas and Gustafsson 2011). Thus, Nar ability to interfere with ER activities raises some concerns on the putative unhealthy side effects of this flavonoid. In addition, ERα and ERβ could be co-expressed within the same organ rendering very difficult to predict the Nar interference on E2 effects. An example is represented by differentiating skeletal muscle.
Although adult skeletal muscle is a stable tissue, it possesses the remarkable ability to rapidly recover and regenerate following injury (Thomas and Gustafsson 2011; Hawke and Garry 2001). In response to injury, undifferentiated satellite cells are activated from a quiescent state and proliferate as myoblasts. Myoblasts, in turn, are withdrawn from the cell cycle and differentiate re-expressing myogenic regulatory factors (MRFs, namely MyoD, Myf5, myogenin, and MRF4) as well as embryonic myofibrillar genes (e.g., the fetal isoform of myosin heavy chain, fMHC). Finally, differentiated myoblasts fuse to form the multinucleated mature muscle fibers, thus contributing to skeletal muscle regeneration (Hawke and Garry 2001; Trapani et al. 2012).
Several endocrine factors, including sex steroid hormones, can influence muscle cell differentiation throughout life via their cognate receptors. In particular, many studies have demonstrated sex differences in skeletal muscle response to injury and in the indices of skeletal muscle damage, inflammation, and repair that are largely attributable to E2 (Bar et al. 1988; McClung et al. 2006). Skeletal muscle expresses both ERs, and in rodents, ERβ is the predominant subtype (Barros and Gustafsson 2011; Galluzzo et al. 2009). ERβ seems to participate to the E2-induced skeletal muscle regeneration after injury even if a suppressive role on glucose transporter (GLUT4) expression has been evidenced (Velders et al. 2012; Barros and Gustafsson 2011). On the other hand, we recently reported that E2 only requires ERα-dependent rapid p38/MAPK and PI3 K/AKT activation to induce L6 myoblast differentiation (Galluzzo et al. 2009). At the present, the impact of Nar on ERα-mediated skeletal muscle cell differentiation is unknown. This study was aimed at determining the impact of the flavonoid Nar on E2-induced differentiation in skeletal muscle cells. Rat L6 myoblasts, mouse C2C12 myoblasts, and mouse satellite cells were used as experimental models.
Materials and methods
Reagents
17β-Estradiol (E2), naringenin (Nar), insulin-like growth factor I (IGF-I), actinomycin, cycloheximide, gentamicin, penicillin, ‘Dulbecco’s modified Eagle’s medium’ (DMEM) (without phenol red), F-10 Ham (N6908), charcoal-stripped fetal calf serum, and the palmitoyl acyltransferase (PAT) inhibitor 2-bromohexadecanoic acid (2-Br) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The ER inhibitor ICI 182,780, the ERα selective agonist, PPT (4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol), the ERβ selective agonist, DPN (2,3-bis(4-hydroxyphenyl)-propionitrile), and the ERβ selective antagonist, THC ((R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol) were obtained from Tocris (Ballwin, MO, USA). The AKT inhibitor, the extracellular regulated kinase (ERK) inhibitor, PD 98059, and the p38 inhibitor, SB 203580, were obtained from Calbiochem (San Diego, CA). Bradford protein assay was obtained from Bio-Rad Laboratories (Hercules, CA, USA). The antiphospho-ERK, anti-AKT, anti-ERK, anti-ERα M20 (N-terminus), anti-ERβ H150, and anticaspase-3 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antiglucose transporter type 4 (Glut-4), antimyogenin, and antifetal myosin heavy chain (fetal MHC) were purchased from Abcam (Cambridge, UK). Antifast MHC MY-32 and antislow MHC NOQ7.5.4D were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-β-tubulin was purchased from MP Biomedicals (Solon, OH, USA). The polyclonal antiphospho-AKT, antiphospho-p38, and anti-p38 antibodies were purchased from New England Biolabs (Beverly, MA, USA). The CDP-Star, chemiluminescence reagent for Western blot was obtained from NEN (Boston, MA, USA). All the other products were from Sigma-Aldrich (St. Louis, MO, USA). Analytical or reagent grade products, without further purification, were used.
Cell culture and cell cycle analysis
Rat myoblast L6 cells (ATCC, Manassas, VA) and mouse myoblasts C2C12 (a generous gift of Prof. Daniela Caporossi, IUSM, Rome Italy) were routinely grown in 5 % CO2 in phenol red-free DMEM medium containing 10 % (v/v) charcoal-stripped fetal calf serum, l-glutamine (2.0 mM), gentamicin (10.0 µg/ml), and penicillin (100.0 U/ml). Cells were passaged every 2 days. Mouse adult primary satellite cells (a generous gift of Dr. Marco Crescenzi, ISS, Rome, Italy) were grown on gelatin-coated dishes in 77 % nutrient mixture F-10 Ham with gentamicin (10.0 µg/ml), and penicillin (100.0 U/ml), 20 % fetal bovine serum, 3 % chicken embryo extract (prepared in Hank’s Balanced Solution), and 2.5 ng/ml basic fibroblast growth factor (Peprotech, Rocky Hill, NJ).
For differentiation, satellite cells were seeded at high density (2.5 × 105) in gelatine-coated 35 mm dishes, and after cell adhesion, growth medium was replaced with DMEM with gentamicin (10.0 µg/ml), penicillin (100.0 U/ml), and 10 % fetal bovine serum. Medium was changed every 24 h (Trapani et al. 2012). L6 and C2C12 undifferentiated myoblasts were treated with differentiation medium containing 2 % (v/v) charcoal-stripped fetal calf serum; myotubes were obtained 7 day after serum concentration reduction (Galluzzo et al. 2009).
In differentiation, medium cells were simultaneously treated either with vehicle (ethanol/PBS 1:10, v/v) or with different E2 concentrations or different concentration of Nar or PPT (final concentration, 10 nM) or DPN (final concentration, 10 nM) or IGF-I (final concentration, 100 ng/ml) or T (final concentration, 1 nM) or DHT (final concentration, 1 nM). Cells were harvested 6, 24, 48, and 72 h or 7 days after treatment with differentiation medium. When indicated, the antiestrogen ICI 182,870 (final concentration 1 µM), the AKT inhibitor (final concentration 1 µM), the p38 inhibitor, SB 203580 (final concentration 5 µM), and the ERβ inhibitor, THC (final concentration 1 µM) were added 30 min before E2 or Nar or IGF-I administration.
L6 myoblasts were harvested with trypsin 24, 48, and 72 h after treatment and fixed with 1 ml ice-cold 70 % ethanol and subsequently stained with 2 mg/ml DAPI/PBS solution. The fluorescence of DNA was measured with DAKO Galaxyflow cytometer equipped with HBO mercury lamp, and the percentage of cells present in sub-G1 phase was calculated using FloMax © Software.
Measurements of reactive oxygen species (ROS)
L6 myoblasts were grown to ~70 % confluence, harvested, and then re-suspended in PBS with 10 µM dichlorodihydrofluorescein diacetate (DCF; Molecular Probes, Eugene, OR, USA) for 30 min at 37 °C in the dark. After additional 30 min, to allow the equilibrium, the fluorescence was measured under continuous gentle magnetic stirring at 37 °C in a PerkinElmer LS-50B spectrofluorimeter. Excitation wavelengths were set at 498 nm and emission at 530 nm, respectively. Cells were treated with 600 µM H2O2 (final concentration) in the presence of either E2 (final concentration, 10 nM) or Nar (final concentration, 1 µM) or vehicle or PPT (final concentration, 10 nM) or DPN (final concentration, 10 nM) for 15 min, and fluorescence was registered as arbitrary unit. In some experiments, the p38 inhibitor, SB 203580 (final concentration 5 µM), and the ERβ inhibitor, THC (final concentration 1 µM) were added 30 min before E2 or Nar or IGF-I administration.
