Abstract
Allogeneic bone marrow transplant is a life-saving procedure for adults and children that have high-risk or relapsed hematological malignancies. Incremental advances in the procedure, as well as expanded sources of donor hematopoietic cell grafts have significantly improved overall rates of success. Yet, the outcomes for patients for whom suitable donors cannot be found remain a significant limitation. These patients may benefit from a hematopoietic cell transplant wherein a relative donor is fully haplotype mismatched. Previously this procedure was limited by graft rejection, lethal graft-versus-host disease, and increased treatment-related toxicity. Recent approaches in haplo-identical transplantation have demonstrated significantly improved outcomes. Based on years of incremental pre-clinical research into this unique form of bone marrow transplant, a range of approaches have now been studied in patients in relatively large phase II trials that will be summarized in this review.
Keywords: Peripheral blood progenitors, Stem cell transplantation, Graft-versus-host disease, Haplo-identical donor, Hematological malignancies
Core tip: Timely donor availability remains a challenge for patients in need of an urgent stem cell transplant. The ability to obtain half matched stem cells from any family member represents a significant breakthrough in the field. This review summarizes some of the current strategies used to substantially improve the outcomes of patients undergoing haplo-identical stem cell transplantation.
INTRODUCTION
Allogeneic bone marrow transplantation (BMT) offers a chance to cure patients that present with high-risk hematological malignancies. These include adults and children with acute myeloid leukemia (AML) in first or latter remission and acute lymphoblastic leukemia (ALL) employing BMT as post remission therapy in first or greater complete remission. Allogeneic BMT can also be considered in eligible patients with chronic lymphocytic leukemia, chronic myeloid leukemia and lymphoma (follicular, large cell, Hodgkin and peripheral T cell). The process of safely performing allogeneic BMT requires the regulated experience of a comprehensive multi-disciplinary team of health care professionals. In principle, the goal is to replace a diseased bone marrow with healthy blood-forming hematopoietic elements from a fully human leukocyte antigen (HLA)-matched healthy donor. At one time allogeneic BMT was routinely associated with a mortality of greater than 40%. Advances in stem cell acquisition and processing, molecular-level typing of unrelated donors and general supportive care that have reduced infectious complications have collectively improved rates of survival. Essential to success is durable engraftment of donor progenitor cells capable of restoring stable hematopoiesis. In addition to engraftment of hematopoietic progenitors, it is now known that donor immune effector cells (including T-lymphocytes) are required for disease eradication and prevention of relapse[1-3]. Specific anti-tumor donor lymphocytes engage in an ongoing immune reaction against residual host malignant cells[4-6]. This graft-versus-tumor (GVT) effect is closely linked to graft-vs-host disease (GVHD)[7-9]. The ability to dissect immune effectors responsible for each process is a element of current BMT research. Recognizing that a successful transplant requires contribution of both donor progenitor and immune effectors has lead to substantial changes in the field. These enhancements include: (1) the design of newer less-toxic preparative regimens[10-13]; and (2) expansion of the sources of donor stem/progenitor cell grafts.
Preparative regimens
Myeloablative transplant conditioning has traditionally been used to “create space” for donor progenitor cells and simultaneously kill residual tumor cells. These preparative protocols employ high-dose chemotherapy often in combination with whole-body irradiation. Significant treatment-related toxicities (TRM) restrict the procedure to young, otherwise-fit patients. Shifting the focus and goals of clinical efficacy from stem cell replacement to maintenance of a transplanted donor immune system has enabled the introduction of less-intense preparative regimens. Milder preparative regimens aim to achieve chimeric engraftment of progenitors as well as donor immune effector cells, including T lymphocytes. Reduced-intensity conditioning (RIC) or non-myeloablative allogeneic BMT is currently performed in a growing number of older patients (up to and beyond 70 years of age) diagnosed with a variety of lymphoid and myeloid neoplasms[13-16]. These hematological malignancies appear to have mixed susceptibility to the GVT effect. Donor immune effector cells impact therapeutic efficacy but also contribute to serious post-transplant side effects, including severe acute and chronic GVHD (aGVHD and cGVHD, respectively). Patient determinants, including remission status, remission duration and disease type, may govern the choice of preparative regimen as well as the type of graft that will contribute to rate of stable engraftment, immune reconstitution, GVL, and GVHD.
Graft sources
Once exclusively obtained by large volume aspiration from the posterior pelvis of a donor, transplantable hematopoietic progenitor cells can now be obtained directly from peripheral blood as well as from fresh umbilical cord blood. These stem cell products are transported world-wide in a highly regulated manner. Common terminology are used to describe stem cells derived from a bone marrow harvest (Hematopoietic Progenitor Cells - Marrow, HPC-M), from a mobilized apheresis peripheral blood product (Hematopoietic Progenitor Cells-Apheresis, HPC-A), from umbilical cord blood (Hematopoietic Progenitor Cells -Cord, HPC-C), or for donor lymphocyte infusion (DLI) (Therapeutic Cells-T). Each source of hematopoietic progenitors exhibits differences in cellular composition which leads to specific biological properties that may be of therapeutic benefit or risk depending on the transplant recipient, type of transplant to be performed as well as the type and immediate status of the hematological malignancy. For example mobilized peripheral blood products HPC-A often have a higher number of CD34+ stem/progenitor cells but also more CD3+ T lymphocytes (1-log higher). Higher CD34+ progenitor cell counts may improve time to engraftment and be used in a non-myeloablative setting but also appear to increase the risk of cGVHD[17-19]. Umbilical cord blood (HPC-C) typically contains a much lower absolute CD34+ progenitor cell count leading to significant delays in engraftment (or rejection); however the immature nature of the donor white blood cells from this source may also reduce the risk of GVHD and allow for some degree of HLA mismatch[20-22].
