Abstract
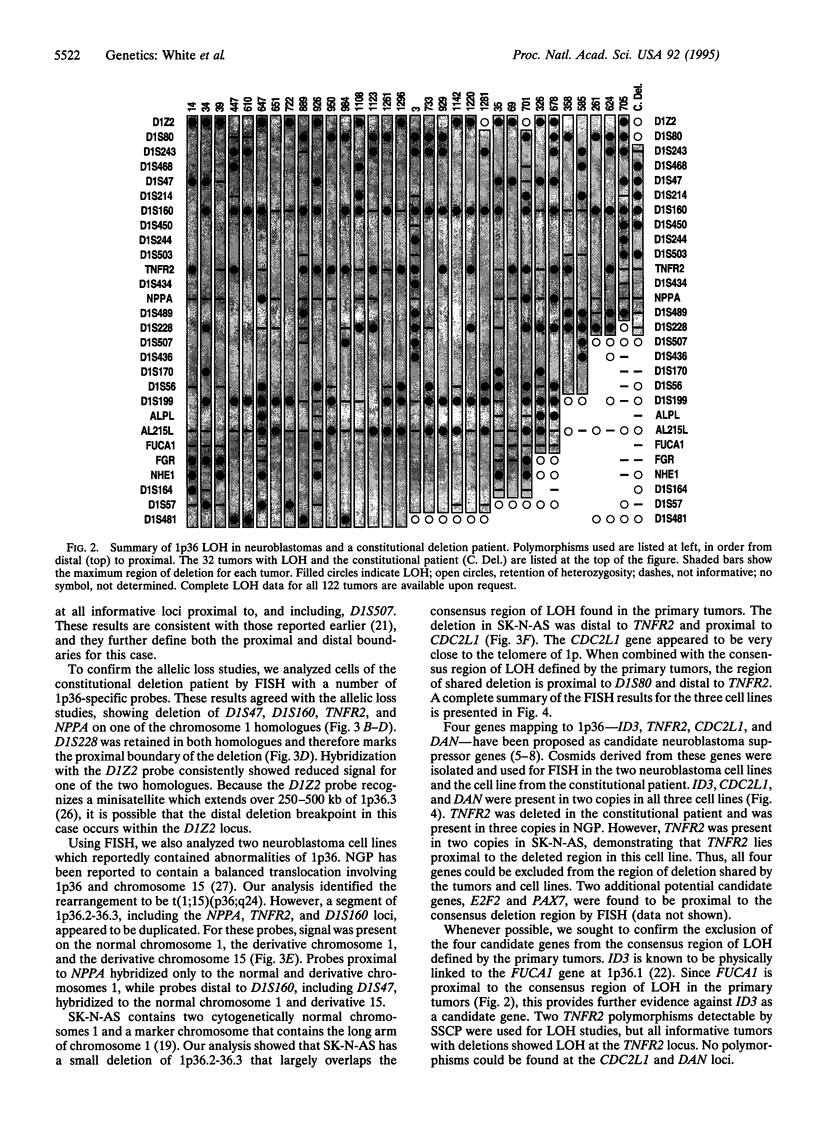
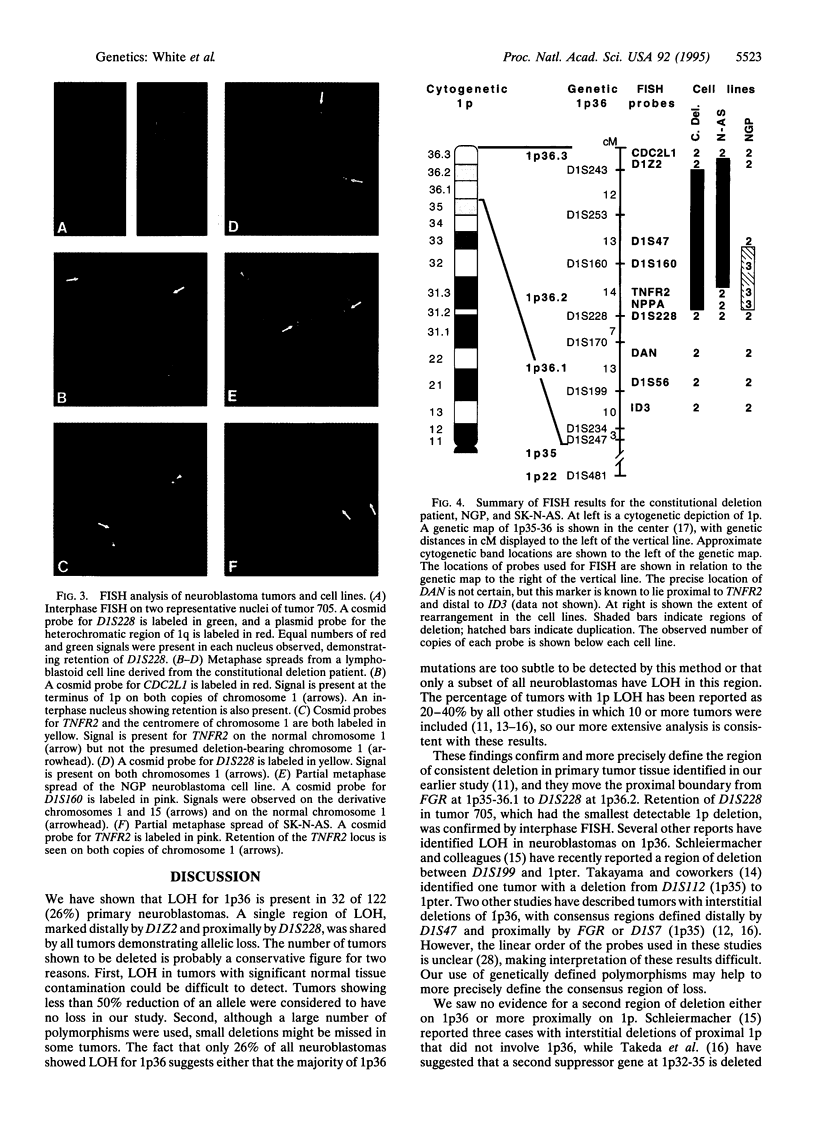
Deletion of the short arm of human chromosome 1 is the most common cytogenetic abnormality observed in neuroblastoma. To characterize the region of consistent deletion, we performed loss of heterozygosity (LOH) studies on 122 neuroblastoma tumor samples with 30 distal chromosome 1p polymorphisms. LOH was detected in 32 of the 122 tumors (26%). A single region of LOH, marked distally by D1Z2 and proximally by D1S228, was detected in all tumors demonstrating loss. Also, cells from a patient with a constitutional deletion of 1p36, and from a neuroblastoma cell line with a small 1p36 deletion, were analyzed by fluorescence in situ hybridization. Cells from both sources had interstitial deletions of 1p36.2-36.3 which overlapped the consensus region of LOH defined by the tumors. Interstitial deletion in the constitutional case was confirmed by allelic loss studies using the panel of polymorphic markers. Four proposed candidate genes--DAN, ID3 (heir-1), CDC2L1 (p58), and TNFR2--were shown to lie outside of the consensus region of allelic loss, as defined by the above deletions. These results more precisely define the location of a neuroblastoma suppressor gene within 1p36.2-36.3, eliminating 33 centimorgans of proximal 1p36 from consideration. Furthermore, a consensus region of loss, which excludes the four leading candidate genes, was found in all tumors with 1p36 LOH.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader S. A., Fasching C., Brodeur G. M., Stanbridge E. J. Dissociation of suppression of tumorigenicity and differentiation in vitro effected by transfer of single human chromosomes into human neuroblastoma cells. Cell Growth Differ. 1991 May;2(5):245–255. [PubMed] [Google Scholar]
- Biegel J. A., White P. S., Marshall H. N., Fujimori M., Zackai E. H., Scher C. D., Brodeur G. M., Emanuel B. S. Constitutional 1p36 deletion in a child with neuroblastoma. Am J Hum Genet. 1993 Jan;52(1):176–182. [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Fong C. T. Molecular biology and genetics of human neuroblastoma. Cancer Genet Cytogenet. 1989 Sep;41(2):153–174. doi: 10.1016/0165-4608(89)90243-4. [DOI] [PubMed] [Google Scholar]