Protein analysis
Protein profiles were analyzed by Western blotting as previously described (Bulzomi et al. 2012). In some experiments, cells were homogenized by using Teflon pestle homogenizer; homogenates were centrifuged at 1,000×g for 10 min to pellet the nuclear fraction. Membrane-rich fractions were obtained by centrifuging the supernatants at 100,000×g for 30 min. Proteins from membrane-rich fraction and from total lysate were then solubilized as described above (Galluzzo et al. 2009). Briefly, 20 µg of protein from lysates were resolved by 7 % SDS–PAGE at 100 V for 60 min. The proteins were subsequently electrophoretically transferred to nitrocellulose for 90 min at 100 V. The nitrocellulose membrane was blocked at room temperature with 3 % BSA in Tris-buffered saline (138 mM NaCl, 27 mM KCl, 25 mM Tris–HCl, 0.05 % Tween-20, pH 6.8) and probed at 4 °C overnight with primary antibodies followed by Trapani et al. (2012) incubation for 1 h with secondary antibodies. The nitrocellulose membrane was probed and then re-probed with anti-β-tubulin or anticaveolin-1 antibodies to normalize total lysate or membrane fractions, respectively. Bound antibodies were visualized using enhanced chemoluminescence detection. All images derived from Western blotting were analyzed with ImageJ (NIH, Bethesda, MD) software for Windows. Each reported value was derived from the ratio between arbitrary units obtained by the protein band and the respective tubulin or caveolin-1 band.
Statistical analysis
Data are expressed as mean ± SD. The difference in parameters was statistically tested for significance with one-way ANOVA followed by Tukey–Kramer post test using GraphPad Instat3 (GraphPad software, Inc., La Jolla, CA) software for Windows. P < 0.05 was considered statistically significant.
Results
Nar effects on skeletal muscle cell differentiation markers
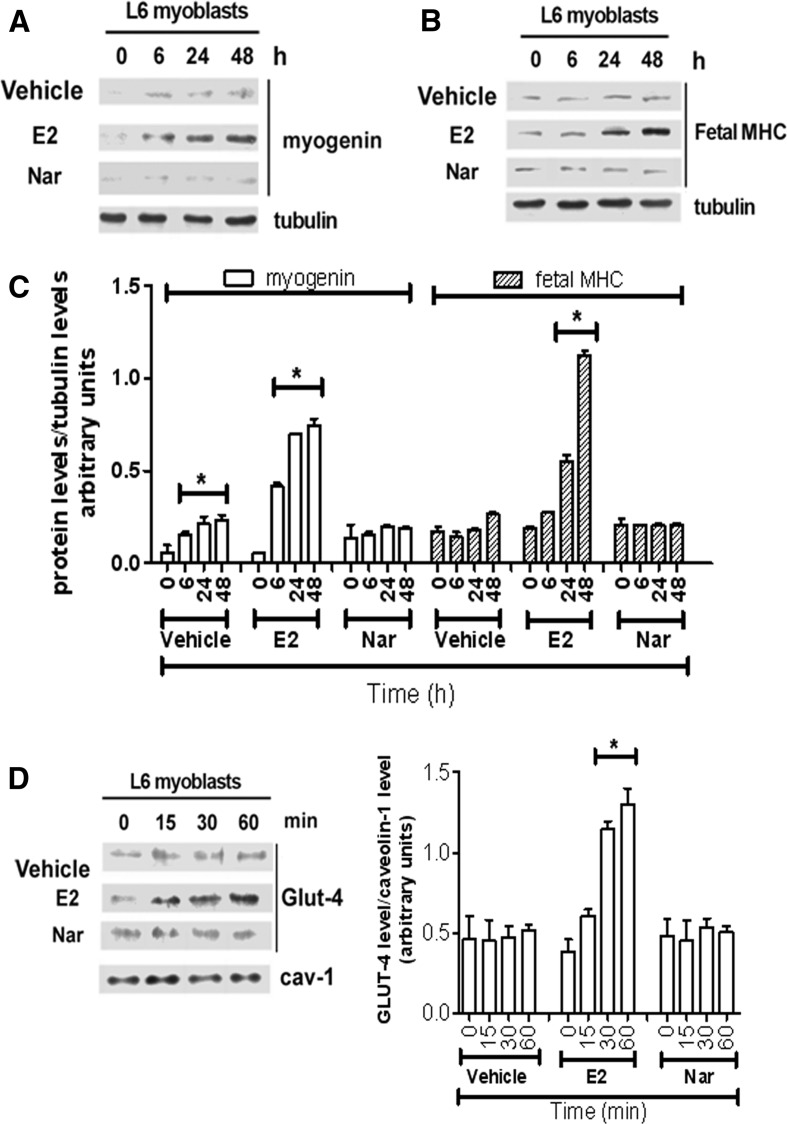
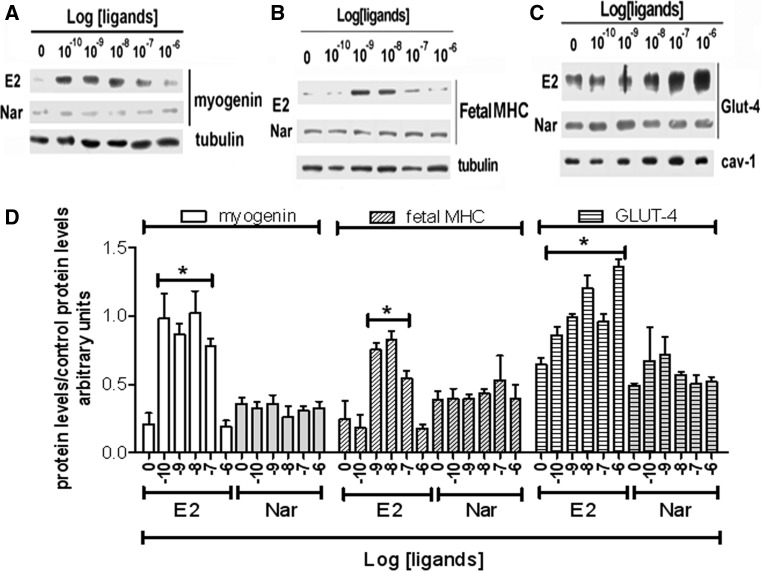
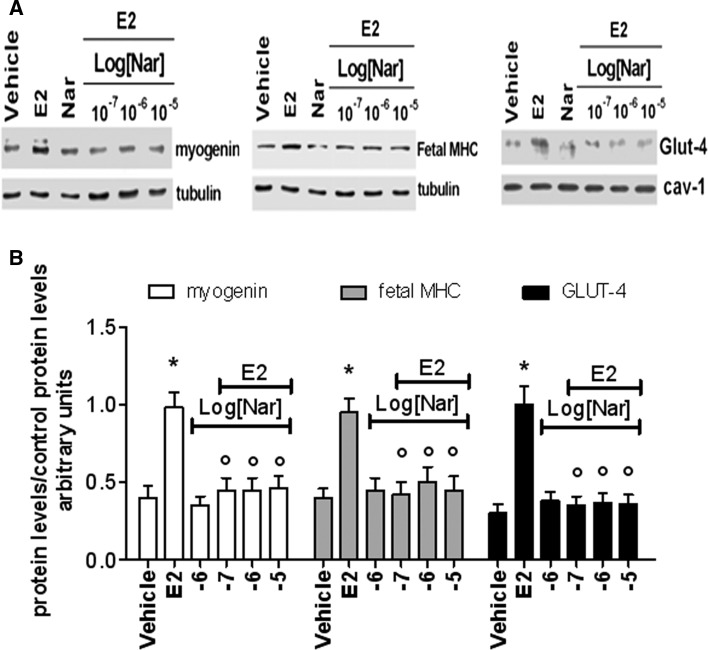
As expected, ERβ is the predominant receptor subtype in growing rat L6 myoblasts (Galluzzo et al. 2009). However, the induction of myoblast differentiation by serum withdrawal is paralleled by an increase of ERα levels, which remain high until 72 h (supplemental figure 1A). In spite of the variation in the ER subtype levels, neither E2 (10 nM) nor Nar (1 µM) modifies the distribution of myoblasts in the cell cycle at any of the tested times (supplemental figure 1B) or activate the cleavage of caspase-3, the effector of apoptosis (17 kDa band, supplemental figure 1C) until 72 h after serum reduction. These data sustain that none of the tested compounds modify myoblast proliferation or apoptosis. On the other hand, just 6 h after serum removal, the levels of myogenin (Myo, a well-known marker of myoblast differentiation) (Hawke and Garry 2001; Trapani et al. 2012) increase, and the levels of this protein are further enhanced in myoblasts treated with 10 nM E2 (Fig. 1a, c). In line with the differentiative effect of E2 already reported in growing L6 cells (Galluzzo et al. 2009), 24 h after serum removal the hormone, the level of the fetal isoform of myosin heavy chain (MHC, a contractile protein marker of skeletal muscle differentiation) doubled over the control (Fig. 1b, c). In contrast, Nar stimulation did not modified Myo or fetal MHC level up to 48 h of stimulation (Fig. 1b, c). A similar effect was reported also for the glucose transporter GLUT-4, a metabolic marker (Fig. 1d). Indeed, only E2 increased the translocation of GLUT-4 to the plasma membrane just 15 min after hormone treatment (Fig. 1d). The E2-induced increase of skeletal muscle differentiation (i.e., Myo and fetal MHC) and metabolism (i.e., GLUT-4) markers, after 48 h of stimulation, was dose-dependent with a maximum detected from 0.1 nM to 10 nM for Myo (Fig. 2a, d), 1 nM to 10 nM for fetal MHC (Fig. 2b, d), and 1 nM to 1 µM for GLUT-4 translocation (Fig. 2c, d), respectively. Notably, Nar had no effect either on Myo and fetal MHC levels or on GLUT-4 translocation at any of the tested concentrations (Fig. 2). However, Nar stimulation (0.1-10 µM) in the presence of an E2 background (10 nM) completely reduced the hormone effects on differentiation marker levels (Fig. 3a, b) in L6 cells, suggesting that Nar could antagonize the E2 effects in rat L6 myoblasts.