Understanding the unique properties of each source of hematopoietic cells helps to determine the anticipated performance for a given transplant recipient. Moreover, efforts to improve efficacy and reduce unwanted toxicities are currently under intense investigation. These include efforts to: (1) increase the dose of CD34+ hematopoietic progenitors in umbilical cord transplantation by combining two separate cord products[23,24] or by performing ex vivo CD34+ stem cell expansions[25]; (2) reduce the number of T-lymphocytes in HPC-A products by ex vivo T cell depletion or by in vivo administration of anti-thymocyte globulin (ATG)[26,27]; and (3) improve engraftment kinetics without cGVHD of HPC-M products by administering G-CSF to the donor prior to marrow harvest[28]. These advances illustrate the growing ability of practitioners to safely manipulate graft sources for maximum clinical benefit.
Identification of a donor
Finding a suitable bone marrow match is based on the HLA system, comprised of genes on chromosome 6. The major histocompatibility complex (MHC) includes two basic classes involved in antigen presentation and subsequent immune activation. MHC class I involves peptide presentation following intracellular digestion, while MHC class II presents extracellular antigens to host T lymphocytes. HLA-A, HLA-B, and HLA-C comprise class I, and HLA-DR, HLA-DQ and HLA-DP are class II. The proteins encoded by HLA define “self” to the host immune system. One set (haplotype) of HLA genes are maternal and the other paternal. From this, any given sibling, excluding an identical twin, will have only a 25% chance of being fully HLA-matched. While matched related siblings remain the best source of donor material, this approach has several world-wide limitations including a significant reduction of family sizes (fertility rates of 1.5-2.0 per family across Europe and North America), a policy of one child families, as well as the health status and potential co-morbidities of older sibling donors. Moreover, lack of sibling donor availability is predicted to become a much greater issue due to reduced family size. It is estimated that the likelihood of finding a sibling match will decline from 53.7% in 2002, to 37.1% in 2009 and 16.6% in 2024[29]. Nonetheless, investigation of family members using low-resolution serological typing (antigen level HLA-A,B, C and allele level HLA-DRB1) remains a standard initial evaluation approach.
If a suitable sibling-match cannot be found, a recipient in need of a transplant will require a search for an unrelated HLA-matched donor. Large national marrow donor programs will canvass for potential volunteers, perform HLA typing and maintain data in an ongoing registry. To be eligible volunteer donors must be in good general health and may be asked to undergo bone marrow harvesting under general anesthesia or daily administration of G-CSF (Filgrastim) followed by large volume leukapheresis. Stem cell donors must be screened to exclude active malignancies, transmissible infectious conditions (HIV, Hepatitis, HTLV-1, West Nile virus, Syphilis), hematological disorders (Sickle Cell Disease), and congenital bleeding disorders. A formal donor assessment will include a comprehensive questionnaire, complete medical history, and medical examination. Once screened and considered eligible, the most pertinent factor that predicts transplant success is donor age. Bone marrow recipients from younger donors (i.e., < 30 years of age) demonstrate improved five-year overall and disease-free survival[30,31]. This survival benefit appears to be the result of lower rates of GVHD when a younger donor is used. A retrospective analysis by the National Marrow Donor Program (NMDP) on over 6900 HLA-matched transplants performed between 1987 to 1999 was conducted to identify unique donor-specific features associated with transplant outcome[30]. In this analysis use of a donor aged 18 to 30 years correlated with a lower cumulative incidence of grade III/IV acute GVHD (P = 0.005) and lower incidence of chronic GVHD at 2 years (P = 0.02). Other studies have suggested a higher rate of chronic GVHD in male recipients transplanted from a multiparous female donor or if mobilized progenitor cells are used[19,30].
Identifying a potential unrelated donor BMT match generally requires high-resolution (HR) HLA typing of both recipient and donor. Studies suggest that employing a molecular (allele level) typing technique can reduce the incidence of severe GVHD and increase survival to levels similar to that seen with a matched-sibling donor[32,33]. Algorithms exist that combine a serological preliminary search (antigen level) with latter confirmatory molecular HR analysis (Figure 1). Efforts to decrease time and cost are dependent on clinical urgency and stability of the primary malignancy[34]. Patients with common alleles and haplotypes have a higher probability of finding a match and generally require fewer pre-screened potential donors to be selected for HR typing (3-5 donors), while those with rare alleles and haplotypes may require as many as 10 or more. High resolution allele level matching for HLA-A, B, C and DRB1 (8/8 match) results in improved survival[35-37]. Additional typing at HLA-DQB1 (10/10 match) and DPB1 loci, as well as DRB3, 4, 5 can be considered. Single loci mismatches at DQB1 and DPB1 appear to be tolerated better than at A, B, C or DRB1. Although it is necessary to minimize the number of allele mismatches, a single allele 7/8 or 9/10 alteration can still be considered. Single mismatch at B or C may be less of a concern than mismatches at A or DRB1 in patients undergoing HPC-M, but not HPC-A transplantation[38,39]. Factors such as the recipient diagnosis, CMV status, age, and sex also need consideration[30].
Figure 1.
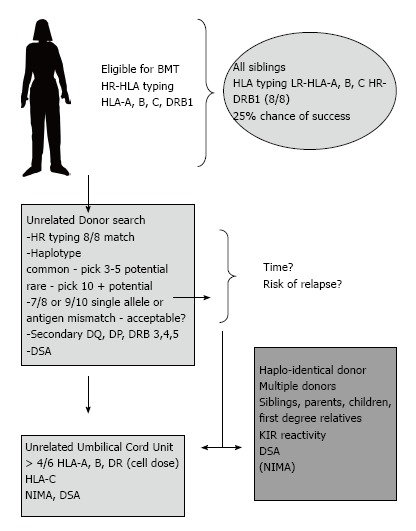
Example of a potential donor search algorithm that aims to expedite finding a suitable stem cell donor. A blend of high and low resolution approaches are employed. In certain situations the likelihood of finding an un-related donor match is low due to rare alleles and haplotypes. Consideration of a haplo-identical BMT may be considered at an earlier stage. Abbreviations: HR: High resolution (allele level); LR: Low resolution (antigen level); NIMA: Non-inherited maternal antigen; KIR: Killer cell Ig-like receptors; DSA: Donor-specific antibodies; BMT: Bone marrow transplant.