- Budowle B., Chakraborty R., Giusti A. M., Eisenberg A. J., Allen R. C. Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet. 1991 Jan;48(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Caron H., van Sluis P., van Roy N., de Kraker J., Speleman F., Voûte P. A., Westerveld A., Slater R., Versteeg R. Recurrent 1;17 translocations in human neuroblastoma reveal nonhomologous mitotic recombination during the S/G2 phase as a novel mechanism for loss of heterozygosity. Am J Hum Genet. 1994 Aug;55(2):341–347. [PMC free article] [PubMed] [Google Scholar]
- Collins A., Keats B. J., Dracopoli N., Shields D. C., Morton N. E. Integration of gene maps: chromosome 1. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4598–4602. doi: 10.1073/pnas.89.10.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., Bruns G. A., Brodeur G. M., Landes G. M., Matise T. C., Seldin M. F., Vance J. M., Weith A. Report and abstracts of the First International Workshop on Human Chromosome 1 Mapping 1994. Bethesda, Maryland, March 25-27, 1994. Cytogenet Cell Genet. 1994;67(3):144–165. [PubMed] [Google Scholar]
- Ellmeier W., Aguzzi A., Kleiner E., Kurzbauer R., Weith A. Mutually exclusive expression of a helix-loop-helix gene and N-myc in human neuroblastomas and in normal development. EMBO J. 1992 Jul;11(7):2563–2571. doi: 10.1002/j.1460-2075.1992.tb05321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H., Ozaki T., Takahashi E., Nomura N., Tabata S., Takahashi H., Ohnuma N., Tanabe M., Iwai J., Yoshida H. Identification of human DAN gene, mapping to the putative neuroblastoma tumor suppressor locus. Oncogene. 1994 Oct;9(10):2785–2791. [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. T., White P. S., Peterson K., Sapienza C., Cavenee W. K., Kern S. E., Vogelstein B., Cantor A. B., Look A. T., Brodeur G. M. Loss of heterozygosity for chromosomes 1 or 14 defines subsets of advanced neuroblastomas. Cancer Res. 1992 Apr 1;52(7):1780–1785. [PubMed] [Google Scholar]