Fig. 1.
Time-course of E2 and Nar effect on differentiation markers in L6 cells. L6 cells were grown in the differentiation medium containing 2 % serum. Western blot analysis of vehicle (DMSO:PBS 0.1:1), E2 (10−8 M), and Nar (10−6 M) treatment on myogenin (a), Fetal MHC (b) levels and related densitometric analyses (c). d Vehicle, E2 and Nar effect on GLUT-4 plasma membrane translocation ( d) and related densitometric analysis. The amount of proteins was normalized by comparison with tubulin (a and b) or cav-1 (d) levels. Data are the mean of five different experiments ± SD. * P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to the vehicle-treated samples
Fig. 2.
Dose response effects of E2 and Nar effect on differentiation markers in L6 cells. a, b, and c, The typical western blots of E2 and Nar dose-dependent (ranging from 10−10 to 10−6 M) effects on myogenin, Fetal MHC levels (24 h of stimulation) and on GLUT-4 translocation to the plasma membrane (30 min of stimulation), respectively. d shows the related densitometric analyses. The amount of proteins was normalized by comparison with tubulin (a and b) or cav-1 (c) levels. Data are the mean of 3 different experiments ± SD. *P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to control samples
Fig. 3.
Effect E2 and Nar coadministration on differentiation markers in L6 cells. L6 cell were maintained in differentiation medium (2 % serum) for 24 h (myogenin and Fetal MHC) or 30 min (GLUT-4) in the presence of either vehicle (DMSO:PBS 0.1:1) or E2 (10−8 M) or Nar (10−6 M) or different concentration of Nar (ranging from 10−7 M to 10−5 M) in the presence of E2 background (10−8 M). a The typical western blot of myogenin and MHC levels, and GLUT-4 plasma membrane translocation and b The related densitometric analyses. The amount of proteins was normalized by comparison with tubulin (myogenin and Fetal MHC) or cav-1 (GLUT-4) levels. Data are the mean of five different experiments ± SD. P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to vehicle-treated samples (asterisk) or to E2-treated samples (open circle)
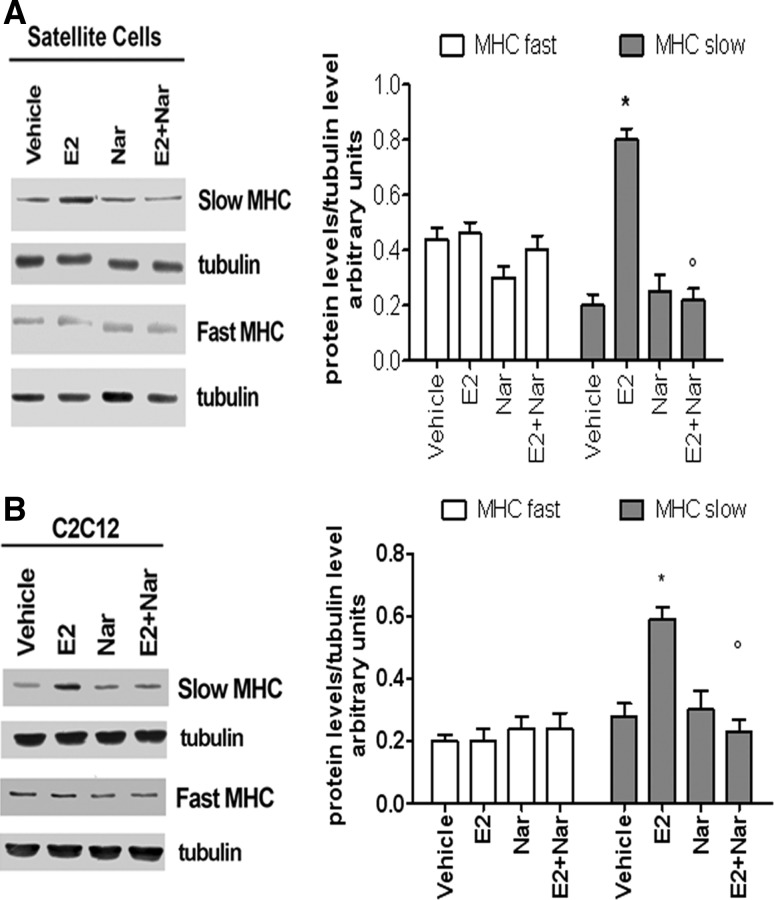
This antagonistic effect of Nar was also confirmed in mouse satellite cells and C2C12 myoblasts. Satellite cells recapitulate several mechanisms of skeletal muscle regeneration and differentiation. Regenerating muscles initially express MHCs that are typical of developing muscle, such as fetal MHCs, but soon they start to express adult isoforms of MHCs (i.e., fast and slow MHC), which identify terminally differentiated myofibers (Zammit et al. 2006; Ciciliot and Schiaffino 2010). This MHC switch is a default program, which also occurs in the absence of the innervation (Ciciliot and Schiaffino 2010). Western blot analyses confirmed the presence of both ER subtypes in satellite cells (Supplemental figure 2A) and revealed that, after 24 h of differentiation stimuli, these cells present higher levels of Myo and fetal MHC (Supplemental figure 2B and 2C) with respect to L6 myoblasts (see Fig. 1). After 48 h of serum withdrawal, the levels of Myo and fetal MHC decreased to disappear 72 h after stimuli, with the contemporary increase of fast and slow MHC isoform levels (Supplemental figure 2B and 2C). Interestingly, E2 treatment (10 nM, 72 h) specifically increased the slow MHC isoform without any effect on fast MHC isoform (Supplemental figure 2B and 2C). This E2 effect is mimicked by 10 nM of ERα agonist PPT but not by the ERβ agonist DPN (10 nM) (Supplemental figure D) demonstrating that, like in L6 myoblasts (Galluzzo et al. 2009), ERα is the receptor subtype involved in E2 effects in satellite cells. Although Nar alone (1 µM, 72 h) did not exert any effect on the level of slow and fast MHC isoforms (Fig. 4a), Nar and E2 co-treatment impaired E2 effect on slow MHC level (Fig. 4a). Similar effect of Nar (1 µM, 48 h) was reported in C2C12 mouse myoblasts (Fig. 4b) sustaining the antagonistic role of this flavonoid in satellite cells and rat and mouse myoblasts.