The ability to perform world-wide searches and identify volunteer donors has dramatically changed the international landscape of BMT. Superior matching as well as enhanced supportive care has improved the overall outcome of matched-unrelated donor (MUD) BMT such that results appear similar to matched-related donor BMT[32,33,38]. Despite this, national registries face considerable challenges and limitations. Increasing allogeneic transplant indications puts greater pressure on the number of world-wide searches. Donor attrition and maintenance of a donor registry requires ongoing organized drives that reach out to younger volunteers and maintain a large potential pool of active registrants. This may vary from country to country; in the United States it is estimated that 1 out of 44 is registered, Canada 1 in 100, while Germany has a donor ratio of 1 to 17 (calculated # of registrants/total population). Moreover, within any given registry, certain ethnicities are often significantly underrepresented[40,41]. Ultimately as many as 25% of all patients requiring BMT will never find a donor and either seek alternative treatments or palliation. This pressing unmet medical need has inspired advances in the use of alternative approaches that include the development of umbilical cord blood (UCB) hematopoietic cell transplant and haplo-identical BMT.
Advances in alternative donor hematopoietic cell transplantation
Umbilical cord blood contains hematopoietic progenitor cells that can be used for allogeneic transplant and immunological reconstitution[42]. Graft composition is a critical element in predicting the short and long-term engraftment performance, rate of rejection, development of GVHD, or ability to provide GVL and prevention of relapse. Potential advantages of pre-stored UCB units include immediate access without donor attrition and an increased availability for ethnic minorities through the use of partially matched UCB products. Conversely, donors of unrelated UCB products are not available if future grafts or donor lymphocyte infusions are required. Numerous international cord banks operate under strict standards of testing, storage, and characterization. A minimum target of 3.0 × 107 nucleated cells per recipient weight per unit of cord blood is generally recommended, while flow cytometry-based measurement of CD34+ cells per recipient weight may be more predictive[42,43]. Most cord products lack sufficient stem cells for most adults or large adolescent recipients. Efforts to accelerate cellular reconstitution following UCB BMT include combining two umbilical cord products (dUCBT) as well as ex vivo expansion[23,25]. When evaluated post-transplant (d 100) typically only one (dominant) cord unit can be identified[24]. In addition to the risk of graft failure, delayed immune reconstitution and increase in infections or relapse, there are theoretical risks that a potential hematological disease, not yet recognized in the newborn, will be transferred to the BMT recipient.
HLA typing of UCB products requires only low resolution serological testing for HLA-A, B, and molecular typing for DRB1 (6 alleles). Units with matches of at least 4/6 are potentially acceptable[44]. Mismatches at DRB1 and C may increase treatment-related mortality. Efforts to safely expand immature progenitors ex vivo without increasing differentiation to committed progenitors are moving towards early phase clinical trials[25].
HAPLO-IDENTICAL BMT IN ADULT PATIENTS
A hematopoietic cell graft (HPC-M or HPC-A) obtained from a family donor that is mismatched at 3/6 loci (HLA-A, B, DRB1) remains a potential for patients who lack a fully matched sibling, 8/8 unrelated donor, or UCB product (patient size, haplotype). Advantages of such a haplo- approach include an expanded potential donor pool that may include parents, siblings, children, and first-degree relatives. Family donors of all ethnicities may be highly motivated, readily available, and willing to donate and be re-mobilized if required. These important theoretical advantages make a compelling case for further development of this approach, especially for patients with high-risk leukemia that are unlikely to maintain a remission during a prolonged unrelated search that may take months. Nonetheless, the ability to safely achieve sustained engraftment of highly HLA-disparate transplanted progenitor cells is a major challenge. Moreover, the risk of lethal aGVHD in the setting of 3/6 HLA disparity is an even greater risk. When GVHD occurs in a fully HLA matched 6/6 sibling transplant it is felt to be the result of minor histocompatibility antigen mismatches, however the nature and extent of immune activation with overt HLA host mismatches will differ in a haplo-identical BMT. Understanding the unique biology, as well as the immediate risk of fatal GVHD, has driven implementation of a variety of relatively effective novel approaches.
Graft-vs-host disease
The essential elements for the development of GVHD include the presence of immunologically competent cells in the hematopoietic cell graft, the presence of transplantation antigens in the host that have not been encountered by the donor, and an inability of the host to destroy the transplanted graft (Billingham’s criteria)[45,46]. Clinical GVHD is generally divided into acute (diffuse maculopapular rash, GI mucosal inflammation, and elevated liver function tests) and chronic GVHD. The diagnosis of chronic GVHD has been recently revised by the National Institutes of Health (NIH) consensus working group report[47]. Clinical signs involve organs or sites that include skin, nails, mouth eyes, genitalia, GI tract, lung and the musculoskeletal system. Specific abnormalities may be either diagnostic (i.e., poikiloderma, esophageal web, bronchiolitis obliterans) or distinctive (i.e., xerostomia, myositis, keratoconjunctivitis sicca). Diagnosis requires at least one diagnostic category or the presence of least one distinctive manifestation confirmed by biopsy or specialized objective test. While classic acute GVHD occurs within 100 d after transplantation some patients continue or relapse beyond this time point often during tapered withdrawal of immunosuppressive agents. Similarly, while classic chronic GVHD may occur in the absence of acute GVHD it may also be present along with acute GVHD (overlap syndrome).
