Abstract
Mesenchymal stem cells (MSCs) derived from human induced pluripotent stem cells (hiPSCs) provide a novel source for generating adipocytes, thus opening new avenues for fundamental research and clinical medicine. We present the adipogenic potential of hiPSCs and the various methods to derive hiPSC-MSCs. We discuss the main characteristic of hiPSC-MSCs, which is their low adipogenic capacity as compared to adult-MSCs. Finally, we propose several hypotheses to explanation this feature, underlying a potential critical role of the micro-environment. We favour the hypothesis that the range of factors or culture conditions required to induce adipocyte differentiation of MSCs derived from adult tissues and from embryonic-like cells could differ.
Keywords: Human induced pluripotent stem cells, Mesenchymal stem cells, Brown adipocytes, White adipocytes, Obesity, Lipodystrophy
Core tip: In this mini-review, we summarized the potential of human induced pluripotent stem cells to generate white and brown adipocytes and we discussed their therapeutic capacities for obesity and lipodystrophy diseases. Then, we described the main approaches to derive human induced pluripotent stem cells-mesenchymal stem cells (hiPSC-MSCs). Finally, we underlined the low adipogenic capacity of hiPSC-MSCs compared to adult-MSCs and proposed several hypothesis to explain this feature.
INTRODUCTION
Mesenchymal stem cells (MSCs) derived from human induced pluripotent stem cells (hiPSCs) provide a novel source for generating adipocytes. hiPSC-MSCs satisfy the minimal criteria for defining MSCs proposed by the International Society for Cellular Therapy, i.e., express certain cell surface markers, adhere to tissue culture plastics and exhibit differentiation towards osteogenic, chondrogenic and adipogenic ligneages[1]. However, hiPSC-MSCs have also been referred to as MSC-like, mesenchymal progenitors or fibroblast-like cells by different authors, including us, because hiPSC-MSCs are not identical to MSCs isolated from adult tissues, as we will discuss. For simplicity, we use the general term MSCs in this review. We present hiPSCs as a unique source of adipocytes, thus opening new avenues for fundamental research and clinical medicine. We also underline the low adipogenic capacity of hiPSC-MSCs compared to adult-MSCs, based on our expertise regarding the study of human MSCs isolated from iPSCs and adipose tissue.
In mammals, two adipose tissue functional types coexist, i.e., brown and white adipose tissues, which are both involved in the energy balance while having opposite functions. White adipose tissue (WAT) is mainly involved in energy storage and mobilization in the form of triglycerides. In contrast, brown adipose tissue (BAT) burns fat and is specialized in energy expenditure. BAT is a key thermogenic organ since brown adipocytes convert nutrients into heat by uncoupling respiration from ATP synthesis. BAT implants were recently shown to improve the metabolic conditions in obese mice and to restore normoglycemia and glucose tolerance in streptozotocin-induced diabetic mice[2]. These recent findings offer promising prospects for basic research and clinical medicine-increasing energy expenditure via recruitment of brown adipocyte progenitors could be a valuable therapeutic approach to counteract obesity and its associated metabolic complications. However, BAT represents a minor fraction of the adipose tissue in humans, is found throughout the body and disappears from most areas with age, persisting only around deeper organs[3]. Human BAT is hard to isolate in this regard, so an alternative cellular source is required to generate brown adipocytes.
Congenital or acquired lipodystrophies result from the loss, degeneration or misdistribution of white adipose tissue, leading to diabetes, severe defects in lipid homeostasis and ectopic fat accumulation. Transplantation of white adipose tissue in a lipoatrophy mouse model has been shown to improve the metabolic parameters[4]. Therefore, isolation of hiPSCs-MSCs differentiating into brown and white adipocytes could provide an unlimited source of adipocytes for autologous cell-based therapy to treat obesity and lipodystrophy diseases.
