Abstract
This review is part three of three and will present an update on the therapeutic options and procedures concerning gastrointestinal (GI) submucosal tumors (SMTs). The aim of this paper is to investigate the treatments of GI SMTs and to present a case of a gastrointestinal stromal tumor (GIST). Literature searches were performed to find information on therapy for GI SMTs. Based on these searches, the optimal therapeutic procedures could be outlined. The choice of treatment of localized tumors is endoscopic resection if possible or, alternatively, laparoscopic resection or surgical resection by an open procedure. However, benign SMTs should only be excised if symptoms are present, and GISTs should be treated with particular precautions. Irresectable or recurrent GISTs may be successfully treated with the tyrosine kinase inhibitor, imatinib.
Keywords: Submucosal tumor, Treatment, Case story, Endoscopic mucosal resection, Imatinib
INTRODUCTION
Surgical resection is the golden standard for treatment of gastrointestinal (GI) submucosal tumors (SMTs). However, new surgical and medical therapeutic options for SMTs have emerged recently. These include, primarily, endoscopic resection and usage of the tyrosine kinase inhibitor, imatinib.
The choice of treatment is based on whether the SMT is thought to be: benign, malignant, exophytic, endophytic, its size, extent and the presence of symptoms. Treatment is mostly not indicated in an asymptomatic, benign SMT, found incidentally. These SMTs are instead controlled by follow-up examinations[1]. On the contrary, malignant SMTs should be excised surgically as a rule[2,3].
Gastrointestinal stromal tumors (GISTs), the most common mesenchymal tumors in the gastrointestinal tract, form a specific problem as they have the ability to metastasize, even though they may appear to be fully benign[4,5]. In recent years it has become clear that the tyrosine kinase inhibitor, imatinib, has a place in the treatment of inoperable, recurrent and metastatic GISTs[2,6-8].
Recent validation of these new treatment options has created a need for a review of the therapeutic options when dealing with SMTs. The aim of the present paper is to update the reader on the different therapeutic possibilities, mostly surgical, regarding various types of SMTs. A case story of a GIST is presented in this context.
ENDOSCOPIC SURGERY
For SMT resection, endoscopic surgical procedures represent an alternative to laparoscopic and conventional open surgical bowel resection procedures in selective cases. Endoscopic ultrasonography (EUS) and multi-slice CT are helpful tools for deciding on which type of surgical procedure should be performed[1]. A comparison between the different methods can be viewed in Table 1.
Table 1.
Various endoscopic therapeutic procedures for the treatment of SMTs
| Indication | Contraindications1 | Complications | Advantages | Disadvantages | |
| SSP[1,56] | SMT < 2 cm; polypoid/pedunculated; sessile with a base < 1-2 cm; intraluminal and originating in muscularis mucosa or submucosa | SMT > 2 cm; originating from the muscularis propria; intramural SMT; extraluminal SMT; located on the lesser curvature, posterior aspect of the stomach body or the cardia | Incomplete resection, hemorrhage, perforation (when the SMT is > 2.5 cm) | High success rate, few complications | See "Complications" |
| SB[1,9,10,57,58] | Same as for SSP | Same as for SSP | Minor bleeding treated with saline inj., metal clips or liquid thrombin | The saline injection prevents full-thickness burning and perforation; high success rate; safe, quick and easy method | If the saline is injected in the surrounding tissue, the SMT will become sessile and therefore more difficult to remove |
| ESMR-L[1] | SMT < 1 cm | SMT > 1 cm; originating from the muscularis propria | No serious complications have been reported | Not restricted by the location of the SMT; achieves deeper resection than SB and conventional EMR and thus a higher rate of curative resection | This technique can only be applied to small SMTs |
| ESMR-C[1] | SMT < 2 cm | SMT > 2 cm; SMTs in the muscularis propria | Minor hemorrhage, though rare. | Simpler and easier version of EMR; high success rate; saline inj., see SB | See "Complications" |
| UT[1,58] | Simple and multicystic SMTs (e.g. lipomas and cystic lymphangiomas) | Vascular tumors | Hemorrhage | Reduced risk of perforation, due to the fact that only the upper half is removed; can be applied to larger tumors | Only applicable in cases of lipomas and cystic lymphangiomas |
| EE-M[1] | Easiest if well capsulated; large SMTs and SMTs in the muscularis propria can be removed by this technique | SMTs with wide bases, severe adhesions or not well capsulated | Minor hemorrhage | Can be used to resect leiomyomas originating from the muscularis propria; sessile or large SMTs > 2 cm can be resected | Very difficult to perform |
| EE-I[1] | Large SMTs and SMTs in the muscularis propria can be removed by this technique | Unknown since this is a new technique | Perforation, minor hemorrhage | Like EE-M this technique is not limited by the size, sessile form or association with the muscularis propria | New method, which means that the efficacy and safety is not known for sure |
These are not absolute contraindications, but should rather be seen as circumstances, where resection is complicated. SSP: standard snare polypectomy; SB: strip biopsy; ESMR-L: resection performed with a ligation device; ESMR-C: endoscopic submucosal tumor resection with a transparent cap; UT: unroofing technique; EE-M: endoscopic enucleation performed with an initial mucosectomy; EE-I: endoscopic enucleation performed with an insulated-tip electrosurgical knife.