The clinical development of the GVHD reaction is complex and involves sequential step-wise immune activation. The primary effector cells are T lymphocytes present in the graft. In the haplo-identical setting, donor T cells may attack disparate non-matched MHC molecules present on the majority of host cells. Donor T cells may be further activated by immunostimulatory cytokines released following tissue damage (gastrointestinal) that results from the preparative regimen[48,49]. Additional activation may occur at the level of the vascular endothelium, co-stimulatory signals originating from antigen presenting cells, and following release of TNFα and other pro-inflammatory cytokines[45,46,50]. T cell effector damage appears to be mediated by perforin-based host target cell lysis and Fas-mediated apoptosis[51,52]. In a standard BMT, treatment options of established GVHD are limited; efforts to prevent GVHD with a combination of a calcineurin inhibitor and methotrexate have proven successful[53,54]. In this setting about one half of patients will develop significant GVHD requiring additional immunosuppressive therapy (corticosteroids).
With a focus on T-lymphocytes, recent advances have been able to dissect the roles of donor T cell subsets present in the graft[55]. These include naive T cells, memory T cells, and regulatory T cells. Naive T cells (CD45RA+/CD62L+) may include cells destined to be alloreactive to the host. Memory T cells (CD45RO+/CD62L+/-) include cells that provide protective anti-microbial immunity post-transplant. Regulatory T cells (CD4+/25+/FoxP3) appear to generally dampen other T cell responses and may be useful in attenuating clinical GVHD. Separation of naive T cells responsible for GVHD from donor T cell subsets responsible for GVT remain relatively elusive but are of obvious clinical importance. The ability to customize, harness, or control the fate of these T cell subsets to mitigate GVHD and retain GVT yet provide adequate post-transplant anti-viral immunity has considerable clinical potential and is of heightened importance in the setting of high-risk leukemia patients undergoing a haplo-identical BMT. These challenges have led to important advances both in laboratory technologies and clinical application of newer agents.
APPROACHES TO HAPLO-IDENTICAL BMT
Given the potential for development of lethal aGVHD, efforts to entirely eliminate alloreactive donor T cells remain a critical first step. It has been suggested that as few as 3 × 104 T cells per recipient weight are capable of causing clinical GVHD[56]. Both in vivo and ex vivo approaches have been developed (Figure 2). Ex vivo strategies include immunomagnetic-based positive selection of CD34+ cells or CD3/19 depletion with preservation of NK and gamma-delta T cells[57,58]. In vivo T cell depletion may be accomplished by early administration of post-transplant cyclophosphamide or by aggressive multi-agent anti-GVHD therapies that include anti-thymocyte globulin (ATG), G-CSF, and triple GVHD prophylaxis as well as recent studies using rapamycin (Table 1).
Figure 2.
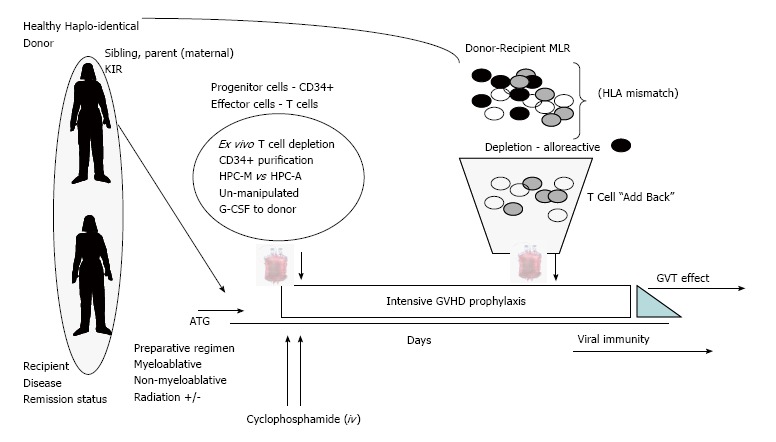
Current approaches to Haplo-identical bone marrow transplant. Recent novel strategies include issues of donor selection, ex vivo complete or partial T cell depletion of HPC-M or HPC-A grafts and the use of anti-thymocyte globulin. Additional strategies include in vivo administration of “high dose” cyclophosphamide and intensive multi-agent GVHD prophylaxis. Finally, in an effort to overcome severe prolonged immune suppression the addition of donor T lymphocytes that have been purged of alloreactive T cells may be of benefit. Abbreviations: DSA: Donor-specific antibodies; MLR: Mixed lymphocyte reaction; HLA: Human leukocyte antigen; GVT: Graft-versus tumor; KIR: Killer cell Ig-like receptors; GVHD: Graft-vs-host disease; ATG: Anti-thymocyte globulin.
Table 1.