Fig. 4.
Effect of E2 and Nar on slow and fast MHC level in satellite cells and in C2C12 myoblasts. Typical Western blot and densitometric analyses of slow and fast MHC levels in satellite cells (a) and in C2C12 myoblasts maintained in differentiation medium for 48 h in the presence of either vehicle (DMSO:PBS 0.1:1) or E2 (10−8 M) or Nar (10−6 M). The amount of proteins was normalized by comparison with tubulin levels. Data represent the mean ± SD of five experiments. P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to vehicle-treated samples (asterisk) or to E2-treated samples (open circle)
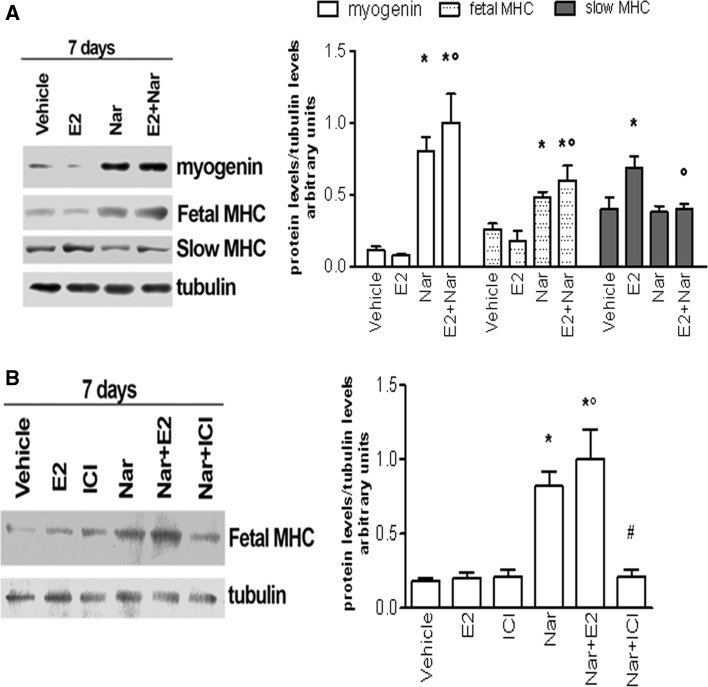
As a whole, these data suggest that Nar delays the skeletal muscle differentiation marker appearance and acts as an E2 antagonist impairing the hormone-induced differentiative effects. This assumption prompted us to verify the long-term Nar and E2 effects on skeletal muscle differentiation markers. As expected from the data of satellite cells (Supplemental figure 2), E2 stimulation (10 nM, 7 days) of L6 myoblasts did not modify the level of either Myo or fetal MHC, although the hormone still increased slow MHC isoform. Intriguingly, a longer stimulation with Nar (1 µM, 7 days) significantly increased both early differentiation markers (i.e., Myo and fetal MHC) even in the presence of E2 (Fig. 5a) without any effect on the late differentiation marker (i.e., slow MHC), while this flavonoid still impaired E2-induced increase of slow MHC. The Nar-induced fetal MHC level requires ER being completely prevented by the pre-treatment with the pure ER inhibitor, ICI 182,870 (1 µM) (Fig. 5b).
Fig. 5.
Long-term Effect of E2 and Nar on differentiation markers in L6 cells. Typical Western blot and densitometric analyses of myogenin, fetal and slow MHC levels in L6 cells (a) maintained in differentiation medium for 7 days in the presence of either vehicle (DMSO:PBS 0.1:1) or E2 (10−8 M) and/or Nar (10−6 M). The amount of proteins was normalized by comparison with tubulin levels. b Typical Western blot and densitometric analyses of fetal MHC levels in L6 cells in differentiation medium for 7 days in the presence of vehicle (DMSO:PBS 0.1:1) or E2 (10−8 M) or Nar (10−6 M) and/or 10−6 M ICI 182,780 (estrogen receptor inhibitor). Data represent the mean ± SD of five experiments. P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to vehicle-treated samples (asterisk) or to E2-treated samples (open circle) or to Nar-treated samples (hash)
Nar action mechanisms in L6 cells
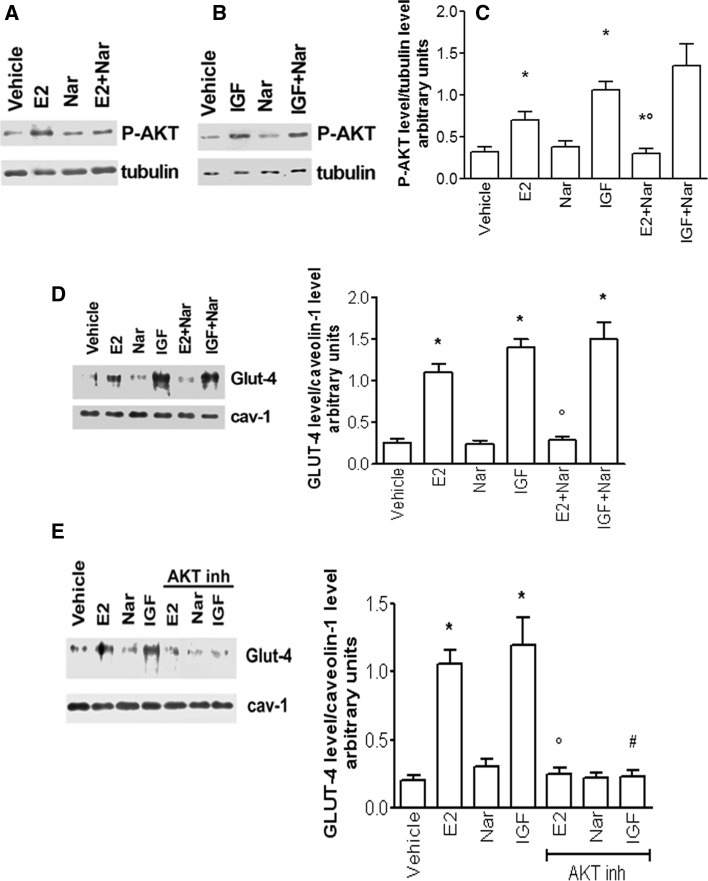
As previously reported (Galluzzo et al. 2009), E2-induced differentiation in proliferating L6 cells requires ERα-mediated AKT and p38 MAPK activation, which, in turn, are necessary for the rapid GLUT-4 plasma membrane translocation and for Myo and fetal MHC increase. Here, we confirm that in differentiating L6 myoblasts, ERα is also necessary for the E2-induced rapid (15 min) activation of both AKT and p38, whereas the ERβ agonist, DPN, just activates p38 without any effect on AKT phosphorylation (Supplemental figure 3). Thus, it is possible that the Nar effects in delaying differentiation rely on the inhibition of AKT and p38 activation. In order to verify this hypothesis, L6 cells were stimulated with Nar, E2 and IGF-I, a well-known muscle trophic factor. As shown in Fig. 6a, b, both E2 and IGF-I rapidly increased (15 min) AKT phosphorylation, whereas Nar did not modify the phosphorylation status of this kinase, but this flavonoid specifically prevented the ability of E2 to activate AKT without any effect on IGF-I-induced AKT activation (Fig. 6a–c). In line with this result, both E2 and IGF-I increased the translocation of GLUT-4 at the plasma membrane (30 min) (Fig. 6d). In addition, cell pre-treatment with AKT inhibitor completely prevented both E2- and IGF-I-induced GLUT-4 translocation to the plasma membrane (Fig. 6e), confirming the pivotal role of this pathway in this event. Intriguingly, no change in GLUT-4 plasma membrane translocation was observed upon Nar stimulation, whereas, only in the presence of E2, Nar completely prevented AKT phosphorylation and GLUT-4 translocation (Fig. 6a, d), suggesting that Nar specifically hampers ERα-mediated AKT phosphorylation.