Standard snare polypectomy is performed with either a one- or a two-channel endoscope. With the one-channel endoscope, the cauterizing snare is placed around the SMT base and pulled as the resection is performed. With the two-channel endoscope, the oral part of the SMT is grasped with a forceps and held, while the snare is placed around the SMT[1].
Strip biopsy is initiated with a submucosal injection of physiologic saline, which may be EUS-guided. This separates the muscularis propria from the luminal layers. After placing a snare around the SMT base, excision is done with electrocoagulation while tightening the snare[1,9,10].
Resection can also be performed with a ligation device. After a submucosal saline injection the SMT is aspirated into the ligation device and the elastic band is released around it. Snare resection is performed with electrocoagulation below the elastic band. Endoscopic SMT resection with a transparent cap is a simpler and easier method[1].
With the unroofing technique, the upper half of the SMT is resected with a snare, creating an opening in the overlying mucosa. In most cases, the remnant SMT resolves spontaneously[1].
Endoscopic enucleation can be performed with an initial mucosectomy. The superficial part of the tumor is removed employing a snare or a cutting knife. Then a biopsy forceps is used to separate the SMT from the surrounding tissue, and the tumor can be removed with a snare[1].
Endoscopic enucleation can also be performed with an insulated-tip electrosurgical knife. Epinephrine injected in the proximal aspect of the SMT detaches it from the overlying tissue. Using a needle-knife, a 3-5 mm diameter hole is made. With the insulated-tip electrosurgical knife introduced though the hole, a longitudinal incision is made in the overlying mucosa and the surrounding tissue is dissected away. The tumor can now be removed en bloc[1].
Some therapeutic interventions can also be performed with push-and-pull enteroscopy in selected cases, which is typically a symptomatic, benign, small intestinal SMT[11].
TREATMENT OF BENIGN SUBMUCOSAL TUMORS
Benign SMTs should generally only be treated if they are symptomatic. In case of asymptomatic SMTs, follow-up examinations seems to be the best approach[1]. Exceptions from this rule (e.g. heterotopic pancreatic tissue) will be dealt with in the following.
Leimyomas
Small, symptomatic, duodenal leiomyomas with benign features can be safely treated with local excision via a longitudinal duodenotomy[12]. If the leiomyoma is located in the esophagus it will often result in progressive dysphagia, in which case enucleation or resection is required[13]. Endoscopic excision is also an option, and even leiomyomas larger than 2cm can be removed by enucleation using a snare, cutting knife or an insulated-tip electrosurgical knife, see above[1]. A case report has shown a successful resection of an esophageal leiomyoma by means of thoracoscopic enucleation[13]. In asymptomatic leiomyomas, follow-up may be preferred[1].
Schwannomas
Since GI Schwannomas are always benign, removal is only indicated in case of severe symptoms[14].
Granular cell tumors
In case of symptoms, endoscopic tumor excision is a good alternative, when the tumor is restricted to the inner layers, as recurrence or metastasis has never been documented in any patients[15,16]. When the tumor also invades the outer layers, EUS can contribute to planning the surgical resection[15].
An investigation of laser therapy for esophageal granular cell tumors included four patients. The method was successful in achieving complete necrosis of the esophageal changes, necessitating four sessions per patient. A mean follow-up period of 66 mo showed no evidence of tumor recurrence. No complications were observed leaving laser therapy as a putative new therapy in selected cases[17].
Heterotopic pancreatic tissue
Malignancy in heterotopic pancreas must be considered, although it is relatively rare[18-21]. If symptoms occur, surgery may be a necessity[18]. Endoscopic resection may be performed either by standard snare polypectomy, strip biopsy, resection performed with a ligation device or by endoscopic submucosal tumor resection with a transparent cap[1]. If heterotopic pancreatic tissue is found incidentally during operation for other reasons, prophylactic resec-tion of the tissue is advisable for prevention of later complications[22].