Summary of clinical approaches to haplo-identical bone marrow transplant
| Ref. | n | Preparative regimen | T-cell depletion/engraftment | GVHD prophylaxis | Acute GVHD | Chronic GVHD | TRM | OS |
| Perugia, | 255 | TBI +/- | Yes | none | 17% | < 5% | 41% | 47%2 |
| Aversa et al[59-61] | MA | 84%-96% | ||||||
| ATG | ||||||||
| Peking, GIAC[69,70] | 250 | MA | No | CsA, MTX | 45% | 31% | 12%-48% | 56%-71% AML1 |
| ATG | G-BM + G-PB | MMF | 25%-60% ALL1 | |||||
| 100% | ||||||||
| Montreal | TBI +/- | Yes | none | 20% | 25% | 15% | 47% | |
| Bastien et al[56] | 19 | MA | T cell | |||||
| ATG | “Add Back” | |||||||
| 100% | ||||||||
| Baltimore | 210 | NMA | No | Tacro, MMF | 27% | 13% | 15% | 40%-45% |
| Studies[64-67] | 87% | PTCyclo | ||||||
| Di Bartolomeo et al[68] | 88 | MA 80% | No | CsA, MTX | 24% | 6% | 36% | 33%-54%1 |
| NMA 20% | G-BM | MMF | ||||||
| ATG | 91% | Basilixumab |
Survival range including standard and high-risk groups;
Survival for patients in complete remission. A variety of approaches have been studied that compare MA-myeloablative to NMA-non myeloablative, T cell depletion of graft, and GVHD prophylaxis. Engraftment rates are high, and GVHD can be attenuated through T cell depletion of the graft or by intensive anti-GVHD prophylaxis, including ATG. GVHD: Graft-vs-host disease; TRM: Treatment related mortality; OS: Overall survival; TBI: Total body irradiation; ATG: Anti-thymocyte globulin; CsA: Cyclosporine A; MTX: Methotrexate; MMF: Mycophenolate mofetil; PTCyclo: Post transplant cyclophosphamide; AML: Acute myeloid leukemia; ALL: Acute lymphoblastic leukemia; Tacro: Tacrolimus.
Aversa et al[59-61] in Perugia, Italy, described a series of incremental approaches to haplo-identical BMT from 1993 to 2006. A series of step-wise approaches focused on patients with high-risk acute myeloid (AML) and lymphoblastic leukemia (ALL). The investigators examined: (1) stem cell sources; (2) graft processing technologies; (3) conditioning regimens; and (4) post-transplant administration of G-CSF. Collectively, the investigative team were able to obtain high doses of CD34+ donor progenitor cells (> 10 × 106/kg. recipient weight) that led to a remarkable rate of successful engraftment. Aversa et al[61] and Reisner et al[62] had previously described the ability of purified CD34+ cells to block the action of residual cytotoxic T lymphocytes leading to tolerance[61]. Moreover when transplanted in very high numbers this “veto effect” could overcome clinical graft rejection by residual host T cells. At the same time newer technologies including CD34-positive selection and use of mobilization agents and peripheral blood progenitor cell collections led to “mega dose” grafts that consistently demonstrated remarkable engraftment of neutrophils and platelets with a low level of graft rejections in a large number of patients. Ex vivo T cell depletion was highly effective (< 0.5 × 105 CD3+ T cells/kg. recipient wt.) with little or no evidence of significant clinical GVHD even in the absence of prophylaxis. Impressive event-free survival rates of up to 48% were noted in AML patients in first complete remission. Higher rates of relapse were seen in ALL patients. Despite these notable clinical and technological advances, prolonged immune reconstitution (CD4+ T lymphocytes) was problematic and non-relapse mortality in the range of 41%. Infections were mostly cytomegalovirus and fungal in origin. Nonetheless, the ability to use rigorous positive selection of “megadose” CD34+ products and achieve timely multi-lineage engraftment, minimal GVHD, and durable survival in some patients became an important clinical platform for future trials.
Following the work of the Perugia group, Roy and colleagues in Montreal devised a novel strategy to safely “add-back” modified donor lymphocytes to hasten immune recovery and provide anti-viral immunity[56]. This strategy involved ex vivo photo-based depletion of alloreactive T cells derived from a donor-recipient mixed lymphocyte reaction (MLR). Working with a highly potent dibromorphodamine photosensitizing compound (TH9402) the team demonstrated accumulation of drug in certain cell types including cancer cells and alloreactive T cells. These cells could then be lysed following exposure to a specific wavelength of visible light (514 nm). The mechanism of ex vivo cellular lysis was shown to involve reactive oxygen species. During an MLR reaction in a haplo-identical setting, donor T lymphocytes respond to donor immune cells and take up TH9402. Non-reactive resting T cells capable of anti-viral immunity do not accumulate the agent and are retained in the infused “add-back” lymphocyte product (ATIR). The characterization of retained cells following photo-depletion differed when HLA-matched or haplo-matched pairs were tested. These experiments demonstrated preservation of CD8+ naive and effector cells in the matched situation and preservation of naive and central memory cells of both CD4+ and CD8+ phenotypes when haplo-identical pairs were studied. When tested in a clinical trial, administration of relatively high doses of photo-depleted T cells post-transplant did not increase grade III-IV GVHD. Patients receiving higher doses of cells demonstrated decreased rates of infection and improved overall survival (47.4%) at a median of 4 years[56]. These encouraging results are now being studied in a multi-institutional phase II setting. A similar approach using anti-CD25 immunotoxin MLR-based purging has been studied in several transplant settings. Similar results indicating successful removal of alloreactive GVHD cells and maintenance of virus-specific T cells have been demonstrated[63].
Ex vivo graft engineering to eliminate GVHD, yet retain GVT and avoid life-threatening infectious complications, is promising but remains investigational, complex, and costly. Kasamon et al[64], Fuchs et al[65], Luznik et al[66], Brunstein et al[67] in Baltimore have developed a similar in vivo platform using post-transplant cyclophosphamide administered shortly following infusion of an un-manipulated haplo-identical mismatched marrow graft. Similar to the ex vivo MLR approach, this strategy exploits the concept of selective depletion of alloreactive immune cells with preservation of resting non-alloreactive cells. Within a period of 24-48 h post-infusion of mismatched HPC-M, alloreactive T cells will rapidly encounter stimulatory host cells. T cells capable of anti-viral immunity will not respond at this stage. Cyclophosphamide will selectively eliminate reactive cells and limit GVHD. Indeed this approach has proven highly feasible in over 200 patients with a range of advanced hematological malignancies. Using a non-myeloablative regimen engraftment was timely (neutrophils 15 d and platelets 24 d) with graft rejections in the range of 13% (autologous marrow recovery). Rates of acute and chronic GVHD were less than 30%. Post-transplant GVHD prophylaxis included tacrolimus and mycophenolate mofetil. Relapse rates were relatively high in a broad range of advanced stage hematological malignancies and overall survival was in the range of 40%-45%.