Fig. 6.
Nar effect on AKT activation and GLUT-4 plasma membrane translocation. Typical Western blot and densitometric analyses of AKT phosphorylation (a, b, and c) and GLUT-4 plasma membrane translocation (d and e) in L6 cells maintained in differentiation medium for 48 h and stimulated for 1 h (a, b, and c) or 30 min (d and e) with either vehicle (DMSO:PBS 0.1:1) or E2 (10−8M) or IGF (100 ng/ml) and/or Nar (10−6 M). When indicated cells have been pre-treated (30 min) with the AKT inhibitor (10−6M). Data represent the mean ± SD of five experiments. P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to vehicle-treated samples (asterisk) or to E2-treated samples (open circle)
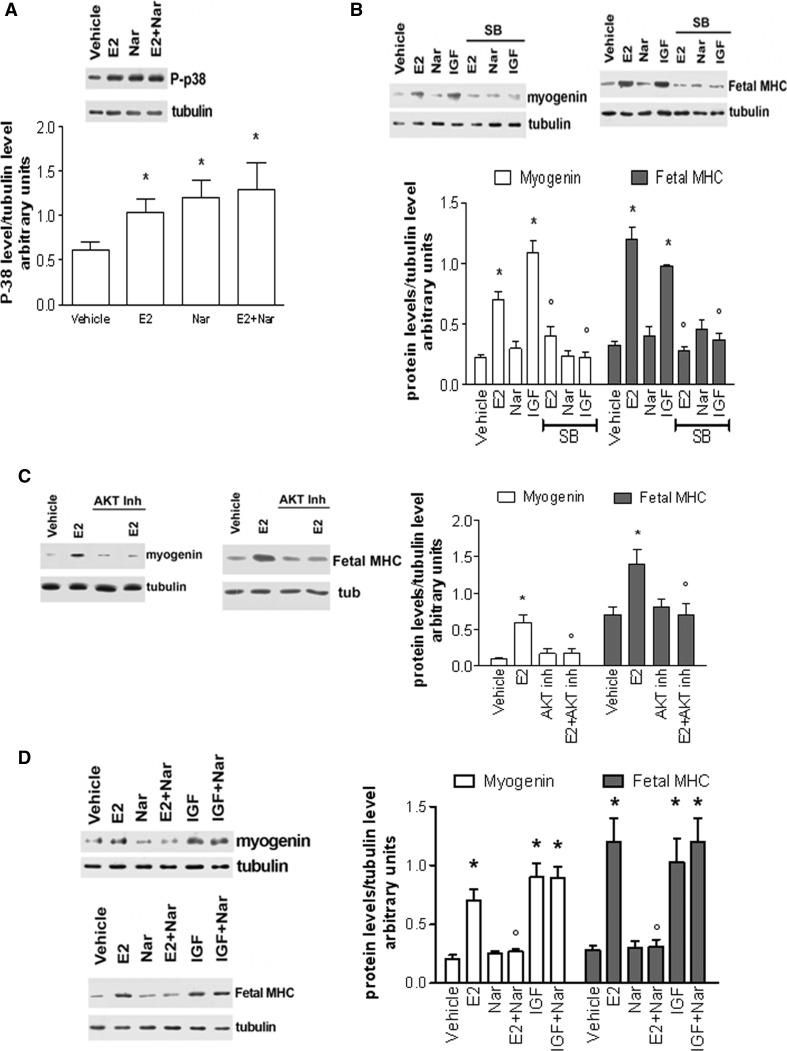
On the other hand, Nar activated, as well as E2, p38 phosphorylation even in the presence of E2 (Fig. 7a). L6 cell pre-treatment with the p38 inhibitor (SB 203580 5 µM) prevented E2- and IGF-induced Myo and MHC increase (Fig. 7b), confirming the involvement of this kinase in E2- and IGF-induced Myo and MHC increase. However, the lack of Myo and MHC increase in Nar-stimulated cells indicate that p38 alone is not sufficient to lead L6 cell to differentiation. Indeed, AKT activation is also requested for the expression of these skeletal muscle differentiation markers as demonstrated by pre-treatment with AKT inhibitor (Fig. 7c). As consequence, Nar only impaired E2-induced Myo and MHC (Fig. 7d) levels in co-stimulation experiments, without affecting IGF-induced differentiation markers (Fig. 7d).
Fig. 7.
Nar effect on p38 activation and myogenin and fetal MHC level. Typical Western blot and densitometric analyses of p 38 phosphorylation (a), myogenin, and fetal MHC levels (b, c, and d) in L6 cells maintained in differentiation medium for 48 h and stimulated for 1 h (a) or 48 h (b, c, and d) with either vehicle (DMSO:PBS 0.1:1) or E2 (10−8 M) or IGF(100 ng/ml) and/or Nar (10−6 M). When indicated cells have been pre-treated (20 min) with p38 inhibitor (SB, 10−6 M, b) or with the AKT inhibitor (10−6 M, c). Data represent the mean ± SD of four experiments. P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to vehicle-treated samples (asterisk) or to E2-treated samples (open circle)
As a whole, these data demonstrate the specific ability of Nar to hamper E2-induced L6 cell differentiation by preventing the ERα-mediated AKT phosphorylation.