Lipomas
Large lipomas may cause massive bleeding or intus-susception[1]. If symptoms occur, the treatment of choice is surgical removal[23-26]. If the tumor is small, endoscopic polypectomy or enucleation may be preferred[23]. Large lipomas can be removed with the unroofing technique[1]. Asymptomatic lipomas should be followed without surgery[24,26] and some of them may in fact resolve spontaneously[1].
Neurofibromas
Neurofibromas are not easy to treat, as they may seem well defined macroscopically, but microscopy often reveals local infiltration. Therefore, these tumors commonly recur after excision[27]. Accordingly surgical resection has to be recommended due to high frequency of recurrence.
VASCULAR TUMORS
Hemangiomas
The therapeutic strategy depends on the size, number, location and symptoms[28]. Endoscopic coagulation or removal of a recurrently bleeding hemangioma may be performed either as an exploratory laparotomy with excision of the hemangioma or as laparoscopic excision with preceding push-and-pull enteroscopy, where the hemangioma is marked with ink[29]. However, in blue rubber-bleb nevi syndrome where multiple hemangiomas may be present, complete eradication may be impossible[28]. Alternatively, endoscopic laser photocoagulation or plasma argon coagulation may be performed[28].
Lymphangiomas
Large, symptomatic lymphangiomas can be removed endoscopically with the unroofing technique[1].
TREATMENT OF MALIGNANT SUBMUCOSAL TUMORS
Leiomyosarcomas
As leiomyosarcomas are considered radio- and chemore-sistant[30], surgical resection remains the only effective treatment and involves both the tumor and adjacent mesentery in small-intestinal leiomyosarcomas[3].
Kaposi’s sarcoma
Kaposi’s sarcoma typically occurs in the coexistence of human herpes virus 8 and HIV[31,32]. The classical Kaposi’s sarcoma is rarely fatal contrary to the much more frequent HIV-associated variant[31]. The treatment is usually dictated by the presence of symptoms[33], and should initially include highly active antiretroviral therapy against HIV with or without specific anti-Kaposi’s sarcoma therapy. This has been shown to halt progression or induce regression. Kaposi’s sarcoma is moderately responsive to radiation and chemotherapy[32].
Metastases
Treatment of metastases is angiographic embolization to control active tumor bleeding, endoscopic removal of the metastases, surgical exploration or medical treatment. If multiple organ involvement is present, and there is no active bleeding, the indication for treatment is questionable[34].
Treatment of gastrointestinal stromal tumors
GISTs stand out as especially complicated to treat compared to other SMTs. Therefore the treatment of these tumors will be described in more detail.
Surgical approaches: laparotomy or laparoscopy?
The first choice of treatment of localized GISTs is complete surgical resection, which seems to be the most important prognostic criterion[2,8,35,36]. The tumor should be removed en-bloc respecting a possible pseudo-capsule to avoid intraperitoneal dissemination[6,36-38], and therefore adjacent organs adherent to the GIST should be resected en-bloc with the tumor[2,36]. GISTs should be resected aggressively with a tumor-free margin[2,6,37,39], and determining this is mostly not much of a problem, since GISTs tend to be exophytic[36]. Re-excision should be considered in case of intramural GISTs that have been excised intra-lesionally and do not infiltrate the serosal surface[2]. A consensus meeting in 2005 concluded that laparoscopic surgery should be avoided, especially in GISTs larger than 2 cm, due to the risk of rupture[2]. Yet recent studies of even very large (up to 15 cm)GISTs showed successful and safe resection in nearly all of the patients employing laparoscopic resection. The reason for unsuccessful laparoscopic treatment (1 patient out of 64) was conversion to laparotomy due to suspected bowel injury when establishing pneumoperitoneum[37,40]. Lymphadenectomy is not a routine procedure owing to the route of malignant spread in GISTs, which is mainly hematological metastasis to the liver[2,6,39]. Hepatectomy for liver metastasis is not recommended as it does not seem to increase survival rates[41]. If the GIST is large or involves large vessels embolization should be considered.