A recent publication from Di Bartolomero and colleagues demonstrates the feasibility of performing haplo-identical BMT using an un-manipulated G-CSF-primed approach in patients with high-risk malignancy[68]. In this series, GVHD prophylaxis was intensive and included ATG, cyclosporine, methotrexate, mycophenylate mofetil, and basiliximab (anti-CD25). Engraftment of neutrophils (21 d) and platelets (28 d) were reasonable. The cumulative incidence of serious acute GVHD was 24% and at 2 years extensive cGVHD was only 6%. The overall 3-year overall survival ranged from 33%-54% (high-risk and standard risk). A large series published by Huang et al[69] and Wu et al[70] also described a similar approach to haplo-identical transplantation using un-manipulated cell grafts with escalated post-transplant immunosuppression. The Peking “GIAC” approach was studied in 250 patients and highlighted the effects of administration of G-CSF to the donor for collection of combined HPC-M and HPC-A grafts, intense immunosuppression (cyclosporine, methotrexate, mycophenolate mofetil and G-CSF), and ATG. Engraftment was rapid with neutrophils and platelets engrafting at 12 and 15 d, respectively. Grade III-IV acute GVHD was 45% and any cGVHD was 31%. Relapse in standard risk AML and ALL was 19.4 and 21.2%, in high-risk AML and ALL was 29.4% and 50.8%. Treatment related mortality ranged from 11.9% to 48.5% and was dependent on risk and disease type. Ultimately investigators suggested these results were comparable to results obtained using an HLA-matched sibling donor[69,70]. In this study investigators administered G-CSF to both the donor and recipient post-transplant. Administration of G-CSF following both autologous and allogeneic BMT has been primarily used to reduce the duration of neutropenia and related complications[71]. In both settings neutrophil recovery is faster resulting in shorter hospitalization for autologous but not allogeneic BMT. Use of G-CSF in allogeneic transplants is otherwise considered safe; however two retrospective studies have raised concern over a possible increase in GVHD[72,73]. Still others have suggested that pre-treatment of T-lymphocytes with G-CSF results in an anti-inflammatory (type-2) cytokine profile that attenuates experimental GVHD severity[74].
Towards the future, Fowler et al[75] at the NIH have recently published a compelling phase 2 study using rapamycin-resistant T cells (2.5 × 107 cells/kg. recipient weight) infused 14 d after hematopoietic cell transplantation for treatment of a variety of refractory hematological malignancies. While that study, on 40 patients of a wide range of ages (18 of whom remained in sustained complete remission up to 84 mo of follow-up), was done in the context of 6/6 HLA-matched sibling donors, it is conceivable that such an approach using their low-intensity conditioning regimen and this specific immune effector product could be adapted to the haplo-transplant setting. Donor lymphocytes in that study demonstrated a consistent and balanced Th1/Th2 profile; incidence probabilities of aGVHD were 20% and 40% at 100 and 180 d post-transplant, respectively.
The studies described above illustrate a broad range of current and future strategies to advance the field of haplo-identical BMT. Ex vivo T cell depletion, selective T cell “add back”, in vivo T cell depletion, and use of intensive GVHD prophylaxis are being actively improved. Feasibility has now been established with reasonable overall survival in a population of high-risk advanced malignancies who lack a traditional matched donor. Previous limitations of graft rejection and unacceptable rates of serious GVHD have been largely overcome. Efforts to enhance GVT and prevent life-threatening viral and fungal infections remain a current focus. Separation and exploitation of the linkage between GVT and GVHD remain a critical next step. It has also been suggested that alloreactive natural killer cells (NK) may be protective against myeloid leukemia relapse. Given that a patient may have several potential haplo-identical donors, it may be possible to choose a donor with heightened NK alloreactivity[76,77]. In addition, administration of donor-derived regulatory T cells may attenuate GVHD yet facilitate GVT effectors[78]. Finally a retrospective analysis of 118 acute leukemia patients undergoing haplo-identical BMT using a parent as a donor suggested improved 5-year EFS when the mother was the donor as compared to the father[79]. When sibling (non-parent) haplo-identical donors were evaluated, the gender of the donor had no effect on outcome. The presence of donor-specific antibodies (DSA) in the recipient may be evaluated to reduce the risk of graft failure[80].
CONCLUSION
At present, allogeneic BMT remains the only chance of cure for adults and children with advanced hematological disease. Transplant indications and eligibility are expanding. Outcomes are improving with reduced-intensity conditioning, HR molecular typing of unrelated donors as well as improved general supportive care measures. Most, but not all patients in need of this life-saving procedure will have a suitable sibling, matched unrelated, or UCB donor graft. Haplo-identical transplantation offers hope to those high-risk patients who face limited treatment options. Despite ethnicity, an expanded pool of motivated donors could be immediately available. A wide range of strategies are currently being explored. Previous serious pitfalls, including graft rejection, severe GVHD, and prolonged immune suppression are becoming less problematic as the science of the field advances. Novel experimental utilization of T regulatory cells, alloreactive NK cells, and other T cell subsets (T-Rapa cells, for example) hold great promise in this rapidly emerging and much needed field.