Differentiation of iPSCs into MSCs, and then into adipocytes, also offers a unique opportunity to determine adipocyte properties according to their embryonic origins, but this issue has yet to be investigated in humans. Indeed, individual white adipose tissue depots are not equivalent, as functional differences have been reported in mice and humans[5]. These distinct properties of individual adipose tissue depots could play a role in obesity-related complications and might explain why the spread of only certain depots is associated with severe metabolic disorders. It has also been observed that the different fat depots are not altered in a similar manner in genetic- and drug-induced lipodystrophies[6]. These regional differences were conserved during in vitro propagation and adipocyte differentiation of MSCs, strongly suggesting that MSCs from different fat depots are indeed inherently different[7]. Recent published data, including ours, suggested that MSC embryonic origins could play a role in these intrinsic differences. Surprisingly little is known about the developmental origin of adipocytes in rodents, and nothing is known in humans. Gesta et al[8] compared gene expression profiles of intra-abdominal and sub-cutaneous adipose tissues in mice and found major differences in the expression of several genes involved in embryonic development and pattern specification. We recently demonstrated that subsets of adipocytes have mesodermic and neuroectodermic origins depending on the location of adipose tissue depots in mouse and quail[9,10]. These data are in full agreement with the demonstration of Takashima et al[11] that MSCs originate both from mesoderm and neuroectoderm. Interestingly, the potential of neuro- and meso-derived MSCs to differentiate and participate in tissue repair differs depending on the embryotic origin[12].
DIFFERENTIATION OF HUMAN-INDUCED PLURIPOTENT STEM CELLS INTO ADIPOCYTES
Differentiation of hiPSCs offers a unique opportunity to purify human MSCs for the purpose of generating brown and white adipocytes, as well as adipocytes of different embryonic origins from the same patients’ somatic cells. Following the pioneer work of Yamanaka’s group on the generation of iPSCs by reprogramming human fibroblasts[13], the capacity of hiPSCs to generate functional adipocytes was first reported by Nakao’s group. The authors showed that hiPSCs have an adipogenic potential comparable to human embryonic stem cells. Interestingly, hiPSC-adipocytes can maintain their functional properties for several weeks after transplantation into nude mice[14,15]. These data revealed that hiPSC-adipocytes could potentially be used to correct metabolic parameters in patients. In these experiments, differentiated hiPSCs, but not MSCs, were transplanted into mice. Indeed, hiPSC differentiated cultures are enriched with adipocytes after adipogenic induction, but also contain several other cell types that are undesirable for transplantation, including immature neural cells and undifferentiated iPSCs that can form teratomas several weeks after transplantation. As indicated by Noguchi and colleagues, transplantation of mature adipocytes alone results in graft loss that could be improved by transplanting adipocyte progenitors[15]. Therefore, purification of hiPSC-MSCs with a high adipogenic capacity is required prior to an hiPSC-based therapeutic approach. Nishio et al[16] developed a procedure to generate functional brown adipocytes at a high frequency from hiPSC using a hematopoietic cocktail to induce hiPSC differentiation. Remarkably, hiPSC-brown adipocytes were able to improve glucose tolerance after transplantation in mice. This report established a link between brown adipocytes and hematopoietic cells, and indicated that hiPSCs could potentially be used to generate brown adipocytes with therapeutic properties. As recently reported, we designed a procedure to derive MSCs with a capacity to differentiate into both brown and white adipocytes[17]. In this latter study, the use of small molecules during hiPSC differentiation revealed that TGFb and retinoic acid pathways regulate the generation of MSC subtypes having a brown or white adipogenic potential, respectively. Differentiation of hiPSCs offers the opportunity to characterize the earliest steps of adipogenesis and identify signalling pathways regulating brown and white fat cell lineages. However, as discussed below, hiPSC-derived MSCs have a low potential to differentiate into adipocytes compared to hMSCs derived from adult adipose tissue.