- Knudson A. G. Antioncogenes and human cancer. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti J. M., Valentine M., Xiang J., Jones B., Amann J., Grenet J., Richmond G., Look A. T., Kidd V. J. Alterations in the PITSLRE protein kinase gene complex on chromosome 1p36 in childhood neuroblastoma. Nat Genet. 1994 Jul;7(3):370–375. doi: 10.1038/ng0794-370. [DOI] [PubMed] [Google Scholar]
- Laureys G., Speleman F., Opdenakker G., Benoit Y., Leroy J. Constitutional translocation t(1;17)(p36;q12-21) in a patient with neuroblastoma. Genes Chromosomes Cancer. 1990 Sep;2(3):252–254. doi: 10.1002/gcc.2870020315. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Saito M., Vidal M., Valentine M., Look T., Harlow E., Dyson N., Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993 Dec;13(12):7813–7825. doi: 10.1128/mcb.13.12.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise T. C., Perlin M., Chakravarti A. Automated construction of genetic linkage maps using an expert system (MultiMap): a human genome linkage map. Nat Genet. 1994 Apr;6(4):384–390. doi: 10.1038/ng0494-384. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Liu X. G., Ikegaki N., White P. S., Yamashiro D. J., Nycum L. M., Biegel J. A., Brodeur G. M. Cloning and chromosomal localization of the human TRK-B tyrosine kinase receptor gene (NTRK2). Genomics. 1995 Jan 20;25(2):538–546. doi: 10.1016/0888-7543(95)80055-q. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Julier C., Wolff R., Holm T., O'Connell P., Leppert M., White R. Characterization of a human 'midisatellite' sequence. Nucleic Acids Res. 1987 Mar 25;15(6):2537–2547. doi: 10.1093/nar/15.6.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasawmy R., Kotea N., Lu C. Y., Sayada C., Baligadoo S., Krishnamoorthy R. Investigation of the polymorphic ScaI site by a PCR-based assay at the human atrial natriuretic peptides (hANP) gene locus. Hum Genet. 1992 Nov;90(3):323–324. doi: 10.1007/BF00220093. [DOI] [PubMed] [Google Scholar]
- Savelyeva L., Corvi R., Schwab M. Translocation involving 1p and 17q is a recurrent genetic alteration of human neuroblastoma cells. Am J Hum Genet. 1994 Aug;55(2):334–340. [PMC free article] [PubMed] [Google Scholar]