Tyrosine kinase inhibitors-imatinib
GISTs are chemo- and radioresistant[42,43]. Immediate medical treatment with the tyrosine kinase inhibitor, imatinib, is indicated in case of metastatic, recurrent or irresectable GISTs[2,6-8]. Imatinib should also be considered in case of equivocal images[2]. The effect can be monitored with combined positron emission tomography (PET) and CT, PET-CT[44]. Imatinib is given as an oral treatment, with a recommended daily dose of 400 mg[2,7]. For lack of response, 600-800 mg/d may be attempted[2]. Treatment with imatinib should be continued until progression, intolerance or patient refusal[2].
Imatinib specifically inhibits a mutated tyrosine kinase receptor (kit-receptor; CD117) that normally regulates cell growth and survival, but a gain-of-function mutation has made it continuously active. However, imatinib has also shown to inhibit platelet derived growth factor receptor alpha mutations (a CD117-related tyrosine kinase receptor) and tumors without mutations[2,7,8,44-47] (e.g. neurofibromatosis type 1-associated GISTs)[48]. A reason for the dramatic effect of imatinib is probably that it inhibits the kit-receptor signaling, which secondarily inhibits the glucose uptake and metabolism and thus cell proliferation[44,49].
The effect of imatinib on GISTs often results in increased tumor size due to hemorrhage, edema and myxoid degeneration and therefore do not correlate to the response criteria of the World Health Organization or of Response Evaluation Criteria in Solid Tumors[2,50]. Decreased metabolism in fluorodeoxyglucose marked PET (FDG-PET), reduction in tumor density (Hounsfield units) in CT and symptomatic improvement all indicate tumor response to imatinib[2]. Long-term studies are still not available. However, patients did not survive for more than 1 year earlier, but with imatinib therapy they now live for more years[51]. High and intermediate risk GISTs should be followed with a CT scan every 3-4 mo for 3 years, then every 6 mo until 5 years and yearly thereafter. Low and very low risk GISTs can be followed every 6 mo for 5 years[2].
The side effects from imatinib tend to be mild and occur rather infrequently[44,50,52]. However, lethal complications such as bleeding may occur[50].
CASE STORY
A 61-year-old woman was hospitalized due to black stools for three days, fainting fits and hematemesis during the past week. The patient suffered from mild epigastric pain and had also experienced nausea.
On examination, the patient was found to be slightly tender corresponding to the epigastrium. Black feces were found at rectal exploration and fresh blood appeared from the stomach tube. Hemoglobin was only 4.5 mmol/L (reference interval: 7-10) at admission.
At standard upper endoscopy a 4 cm × 4 cm SMT was revealed in the anterior wall of the stomach, close to the cardia. It had a fibrin-coated ulceration showing stigmata of hemorrhage. The biopsies were inconclusive, due to lack of submucosal representation.
A CT scan confirmed the gastric mass, but also revealed a mass in the left adrenal gland. It was not possible to take a biopsy from the latter by ultrasound due to lack of visualization. Neither was it possible by CT owing to the fact that there was no free window to reach the tumor without serious risk of lung damage.
The patient was referred for resection of both tumors by an open surgical procedure. Postoperatively, an explorative laparotomy was performed due to non-specific hemorrhage. Furthermore, bilateral, moderate pleura exudates were found, however not requiring drainage. Apart from this, the postoperative course was uneventful.
Macroscopic examination showed a tumor size of 45 mm × 40 mm × 36 mm with a cystic lumen of 37 mm containing blood and mucus. The consistency of the tumor tissue was firm, it had a capsule-like structure with fibrous septa and the color was mixed gray-yellow and brown. Microscopically, the tumor tissue was whirled with distinct palisading nuclei (Figure 1). The cells were spindle shaped with mild nuclear atypia and few mitoses (1-2/50 high power fields). There was significant edema and mild, diffuse lymphocyte infiltration. Furthermore, central degeneration with sequelae from hemorrhage, fibrosis and coagulation necrosis surrounding vascular structures was seen. Additionally, multiple small, thin-walled cysts looking like dilated lymph vessels and invaginated serosal surface was found. No tumor necrosis was seen.
Figure 1.
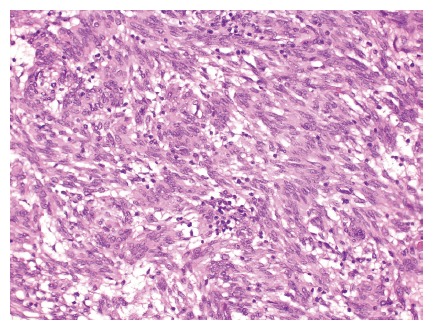
Histological findings of the gastrointestinal stromal tumor described in the case are presented showing spindle shaped cells with mild, nuclear atypia, few mitoses and slight, diffuse lymphocyte infiltration is seen (HE, x 100). Courtesy of B. Vainer.