ADIPOGENIC CAPACITY OF hIPSC-DERIVED MSCS
Chen et al[18] first underlined the limited capacity of hiPSC-MSCs to undergo adipocyte differentiation, a feature that has often been observed by authors but not always highlighted. Ahfeldt et al[19] were able to generate pure brown or white adipocytes from hiPSCs, but only following genetic modification of MSCs. These artificially programmed hMSCs are of great interest from a therapeutic standpoint, but cannot be applied to investigate ontogenesis of human adipocytes. The fact that MSCs must be transduced with adipogenesis master genes clearly illustrates the low adipogenic potential of hiPSC-derived MSCs. This low adipogenic potential is illustrated in Figure 1. As shown, transduction of hiPSC-MSCs with a vector expressing an adipogenic gene, such as C/EBP-LAP, dramatically enhanced the MSC adipogenic potential, as monitored with the FABP4 adipogenic marker, but still at a lower level compared to MSCs derived from adipose tissue, also named adipose-derive stem cells (ASCs). Interestingly, some authors claim that the low differentiation capacity is limited to adipogenesis since hiPSC- and hESC-MSCs are able to differentiate towards chondrogenic and osteogenic lineages at high levels[20-22]. MSCs are abundant in adipose tissue, can be easily harvested using liposuction and have a considerable expansion potential ex vivo, particularly when isolated from young donors[23]. Adipose tissue derived MSCs are currently being investigated in autologous transplantations to improve revascularization and tissue perfusion in ischemic limbs[24-26]. Results so far suggest that the efficiency of adipose tissue-MSCs in regenerative medicine could rely more on their cytokine secretory functions and potential use as immunomodulators than on their differentiation potential[27]. The therapeutic potential of MSCs derived from hiPSCs and from human adipose tissue should therefore now be carefully compared.
Figure 1.
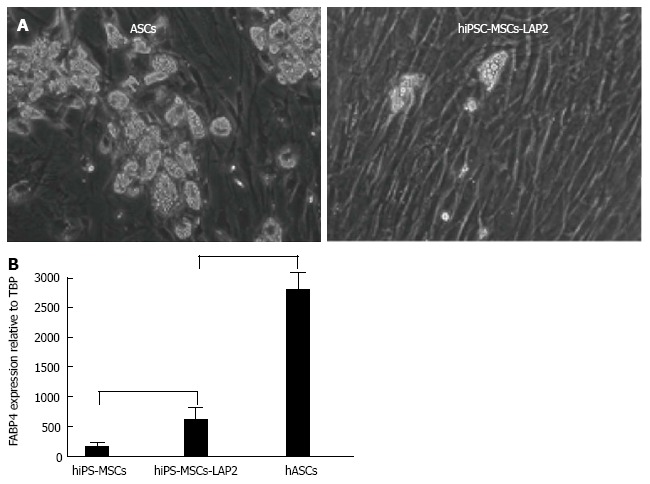
Adipogenic capacities of mesenchymal stem cells derived from human induced pluripotent stem cells and from human adipose tissue (adipose-derive stem cells). A: Microphographic images showing adipocytes generated from hiPSC-MSCs transduced with C/EBPb LAP (left) and from adult ASCs (right); B: Adipocyte differentiation potentials quantified via FABP4 adipogenic gene expression. MSCs: Mesenchymal stem cells; hiPSCs: Human induced pluripotent stem cells; ASCs: Adipose-derive stem cells.
In recent years, various groups have successfully derived MSCs from hESC and hiPSC using a range of methods. There are two main approaches to differentiate pluripotent stem cells into MSCs (Figure 2).
Figure 2.
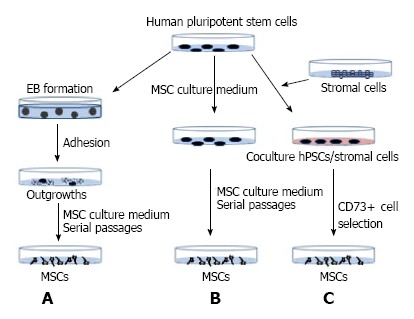
Mesenchymal stem cell derivation strategies. MSCs were derived from hiPSCs following (A) or not (B) embryoid body formation. Alternatively, hiPSCs were maintained in co-culture with murine stromal cells, and then MSCs were derived following selection of CD73-positive cells (C). MSCs: Mesenchymal stem cells; hiPSCs: Human induced pluripotent stem cells.