- Schleiermacher G., Peter M., Michon J., Hugot J. P., Vielh P., Zucker J. M., Magdelénat H., Thomas G., Delattre O. Two distinct deleted regions on the short arm of chromosome 1 in neuroblastoma. Genes Chromosomes Cancer. 1994 Aug;10(4):275–281. doi: 10.1002/gcc.2870100409. [DOI] [PubMed] [Google Scholar]
- Shapiro D. N., Sublett J. E., Li B., Valentine M. B., Morris S. W., Noll M. The gene for PAX7, a member of the paired-box-containing genes, is localized on human chromosome arm 1p36. Genomics. 1993 Sep;17(3):767–769. doi: 10.1006/geno.1993.1404. [DOI] [PubMed] [Google Scholar]
- Takayama H., Suzuki T., Mugishima H., Fujisawa T., Ookuni M., Schwab M., Gehring M., Nakamura Y., Sugimura T., Terada M. Deletion mapping of chromosomes 14q and 1p in human neuroblastoma. Oncogene. 1992 Jun;7(6):1185–1189. [PubMed] [Google Scholar]
- Takeda O., Homma C., Maseki N., Sakurai M., Kanda N., Schwab M., Nakamura Y., Kaneko Y. There may be two tumor suppressor genes on chromosome arm 1p closely associated with biologically distinct subtypes of neuroblastoma. Genes Chromosomes Cancer. 1994 May;10(1):30–39. doi: 10.1002/gcc.2870100106. [DOI] [PubMed] [Google Scholar]
- Van Roy N., Laureys G., Cheng N. C., Willem P., Opdenakker G., Versteeg R., Speleman F. 1;17 translocations and other chromosome 17 rearrangements in human primary neuroblastoma tumors and cell lines. Genes Chromosomes Cancer. 1994 Jun;10(2):103–114. doi: 10.1002/gcc.2870100205. [DOI] [PubMed] [Google Scholar]
- Van Roy N., Laureys G., Versteeg R., Opdenakker G., Speleman F. High-resolution fluorescence mapping of 46 DNA markers to the short arm of human chromosome 1. Genomics. 1993 Oct;18(1):71–78. doi: 10.1006/geno.1993.1427. [DOI] [PubMed] [Google Scholar]
- Weith A., Martinsson T., Cziepluch C., Brüderlein S., Amler L. C., Berthold F., Schwab M. Neuroblastoma consensus deletion maps to 1p36.1-2. Genes Chromosomes Cancer. 1989 Nov;1(2):159–166. doi: 10.1002/gcc.2870010209. [DOI] [PubMed] [Google Scholar]
- White P. S., Kaufman B. A., Marshall H. N., Brodeur G. M. Use of the single-strand conformation polymorphism technique to detect loss of heterozygosity in neuroblastoma. Genes Chromosomes Cancer. 1993 Jun;7(2):102–108. doi: 10.1002/gcc.2870070207. [DOI] [PubMed] [Google Scholar]