Footnotes
P- Reviewer: Scatena R, Shao R S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
References
- 1.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 2.Gale RP, Horowitz MM, Ash RC, Champlin RE, Goldman JM, Rimm AA, Ringdén O, Stone JA, Bortin MM. Identical-twin bone marrow transplants for leukemia. Ann Intern Med. 1994;120:646–652. doi: 10.7326/0003-4819-120-8-199404150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Nishida T, Hudecek M, Kostic A, Bleakley M, Warren EH, Maloney D, Storb R, Riddell SR. Development of tumor-reactive T cells after nonmyeloablative allogeneic hematopoietic stem cell transplant for chronic lymphocytic leukemia. Clin Cancer Res. 2009;15:4759–4768. doi: 10.1158/1078-0432.CCR-09-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martino R, Caballero MD, Pérez-Simón JA, Canals C, Solano C, Urbano-Ispízua A, Bargay J, Léon A, Sarrá J, Sanz GF, Moraleda JM, Brunet S, San Miguel J, Sierra J. Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood. 2002;100:2243–2245. doi: 10.1182/blood-2002-02-0400. [DOI] [PubMed] [Google Scholar]
- 5.Collins RH, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, Goodman SA, Wolff SN, Hu W, Verfaillie C, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 6.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 7.Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, Storb R. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 8.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 9.Claret EJ, Alyea EP, Orsini E, Pickett CC, Collins H, Wang Y, Neuberg D, Soiffer RJ, Ritz J. Characterization of T cell repertoire in patients with graft-versus-leukemia after donor lymphocyte infusion. J Clin Invest. 1997;100:855–866. doi: 10.1172/JCI119601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett J, Childs R. Non-myeloablative stem cell transplants. Br J Haematol. 2000;111:6–17. doi: 10.1046/j.1365-2141.2000.02405.x. [DOI] [PubMed] [Google Scholar]
- 11.Uzunel M, Mattsson J, Brune M, Johansson JE, Aschan J, Ringdén O. Kinetics of minimal residual disease and chimerism in patients with chronic myeloid leukemia after nonmyeloablative conditioning and allogeneic stem cell transplantation. Blood. 2003;101:469–472. doi: 10.1182/blood-2002-02-0571. [DOI] [PubMed] [Google Scholar]
- 12.Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, Sandmaier BM, Storb R. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 13.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, Lee SJ, Windawi S, Ritz J, Stone RM, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 14.Corradini P, Zallio F, Mariotti J, Farina L, Bregni M, Valagussa P, Ciceri F, Bacigalupo A, Dodero A, Lucesole M, et al. Effect of age and previous autologous transplantation on nonrelapse mortality and survival in patients treated with reduced-intensity conditioning and allografting for advanced hematologic malignancies. J Clin Oncol. 2005;23:6690–6698. doi: 10.1200/JCO.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 15.McClune BL, Weisdorf DJ. Reduced-intensity conditioning allogeneic stem cell transplantation for older adults: is it the standard of care? Curr Opin Hematol. 2010;17:133–138. doi: 10.1097/MOH.0b013e3283366ba4. [DOI] [PubMed] [Google Scholar]
- 16.Hermann S, Klein SA, Jacobi V, Thalhammer A, Bialleck H, Duchscherer M, Wassmann B, Hoelzer D, Martin H. Older patients with high-risk fungal infections can be successfully allografted using non-myeloablative conditioning in combination with intensified supportive care regimens. Br J Haematol. 2001;113:446–454. doi: 10.1046/j.1365-2141.2001.02747.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, Russell N, Apperley JF, Gorin NC, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–767. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 18.Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, Beksac M, Hasenclever D, Socié G, Schmitz N. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol. 2010;11:331–338. doi: 10.1016/S1470-2045(09)70352-3. [DOI] [PubMed] [Google Scholar]
- 19.Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 21.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 22.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, Sirvent A, Champlin RE, Chao N, Gee AP, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sideri A, Neokleous N, Brunet De La Grange P, Guerton B, Le Bousse Kerdilles MC, Uzan G, Peste-Tsilimidos C, Gluckman E. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96:1213–1220. doi: 10.3324/haematol.2010.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, Verfaillie CM, Wagner JE. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 25.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, Alousi A, Saliba R, McMannis JD, Kaur I, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 27.Socié G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 28.Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98:3186–3191. doi: 10.1182/blood.v98.12.3186. [DOI] [PubMed] [Google Scholar]
- 29.Allan DS, Takach S, Smith S, Goldman M. Impact of declining fertility rates in Canada on donor options in blood and marrow transplantation. Biol Blood Marrow Transplant. 2009;15:1634–1637. doi: 10.1016/j.bbmt.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, Hegland J, Kamani N, Kernan NA, King R, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 31.Davies SM, Kollman C, Anasetti C, Antin JH, Gajewski J, Casper JT, Nademanee A, Noreen H, King R, Confer D, et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the national marrow donor program. Blood. 2000;96:4096–4102. [PubMed] [Google Scholar]
- 32.Gupta V, Tallman MS, He W, Logan BR, Copelan E, Gale RP, Khoury HJ, Klumpp T, Koreth J, Lazarus HM, et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood. 2010;116:1839–1848. doi: 10.1182/blood-2010-04-278317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore J, Nivison-Smith I, Goh K, Ma D, Bradstock K, Szer J, Durrant S, Schwarer A, Bardy P, Herrmann R, et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2007;13:601–607. doi: 10.1016/j.bbmt.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 34.Petersdorf EW, Anasetti C, Martin PJ, Gooley T, Radich J, Malkki M, Woolfrey A, Smith A, Mickelson E, Hansen JA. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 35.Speiser DE, Tiercy JM, Rufer N, Grundschober C, Gratwohl A, Chapuis B, Helg C, Löliger CC, Siren MK, Roosnek E, et al. High resolution HLA matching associated with decreased mortality after unrelated bone marrow transplantation. Blood. 1996;87:4455–4462. [PubMed] [Google Scholar]