Immunohistochemically, the tumor tissue was strongly reactive for CD117 and CD34 (Figures 2 and 3) with smaller areas being positive for smooth muscle actin, and negative for desmin, S-100 and cytokeratin. MIB-1 (Ki-67, proliferation marker) was reactive in 1%-2% of the cells.
Figure 2.
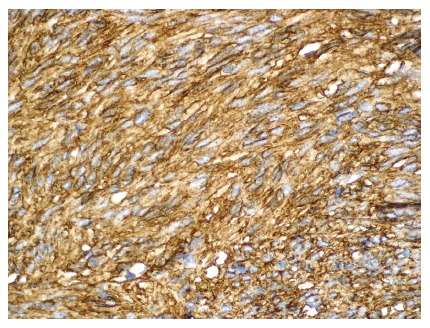
Histological findings of the gastrointestinal tumor described in the case are presented showing a positive CD34 immunoreaction (x 100). Courtesy of B. Vainer.
Figure 3.
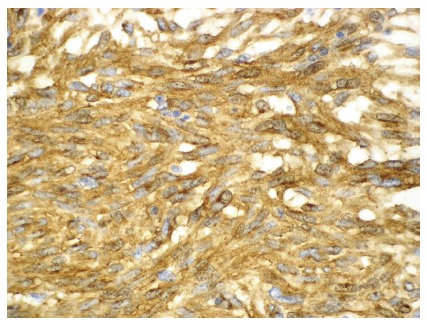
Histological findings of the gastrointestinal stromal tumor described in the case are presented showing a positive CD117 immunoreaction (x 200). Courtesy of B. Vainer.
In conclusion, the tumor was found to be a low-risk GIST with microscopically confirmed free resection margins. The adrenal tumor was a cortical adenoma and was not related to the GIST.
DISCUSSION
The choice of surgical procedure is dictated by the clinical condition of the patient, the type, size, shape, location and extent of the GI SMT. In general, benign appearing, asymptomatic SMTs should be evaluated by follow-up examinations, whereas surgical resection should be reserved for symptomatic SMTs or those suspicious of malignancy, including all GISTs[53,54].
The surgical procedure can either be performed endoscopically for intraluminal growing SMTs, laparo-scopically for SMTs with extraluminal growth or through laparotomy for SMTs suspected to be malignant[39,55]. Employing endoscopic resection, there is an increased risk of perforation and hemorrhage, if the SMT is located near the serosa, but this may be prevented by the application of metal clips[1]. The availability for expertise in endoscopic and laparoscopic procedures will be a limiting factor until these techniques have been implemented. Referral to expert centers is therefore a necessity.
GISTs should always be removed, since all of these tumors can potentially metastasize. The laparoscopic and open surgical resection procedure with a “gentle-touch technique” is recommended in order to reduce the risk of hemorrhage and intra-peritoneal dissemination, as GISTs tend to have a friable consistency. Medical treatment with a tyrosine kinase inhibitor (i.e. imatinib) is indicated for recurrent or irresectable GISTs as this treatment has proven very effective, safe and tolerable. Follow-up with CT in patients with GISTs is recommended.
In the presented case, the GIST was excised in toto and adrenectomy was performed in the same intervention. Hemostasis was achieved. The following night, acute operation was performed due to hemorrhage, which arose from the adrenectomy area. The recommended follow-up interval for patients with low-risk GISTs like the present case is CT scans every 6 mo.