One strategy involves embryoid body (EB) formation. In this approach, suspension cultures allow pluripotent stem cells to form 3-dimensional structures called EB (Figure 2A). This step models the in vitro embryonic development with the commitment of cells into the three primary germ layers. The duration of this stage may range from 7 to 14 d. EBs are then seeded onto culture dishes and, after a proliferation step, outgrowth cells are maintained in a mesenchymal culture medium. Subsequently, adherent cells display a fibroblast-like morphology and acquire specific MSC markers after serial passages[28,29]. An alternative strategy involves direct differentiation of pluripotent stem cells without the EB step (Figure 2B). Pluripotent stem cells are dissociated into single-cell suspensions and then maintained in mesenchymal medium for several weeks. The resulting cell cultures are enriched for MSCs through serial cell passaging[30]. Another version of this protocol relies on the spontaneous differentiation of pluripotent stem cells into MSCs[31]. Different research groups have developed additional steps to improve this method. For instance, before single-cell suspension, hiPSCs are committed towards MSC differentiation via treatment with small molecules, such as inhibitors of the TGFb pathway[18]. Using small molecules with the EB method has been shown to promote the generation of two MSC subtypes having a selective potential towards brown and white adipocyte lineages[17]. Growing hiPSCs on a fibrillar-collagen matrix has also been shown to improve their differentiation into MSCs[32]. Coculture of pluripotent stem cells with murine stromal cells, followed by the selection of CD73-positive cells[33], is another way to derive MSCs (Figure 2C). Interestingly, MSCs from mesoderm or from neuroectoderm origins can be derived depending on the stromal cells used[34,35]. Finally, MSCs derived from different hiPSC lines generated from different donors, or from the same hiPSC clone using different derivation approaches, have the same adipogenic features[30,31]. MSC adipogenic characteristics are therefore not dependent on the derivation method used.
Several hypotheses could be put forward to explain the low hiPSC-MSC adipogenic capacity compared to adult MSCs. Interestingly, the low differentiation potential is not restricted to hiPSC-MSCs since MSCs derived from human embryonic stem cells (hESCs) display the same feature, thus ruling out the possibility that the low hiPSC-MSC adipogenic capacity could be due to the reprogramming process or to an epigenetic mechanism. The fact that hiPSCs generated from adipose-derived stem cells or from neural stem cells displayed a similar adipogenic capacity is in agreement with this hypothesis[17]. One possibility is that the criteria to identify hiPSC-MSCs and adult MSCs could differ. For instance, individual cell surface markers used to characterize MSCs are also expressed by non-MSCs such as by fibroblasts[36]. Therefore, it could be important to select hiPSC-MSCs based on the co-expression of several mesenchymal markers and not on CD73 expression only. We favour the hypothesis that the range of factors or culture conditions required to induce adipocyte differentiation of MSCs derived from adult tissues and from embryonic-like cells could differ. The low hiPSC-MSC adipogenic capacity is a reminiscence of an earlier observation reported by Han et al[37]. The authors observed that epididymal adipose tissue, which undergoes postnatal development in mouse, is composed of multipotent progenitor cells that meet the MSC criteria but lack an adipogenic capacity in vitro. In contrast to cells derived from other fat pads, epididymal fat cells require a three-dimensional culture conditions and a different micro-environment to undergo differentiation. These results underline that the micro-environment has a critical role in differentiation but could differ for adult and embryonic-like cells. We have observed that hiPSCs can generate nice adipocyte colonies when MSCs are not derived but are maintained in an iPSC environment. As shown in Figure 3, adipocytes are close to non-adipose cells in hiPSC-differentiated cultures. As isolated MSCs have been found to have a low adipogenic capacity, we propose that these non-adipogenic cells are required for full differentiation of MSCs into adipocytes. The identification of these non-adipogenic cells and factors that they secrete could be of a great interest.
Figure 3.
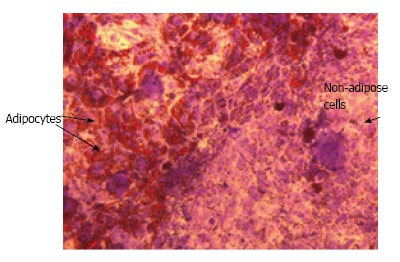
A niche for adipocytes when mesenchymal stem cells are maintained in the human induced pluripotent stem cells environment. Differentiated hiPSCs stained with Oil Red O, a specific stain for lipid droplets, to reveal adipocytes, and with violet crystal, a non specific stain, to reveal other cells. hiPSCs: Human induced pluripotent stem cells.