Nar effect on ERβ-mediated functions in L6 myoblasts
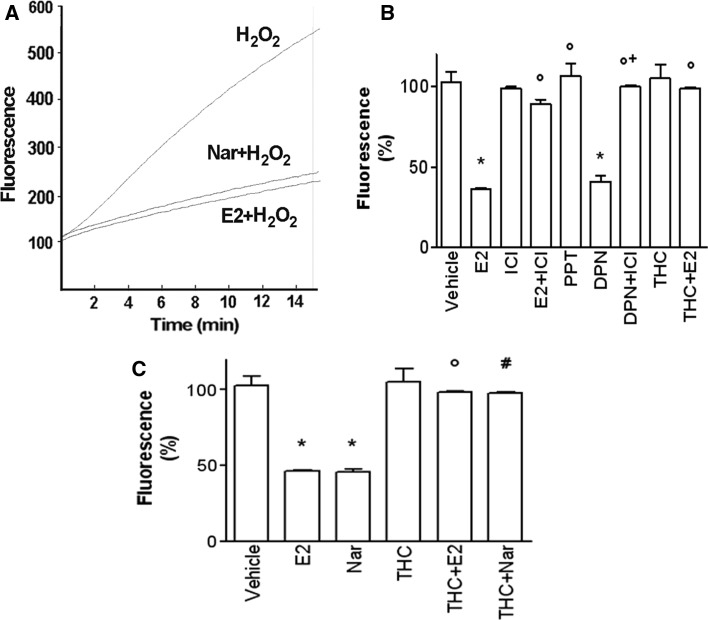
Reactive oxygen species (ROS) production physiologically occurs in resting and contracting muscle as well as during skeletal muscle differentiation as a by-product of mitochondrial respiration. However, when ROS levels overwhelm the endogenous antioxidant defense systems (Ji 2001), the resulting oxidative stress inhibits myoblast differentiation. P38 MAPK mediates oxidative stress-sensitive cell signaling pathways in several tissues including skeletal muscle (Powers et al. 2010). The evidence that both E2 and Nar, described as strong antioxidant (Tiidus et al. 2005), rapidly activates p38 prompted us to evaluate Nar effect, in comparison with E2, on H2O2-induced ROS production and the contribution of each ER isoform in mediating this putative effect. L6 cells were exposed to H2O2 (600 µM) for 15 min, and then, ROS generation was measured (Fig. 8a). E2 (10 nM) or Nar (1 µM) stimulation for 24 h before H2O2 addition caused a marked decrease in ROS generation (Fig. 8a). E2 antioxidant effects were completely mimicked by the ERβ selective agonists DPN, whereas the ERα selective agonist, PPT, was unable to prevent the ROS production (Fig. 8b). Moreover, the pure antagonists of ERs, ICI, and the ERβ selective antagonist, THC, completely prevented both E2- and Nar-induced decrease in ROS generation (Fig. 8b, c). These data suggest that, like E2, ERβ is involved in the Nar protective effect against H2O2-induced ROS production.
Fig. 8.
Nar and E2 effect on H2O2-induced ROS production in L6 myoblasts. L6 cells, maintained in differentiation medium for 48 h, were pre-treated, as indicated, with either vehicle (DMSO:PBS 0.1:1) or E2 (10−8 M) or Nar (10−6 M) or estrogen receptor inhibitor (ICI 182,780, ICI, 10−6 M) or the ERα agonist (PPT, 10−8 M) or the ERβ agonist (DPN, 10−8 M) or the ERβ antagonist THC (10−6 M) before exposition to H2O2 (2 × 10−4 M) for 15 min. In a typical original output (arbitrary units) of the registrations captured by the spectrofluorimeter during 15 min substance administration are reported. In b and c are reported data expressed as % of variation between H2O2-stimulated fluorescence versus basal fluorescence for each stimulation. Data are the mean ± SD of three experiments. P < 0.001 was calculated with ANOVA followed by Tukey–Kramer post test with respect to vehicle-treated samples (asterisk) or to E2-treated samples (open circle) or to DPN-treated samples (plus sign)or to Nar-treated samples (hash)
Discussion
In the present study, we report, for the first time, the ability of Nar to delay, without impeding, the differentiation of skeletal muscle cells preserving these cells from the cytotoxic effects of oxidative stress injury. The finding that 1 µM Nar protects skeletal muscle against oxidative stress could be of importance even in humans as consumption of orange or grapefruit juice (8 ml/kg body weight) increases up to 0.6 or 6 µM, respectively, the plasma Nar levels (Erlund et al. 2001; Zygmunt et al. 2010). Thus, naringenin concentrations close to that could be reached in the plasma after a meal rich in citrus fruits (e.g., 1 µM) may play a significant role in skeletal muscle cells. Our results together with the recent observations that Nar can directly activate glucose uptake in cultured skeletal muscle (Zygmunt et al. 2010) as well as improve insulin action in skeletal muscle reduce plasma free fatty acid levels, and hepatic gluconeogenesis in fructose-fed insulin resistant rats (Kannappan and Anuradha 2010) sustains that skeletal muscle is a target tissue for this flavonoid. Conversely, contrasting effect of Nar on glucose transport in monocytes (Park 1999), in human breast cancer cells (Harmon and Patel 2004), and in rat adipocytes (Harmon and Patel 2003) has been reported, suggesting a tissue-specific effect for this flavanone. However, these differences may be also due to the Nar concentration used, ranging from 1 to 100 µM, and/or to the cell lines used in each study, which could differently metabolize Nar and could be supplied with diverse receptor pattern including estrogen receptors. Indeed, like other flavonoids, Nar is well known as an estrogenic and antiestrogenic substance in dependence from the subtype of ER presents (Totta et al. 2004; Galluzzo et al. 2008; Kretzschmar et al. 2010; Amer et al. 2012; Swarnkar et al. 2012). In particular, our studies in cancer cells demonstrated that Nar hampers E2-related cancer cell progression by inhibiting the rapid ERα-dependent signal transduction pathways important for E2-induced cancer cell cycle progression (Totta et al. 2004; Galluzzo et al. 2009). On the other hand, flavonoids act as E2 mimetic in the presence of ERβ subtype (Totta et al. 2004; Bulzomi et al. 2012). Whether similar action mechanisms are also activated by Nar in noncancerous cells is completely unknown. Skeletal muscle differentiation represents a good experimental model to reach this aim in that it has been established that rodent (Barros et al. 2006; Galluzzo et al. 2009) and human (Lemoine et al. 2003; Wiik et al. 2003, 2009) skeletal muscles contain both functional ER subtypes.
Several studies indicate that E2 deficiency inhibits the muscle regeneration, and E2 supplementation enhances muscle mass and strength (Wust et al. 2008) accelerating skeletal muscles regeneration (McClung et al. 2006; Brown et al. 2005). In addition, previous data from our laboratory show the ability of E2 to increase the levels of well-known skeletal muscle differentiation (i.e., Myo and fetal MHC) and metabolic (i.e., GLUT-4 translocation) markers in growing L6 rat myoblasts (i.e., 10 % serum) (Galluzzo et al. 2009). In good agreement with the literature (McClung et al. 2006; Brown et al. 2005; Thomas et al. 2010; Barros and Gustafsson 2011), this E2 effect is dependent on the receptor subtype α signaling initiating at the plasma membrane (Galluzzo et al. 2009), although the involvement of ERβ in the expression of satellite cell and muscle regeneration markers (i.e., MHC embryonic, MyoD, Pax7) after injury with a myotoxin (notexin) has been also reported (Velders et al. 2012). Here, we also confirm this E2 effect in differentiating conditions. In fact, E2, like IGF-I, rapidly increases both the activation of AKT, linked to muscle development, regeneration, and hypertrophy (Lawlor and Rotwein 2000; Rommel et al. 2001; Sandri et al. 2004) and the p38/MAPK signaling pathway, which is crucial for the transcriptional control of skeletal muscle differentiation and for the fusion of myoblasts into myotubes (Lluis et al. 2006). Although several studies suggest that the two pathways are parallel (Keren et al. 2006), present data demonstrate that p38 MAPK activation is not sufficient to enhance myoblast differentiation, highlighting the pivotal role of ERα-activated AKT in promoting E2-mediated myoblast differentiation.