- 36.Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoko H, Yoshida T, Kimura A, Akaza T, Kamikawaji N, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 37.Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, Chehata S, Esperou H, Vernant JP, Michallet M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, Fernandez-Vina M, Flomenberg N, Horowitz M, Hurley CK, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 39.Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M, Gajewski J, Hale GA, Horan J, Battiwalla M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beatty PG, Mori M, Milford E. Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation. 1995;60:778–783. [PubMed] [Google Scholar]
- 41.Mori M, Beatty PG, Graves M, Boucher KM, Milford EL. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Program Donor Registry. Transplantation. 1997;64:1017–1027. doi: 10.1097/00007890-199710150-00014. [DOI] [PubMed] [Google Scholar]
- 42.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115:1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 44.Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, Brown M, Champlin RE, Garcia-Lopez J, Hattersely G, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12:1214–1221. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 47.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res. 2002;8:441–448. doi: 10.1179/096805102125001046. [DOI] [PubMed] [Google Scholar]
- 49.Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115:1865–1872. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Baker MB, Altman NH, Podack ER, Levy RB. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med. 1996;183:2645–2656. doi: 10.1084/jem.183.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda Y, Levy RB, Reddy P, Liu C, Clouthier SG, Teshima T, Ferrara JL. Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood. 2005;105:2023–2027. doi: 10.1182/blood-2004-08-3036. [DOI] [PubMed] [Google Scholar]
- 53.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 54.Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Hansen J. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68:119–125. [PubMed] [Google Scholar]
- 55.Riddell SR, Appelbaum FR. Graft-versus-host disease: a surge of developments. PLoS Med. 2007;4:e198. doi: 10.1371/journal.pmed.0040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bastien JP, Roy J, Roy DC. Selective T-cell depletion for haplotype-mismatched allogeneic stem cell transplantation. Semin Oncol. 2012;39:674–682. doi: 10.1053/j.seminoncol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Bethge WA, Faul C, Bornhäuser M, Stuhler G, Beelen DW, Lang P, Stelljes M, Vogel W, Hägele M, Handgretinger R, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis. 2008;40:13–19. doi: 10.1016/j.bcmd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Handgretinger R, Klingebiel T, Lang P, Schumm M, Neu S, Geiselhart A, Bader P, Schlegel PG, Greil J, Stachel D, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27:777–783. doi: 10.1038/sj.bmt.1702996. [DOI] [PubMed] [Google Scholar]
- 59.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 60.Aversa F, Reisner Y, Martelli MF. The haploidentical option for high-risk haematological malignancies. Blood Cells Mol Dis. 2008;40:8–12. doi: 10.1016/j.bcmd.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 62.Rachamim N, Gan J, Segall H, Krauthgamer R, Marcus H, Berrebi A, Martelli M, Reisner Y. Tolerance induction by “megadose” hematopoietic transplants: donor-type human CD34 stem cells induce potent specific reduction of host anti-donor cytotoxic T lymphocyte precursors in mixed lymphocyte culture. Transplantation. 1998;65:1386–1393. doi: 10.1097/00007890-199805270-00017. [DOI] [PubMed] [Google Scholar]
- 63.Montagna D, Yvon E, Calcaterra V, Comoli P, Locatelli F, Maccario R, Fisher A, Cavazzana-Calvo M. Depletion of alloreactive T cells by a specific anti-interleukin-2 receptor p55 chain immunotoxin does not impair in vitro antileukemia and antiviral activity. Blood. 1999;93:3550–3557. [PubMed] [Google Scholar]
- 64.Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J, Morris LE, Crilley PA, O’Donnell PV, Rossiter N, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuchs EJ. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematology Am Soc Hematol Educ Program. 2012;2012:230–236. doi: 10.1182/asheducation-2012.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, Devine SM, Wingard JR, Aljitawi OS, Cutler CS, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, Adorno G, Angelini S, Andreani M, De Felice L, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121:849–857. doi: 10.1182/blood-2012-08-453399. [DOI] [PubMed] [Google Scholar]
- 69.Huang XJ, Chang YJ. Unmanipulated HLA-mismatched/haploidentical blood and marrow hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:197–204. doi: 10.1016/j.bbmt.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Wu T, Lu DP. Unmanipulated haploidentical blood and marrow transplantation: where we are. Hong Kong Med J. 2009;15:27–30. [PubMed] [Google Scholar]
- 71.Trivedi M, Martinez S, Corringham S, Medley K, Ball ED. Optimal use of G-CSF administration after hematopoietic SCT. Bone Marrow Transplant. 2009;43:895–908. doi: 10.1038/bmt.2009.75. [DOI] [PubMed] [Google Scholar]
- 72.Ringdén O, Labopin M, Gorin NC, Le Blanc K, Rocha V, Gluckman E, Reiffers J, Arcese W, Vossen JM, Jouet JP, et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2004;22:416–423. doi: 10.1200/JCO.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 73.Remberger M, Naseh N, Aschan J, Barkholt L, LeBlanc K, Svennberg P, Ringdén O. G-CSF given after haematopoietic stem cell transplantation using HLA-identical sibling donors is associated to a higher incidence of acute GVHD II-IV. Bone Marrow Transplant. 2003;32:217–223. doi: 10.1038/sj.bmt.1704108. [DOI] [PubMed] [Google Scholar]
- 74.Pan L, Delmonte J, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 75.Fowler DH, Mossoba ME, Steinberg SM, Halverson DC, Stroncek D, Khuu HM, Hakim FT, Castiello L, Sabatino M, Leitman SF, et al. Phase 2 clinical trial of rapamycin-resistant donor CD4+ Th2/Th1 (T-Rapa) cells after low-intensity allogeneic hematopoietic cell transplantation. Blood. 2013;121:2864–2874. doi: 10.1182/blood-2012-08-446872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 77.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 79.van Rood JJ, Loberiza FR, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, Champlin RE, Gale RP, Ringdén O, Hows JM, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 80.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, Vierra-Green C, Anasetti C. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115:2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]