CONCLUSION
The potential of hiPSCs to generate MSCs having an adipogenic capacity represents a powerful cellular model for studying brown and white adipocyte ontogenesis and comparing the properties of adipocytes derived from mesoderm or neuroectoderm. This also provides a basis for investigating factors involved in the recruitment of MSCs having different potentials in normal and pathological contexts.
From a clinical standpoint, many issues have to be resolved before using hiPSC-MSCs in cell-based therapeutic for obesity and lipodystrophy diseases. However, the differentiation of iPS cells towards the adipogenic lineage offers a unique opportunity to purify white and brown adipocytes from patients, which could lead to the development of autologous transplantation procedures to treat obese and lipodystrophic patients. It would be essential to determine the factors underlying the low adipogenic capacity of hiPSC-MSCs. They are functionally distinct from adult hMSCs and the challenge will be to determine the cellular and molecular events necessary to prime hiPSCs towards the adipogenic lineage at a high level.
Footnotes
Supported by Fondation ARC and ANRS
P- Reviewer: Chen LY, Zhang Q S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674–682. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 2012;8:55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- 11.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135:2845–2854. doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Taura D, Noguchi M, Sone M, Hosoda K, Mori E, Okada Y, Takahashi K, Homma K, Oyamada N, Inuzuka M, et al. Adipogenic differentiation of human induced pluripotent stem cells: comparison with that of human embryonic stem cells. FEBS Lett. 2009;583:1029–1033. doi: 10.1016/j.febslet.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi M, Hosoda K, Nakane M, Mori E, Nakao K, Taura D, Yamamoto Y, Kusakabe T, Sone M, Sakurai H, et al. In vitro characterization and engraftment of adipocytes derived from human induced pluripotent stem cells and embryonic stem cells. Stem Cells Dev. 2013;22:2895–2905. doi: 10.1089/scd.2013.0113. [DOI] [PubMed] [Google Scholar]
- 16.Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394–406. doi: 10.1016/j.cmet.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Mohsen-Kanson T, Hafner AL, Wdziekonski B, Takashima Y, Villageois P, Carrière A, Svensson M, Bagnis C, Chignon-Sicard B, Svensson PA, et al. Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem Cells. 2014;32:1459–1467. doi: 10.1002/stem.1607. [DOI] [PubMed] [Google Scholar]
- 18.Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1:83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, Camahort R, Xia F, Shay J, Rhee EP, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14:209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- 21.Boyd NL, Robbins KR, Dhara SK, West FD, Stice SL. Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng Part A. 2009;15:1897–1907. doi: 10.1089/ten.tea.2008.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson C, Emanuelsson K, Wessberg F, Kajic K, Axell MZ, Eriksson PS, Lindahl A, Hyllner J, Strehl R. Human embryonic stem cell-derived mesenchymal progenitors--potential in regenerative medicine. Stem Cell Res. 2009;3:39–50. doi: 10.1016/j.scr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 27.Gimble JM, Nuttall ME. Adipose-derived stromal/stem cells (ASC) in regenerative medicine: pharmaceutical applications. Curr Pharm Des. 2011;17:332–339. doi: 10.2174/138161211795164220. [DOI] [PubMed] [Google Scholar]
- 28.Brown SE, Tong W, Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189:256–260. doi: 10.1159/000151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee EJ, Lee HN, Kang HJ, Kim KH, Hur J, Cho HJ, Lee J, Chung HM, Cho J, Cho MY, et al. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part A. 2010;16:705–715. doi: 10.1089/ten.tea.2008.0596. [DOI] [PubMed] [Google Scholar]
- 30.Hynes K, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev. 2014;23:1084–1096. doi: 10.1089/scd.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diederichs S, Tuan RS. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev. 2014;23:1594–1610. doi: 10.1089/scd.2013.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One. 2012;7:e33225. doi: 10.1371/journal.pone.0033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18:1059–1070. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]