Our results clearly indicate that Nar enhances Myo and fetal MHC levels only after long time of exposition (i.e., after 7 days), while Nar completely hampers E2 ability to induce myoblast and satellite cell differentiation. Notably, Nar does not prevent IGF-I-induced AKT activation, and in turn, it does not affect IGF-I-dependent differentiation. These data strongly indicate that Nar, like in cancer cells (Totta et al. 2004; Galluzzo et al. 2008), specifically hampers ERα-mediated AKT activation and its downstream pathways (i.e., GLUT-4 translocation, Myo and MHC expression) without any direct effect on the kinase activity. In line with these results, the intake of prenylated metabolite of Nar (8-prenyl naringenin), full agonist on ERα and pronounced antagonist on ERβ (Roelens et al. 2006), accelerates AKT phosphorylation preventing muscle atrophy, promoting post-natal growth, and delaying the degradation of skeletal muscles in denervated mice (Mukai et al. 2012). Intriguingly, no effect was reported by Nar intake on AKT activation and muscle recovery in the same mice (Mukai et al. 2012).
However, despite of its antagonistic effects on E2:ERα complex-mediated differentiation, Nar does not impair muscle homeostasis and does not increase myoblast apoptosis. Our data demonstrate that Nar exerts a protective role on muscle, preventing ROS-induced damage. In muscle cells, ROS are continually generated. It is believed that these molecules have a well-established role as physiological modulators of skeletal muscle functions, including development, metabolism, and contractile functions. Moreover, studies in the past two decades suggest that, during strenuous muscle activity, in some pathological conditions or in aging, the generation of ROS in the skeletal muscle cells may be elevated to a level that overwhelms the antioxidant defense systems (Ji 1995, 2001) and can contribute to the development of muscle fatigue, inflammation, and degeneration (Meydani et al. 1992). When rat myoblasts were exposed to moderate or high intensity H2O2-induced oxidative stress for 1–6 h, cell death induction became evident and the activation of the apoptotic pathway could be evaluated 2 h after treatment (Caporossi et al. 2003). Both E2 and Nar exert a protective effect on H2O2-induced ROS production via ERβ activities. According to these data, E2:ERβ complex-mediated cytoprotection from oxidative stress has been reported also in ARPE cells (Giddabasappa et al. 2010). As a consequence, our data strongly support that Nar-mediated skeletal muscle protection from ROS-induced oxidative stress depends on its ERβ agonist activity rather than a free radical quenching compound activity.
As a whole, data reported here strongly sustain that although Nar action mechanisms include the impairment of ERα signals which drive muscle cells to differentiation, the protective effects triggered by Nar in the presence of ERβ could balance this negative effect avoiding the toxic effects produced by oxidative stress. Very recently, it has been reported that chronic exposition to naringin (the precursor of naringenin) for 6 months was well tolerated in Sprague-Dawley rats and did not cause either lethality or toxic clinical symptoms and changes in both sexes (Li et al. 2014). The authors propose that the no-observed-adverse-effect level (NOAEL) of naringin could be greater than 1,250 mg/kg/day following daily oral administrations. Using the body surface area normalization method, authors define that the dose of 1,250 mg/kg naringin in rat corresponds to 12 g/60 kg in humans (Li et al. 2014). Certainly, more experiments are needed to verify naringin and Nar effects in humans; in particular, the Nar bio-availability, the Nar concentration in the human blood that could be different in dependence on citrus-derived juices ingestion as daily or occasionally consumed, and the Nar metabolism that could produce active metabolites as already described for other flavonoids (Totta et al. 2005) are all necessary data to better define the effects of Nar on human health. Until then, our data obtained in cultured skeletal muscle cells open a new field of investigation indicating the role of Nar in the modulation of skeletal muscle differentiation.
Electronic supplementary material
Acknowledgments
Authors whish to thank Dr. M. Crescenzi (Department of Environment and Primary Prevention, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy) and Prof. Daniela Caporossi (University of Rome “Foro Italico”, Piazza L. de Bosis 6, 00135, Rome, Italy) for Satellite cells and C2C12 myoblasts, respectively. This work was supported by grants from the University Roma Tre (to M.M.).
References
- Amer DA, Jahne M, Weigt C, Kretzschmar G, Vollmer G. Effect of 17beta-estradiol and flavonoids on the regulation of expression of newly identified oestrogen responsive genes in a rat raphe nuclei-derived cell line. J Cell Physiol. 2012;227:3434–3445. doi: 10.1002/jcp.24044. [DOI] [PubMed] [Google Scholar]
- Arul D, Subramanian P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol Oncol Res. 2013;19:763–770. doi: 10.1007/s12253-013-9641-1. [DOI] [PubMed] [Google Scholar]
- Bar PR, Amelink GJ, Oldenburg B, Blankenstein MA. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci. 1988;42:2677–2681. doi: 10.1016/0024-3205(88)90243-3. [DOI] [PubMed] [Google Scholar]
- Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc Natl Acad Sci USA. 2006;103:1605–1608. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Bengmark S, Mesa MD, Gil A. Plant-derived health: the effects of turmeric and curcuminoids. Nutr Hosp. 2009;24:273–281. [PubMed] [Google Scholar]
- Brown M, Foley A, Ferreria JA. Ovariectomy, hindlimb unweighting, and recovery effects on skeletal muscle in adult rats. Aviat Space Environ Med. 2005;76:1012–1018. [PubMed] [Google Scholar]
- Bulzomi P, Bolli A, Galluzzo P, Leone S, Acconcia F, Marino M. Naringenin and 17beta-estradiol coadministration prevents hormone-induced human cancer cell growth. IUBMB Life. 2010;62:51–60. doi: 10.1002/iub.279. [DOI] [PubMed] [Google Scholar]
- Bulzomi P, Galluzzo P, Bolli A, Leone S, Acconcia F, Marino M. The pro-apoptotic effect of quercetin in cancer cell lines requires ERbeta-dependent signals. J Cell Physiol. 2012;227:1891–1898. doi: 10.1002/jcp.22917. [DOI] [PubMed] [Google Scholar]
- Caporossi D, Ciafre SA, Pittaluga M, Savini I, Farace MG. Cellular responses to H(2)O(2) and bleomycin-induced oxidative stress in L6C5 rat myoblasts. Free Radic Biol Med. 2003;35:1355–1364. doi: 10.1016/j.freeradbiomed.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des. 2010;16:906–914. doi: 10.2174/138161210790883453. [DOI] [PubMed] [Google Scholar]
- Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131:235–241. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- Galluzzo P, Marino M. Nutritional flavonoids impact on nuclear and extranuclear estrogen receptor activities. Genes Nutr. 2006;1:161–176. doi: 10.1007/BF02829966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzo P, Ascenzi P, Bulzomi P, Marino M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor alpha-palmitoylation. Endocrinology. 2008;149:2567–2575. doi: 10.1210/en.2007-1173. [DOI] [PubMed] [Google Scholar]
- Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M. 17beta-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-alpha-mediated signals. Am J Physiol Cell Physiol. 2009;297:C1249–C1262. doi: 10.1152/ajpcell.00188.2009. [DOI] [PubMed] [Google Scholar]
- Giddabasappa A, Bauler M, Yepuru M, Chaum E, Dalton JT, Eswaraka J. 17-beta estradiol protects ARPE-19 cells from oxidative stress through estrogen receptor-beta. Invest Ophthalmol Vis Sci. 2010;51:5278–5287. doi: 10.1167/iovs.10-5316. [DOI] [PubMed] [Google Scholar]
- Harmon AW, Patel YM. Naringenin inhibits phosphoinositide 3-kinase activity and glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;305:229–234. doi: 10.1016/S0006-291X(03)00720-4. [DOI] [PubMed] [Google Scholar]
- Harmon AW, Patel YM. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: a mechanism for impaired cellular proliferation. Breast Cancer Res Treat. 2004;85:103–110. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology (1985) J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Huong DT, Takahashi Y, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition. 2006;22:546–552. doi: 10.1016/j.nut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Kim HK, Kim HJ, Do GM, Jeong TS, Park YB, Choi MS. Hypocholesterolemic and antioxidative effects of naringenin and its two metabolites in high-cholesterol fed rats. Transl Res. 2007;149:15–21. doi: 10.1016/j.trsl.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ji LL. Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med. 1995;18:1079–1086. doi: 10.1016/0891-5849(94)00212-3. [DOI] [PubMed] [Google Scholar]
- Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928:236–247. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr. 2004;134:2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- Kannappan S, Anuradha CV. Naringenin enhances insulin-stimulated tyrosine phosphorylation and improves the cellular actions of insulin in a dietary model of metabolic syndrome. Eur J Nutr. 2010;49:101–109. doi: 10.1007/s00394-009-0054-6. [DOI] [PubMed] [Google Scholar]
- Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Kretzschmar G, Zierau O, Wober J, Tischer S, Metz P, Vollmer G. Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J Steroid Biochem Mol Biol. 2010;118:1–6. doi: 10.1016/j.jsbmb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Rotwein P. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol Cell Biol. 2000;20:8983–8995. doi: 10.1128/MCB.20.23.8983-8995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor α mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35:439–443. doi: 10.1249/01.MSS.0000053654.14410.78. [DOI] [PubMed] [Google Scholar]
- Li P, Wang S, Guan X, Cen X, Hu C, Peng W, Wang Y, Su W. Six months chronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem Toxicol. 2014;66:65–75. doi: 10.1016/j.fct.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Lluis F, Perdiguero E, Nebreda AR, Munoz-Canoves P. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 2006;16:36–44. doi: 10.1016/j.tcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Marino M, Pellegrini M, La Rosa P, Acconcia F. Susceptibility of estrogen receptor rapid responses to xenoestrogens: physiological outcomes. Steroids. 2012;77:910–917. doi: 10.1016/j.steroids.2012.02.019. [DOI] [PubMed] [Google Scholar]
- McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy (1985) J Appl Physiol. 2006;100:2012–2023. doi: 10.1152/japplphysiol.01583.2005. [DOI] [PubMed] [Google Scholar]
- Meiyanto E, Hermawan A, Anindyajati A. Natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pac J Cancer Prev. 2012;13:427–436. doi: 10.7314/APJCP.2012.13.2.427. [DOI] [PubMed] [Google Scholar]
- Meydani M, Evans W, Handelman G, Fielding RA, Meydani SN, Fiatarone MA, Blumberg JB, Cannon JG. Antioxidant response to exercise-induced oxidative stress and protection by vitamin E. Ann N Y Acad Sci. 1992;669:363–364. doi: 10.1111/j.1749-6632.1992.tb17124.x. [DOI] [PubMed] [Google Scholar]
- Mukai R, Horikawa H, Fujikura Y, Kawamura T, Nemoto H, Nikawa T, Terao J. Prevention of disuse muscle atrophy by dietary ingestion of 8-prenylnaringenin in denervated mice. PLoS One. 2012;7:e45048. doi: 10.1371/journal.pone.0045048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB. Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochem Biophys Res Commun. 1999;260:568–574. doi: 10.1006/bbrc.1999.0890. [DOI] [PubMed] [Google Scholar]
- Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256:128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Roelens F, Heldring N, Dhooge W, Bengtsson M, Comhaire F, Gustafsson JA, Treuter E, De Keukeleire D. Subtle side-chain modifications of the hop phytoestrogen 8-prenylnaringenin result in distinct agonist/antagonist activity profiles for estrogen receptors alpha and beta. J Med Chem. 2006;49:7357–7365. doi: 10.1021/jm060692n. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M, Cohen M, Soto-Gutierrez A, Yagi H, Wang H, Goldwasser J, Lee-Parsons CW, Benny-Ratsaby O, Yarmush ML, Nahmias Y. Enhancement of naringenin bioavailability by complexation with hydroxypropyl-beta-cyclodextrin [corrected] PLoS One. 2011;6:e18033. doi: 10.1371/journal.pone.0018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarnkar G, Sharan K, Siddiqui JA, Mishra JS, Khan K, Khan MP, Gupta V, Rawat P, Maurya R, Dwivedi AK, Sanyal S, Chattopadhyay N. A naturally occurring naringenin derivative exerts potent bone anabolic effects by mimicking oestrogen action on osteoblasts. Br J Pharmacol. 2012;165:1526–1542. doi: 10.1111/j.1476-5381.2011.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- Thomas A, Bunyan K, Tiidus PM. Oestrogen receptor-alpha activation augments post-exercise myoblast proliferation. Acta Physiol (Oxf) 2010;198:81–89. doi: 10.1111/j.1748-1716.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Deller M, Liu XL. Oestrogen influence on myogenic satellite cells following downhill running in male rats: a preliminary study. Acta Physiol Scand. 2005;184:67–72. doi: 10.1111/j.1365-201X.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- Totta P, Acconcia F, Leone S, Cardillo I, Marino M. Mechanisms of naringenin-induced apoptotic cascade in cancer cells: involvement of estrogen receptor alpha and beta signalling. IUBMB Life. 2004;56:491–499. doi: 10.1080/15216540400010792. [DOI] [PubMed] [Google Scholar]
- Totta P, Acconcia F, Virgili F, Cassidy A, Weinberg PD, Rimbach G, Marino M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activities of estrogen receptor-beta in cultured human cancer cells. J Nutr. 2005;135:2687–2693. doi: 10.1093/jn/135.11.2687. [DOI] [PubMed] [Google Scholar]
- Trapani L, Segatto M, La Rosa P, Fanelli F, Moreno S, Marino M, Pallottini V. 3-hydroxy 3-methylglutaryl coenzyme A reductase inhibition impairs muscle regeneration. J Cell Biochem. 2012;113:2057–2063. doi: 10.1002/jcb.24077. [DOI] [PubMed] [Google Scholar]
- Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26:1909–1920. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- Virgili F, Acconcia F, Ambra R, Rinna A, Totta P, Marino M. Nutritional flavonoids modulate estrogen receptor alpha signaling. IUBMB Life. 2004;56:145–151. doi: 10.1080/15216540410001685083. [DOI] [PubMed] [Google Scholar]
- Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012;31:323–351. doi: 10.1007/s10555-012-9347-y. [DOI] [PubMed] [Google Scholar]
- Wiik A, Glenmark B, Ekman M, Esbjörnsson-Liljedahl M, Johansson O, Bodin K, Enmark E, Jansson E. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand. 2003;179:381–387. doi: 10.1046/j.0001-6772.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- Wiik A, Hellsten Y, Berthelson P, Lundholm L, Fischer H, Jansson E. Activation of estrogen response elements is mediated both via estrogen and muscle contractions in rat skeletal muscle myotubes. Am J Physiol Cell Physiol. 2009;296:C215–C220. doi: 10.1152/ajpcell.00148.2008. [DOI] [PubMed] [Google Scholar]
- Wust RC, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol. 2008;93:843–850. doi: 10.1113/expphysiol.2007.041764. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zygmunt K, Faubert B, MacNeil J, Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun. 2010;398:178–183. doi: 10.1016/j.bbrc.2010.06.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.