ACKNOWLEDGMENTS
We thank pathologist Anne Mellon Mogensen for her descriptions of the GIST presented in the case, pathologist Ben Vainer for photographs of the same GIST and pathologist Katalin Kiss for her proof reading of the case story.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu Y
References
- 1.Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2005;37:646–654. doi: 10.1055/s-2005-861477. [DOI] [PubMed] [Google Scholar]
- 2.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 3.Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol. 2001;33:267–282. doi: 10.1097/00004836-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–483. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 6.Lau S, Tam KF, Kam CK, Lui CY, Siu CW, Lam HS, Mak KL. Imaging of gastrointestinal stromal tumour (GIST) Clin Radiol. 2004;59:487–498. doi: 10.1016/j.crad.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura Y, Miettinen M, Hirota S, Kanakura Y. Gastrointestinal stromal tumor (GIST): from pathology to molecular target therapy. Tokyo: Japan Scientific Societies Press; 2004. [Google Scholar]
- 8.Reddy MP, Reddy P, Lilien DL. F-18 FDG PET imaging in gastrointestinal stromal tumor. Clin Nucl Med. 2003;28:677–679. doi: 10.1097/01.RLU.0000079395.25949.c1. [DOI] [PubMed] [Google Scholar]
- 9.Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, Kohgo Y. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc. 2002;55:44–49. doi: 10.1067/mge.2002.119871. [DOI] [PubMed] [Google Scholar]
- 10.Sun S, Wang M, Sun S. Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors. Endoscopy. 2002;34:82–85. doi: 10.1055/s-2002-19386. [DOI] [PubMed] [Google Scholar]
- 11.Ell C, May A, Nachbar L, Cellier C, Landi B, di Caro S, Gasbarrini A. Push-and-pull enteroscopy in the small bowel using the double-balloon technique: results of a prospective European multicenter study. Endoscopy. 2005;37:613–616. doi: 10.1055/s-2005-870126. [DOI] [PubMed] [Google Scholar]
- 12.Rice DC, Bakaeen F, Farley DR, Unni KK, van Heerden JA. Surgical management of duodenal leiomyomas. World J Surg. 2001;25:562–566. doi: 10.1007/s002680020083. [DOI] [PubMed] [Google Scholar]
- 13.Ertem M, Baca B, Doğusoy G, Ergüney S, Yavuz N. Thoracoscopic enucleation of a giant submucosal tumor of the esophagus. Surg Laparosc Endosc Percutan Tech. 2004;14:87–90. doi: 10.1097/00129689-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Inagawa S, Hori M, Shimazaki J, Matsumoto S, Ishii H, Itabashi M, Adachi S, Kawamoto T, Fukao K. Solitary schwannoma of the colon: report of two cases. Surg Today. 2001;31:833–838. doi: 10.1007/s005950170060. [DOI] [PubMed] [Google Scholar]
- 15.Palazzo L, Landi B, Cellier C, Roseau G, Chaussade S, Couturier D, Barbier J. Endosonographic features of esophageal granular cell tumors. Endoscopy. 1997;29:850–853. doi: 10.1055/s-2007-1004320. [DOI] [PubMed] [Google Scholar]
- 16.Nakachi A, Miyazato H, Oshiro T, Shimoji H, Shiraishi M, Muto Y. Granular cell tumor of the rectum: a case report and review of the literature. J Gastroenterol. 2000;35:631–634. doi: 10.1007/s005350070064. [DOI] [PubMed] [Google Scholar]
- 17.Norberto L, Urso E, Angriman I, Ranzato R, Erroi F, Marino S, Tosato S, Ruffolo C, D'Amico DF. Yttrium-aluminum-garnet laser therapy of esophageal granular cell tumor. Surg Endosc. 2002;16:361–362. doi: 10.1007/s00464-001-4211-0. [DOI] [PubMed] [Google Scholar]
- 18.Day D, Jass J, Price AB, Shepherd NA, Sloan JM, Talbot IC, Warren BF, Williams GT. Morson & Dawson's Gastrointestinal Pathology. Massachusetts: Blackwell Science Ltd; 2003. pp. 205–209, 383-388, 615. [Google Scholar]
- 19.Sun Y, Wasserman PG. Acinar cell carcinoma arising in the stomach: a case report with literature review. Hum Pathol. 2004;35:263–265. doi: 10.1016/j.humpath.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Maekawa T, Sakai T, Shirakusa T. Transgastrostomal endoscopic surgery for early gastric carcinoma and submucosal tumor. Surg Endosc. 1999;13:361–364. doi: 10.1007/s004649900990. [DOI] [PubMed] [Google Scholar]
- 21.Ikematsu Y, Nishiwaki Y, Kida H, Iwaoka Y, Nagashima S, Ozawa T, Hasegawa S, Okawada T, Waki S. Gastric outlet obstruction caused by a heterotopic pancreas in a pregnant woman: report of a case. Surg Today. 2003;33:952–955. doi: 10.1007/s00595-003-2614-3. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Tsunoda T, Eto T, Yamada M, Tajima Y, Shimogama H, Yamaguchi T, Matsuo S, Izawa K. Diagnosis and management of heterotopic pancreas. Int Surg. 1993;78:32–35. [PubMed] [Google Scholar]
- 23.Mouës CM, Steenvoorde P, Viersma JH, van Groningen K, de Bruïne JF. Jejunal intussusception of a gastric lipoma: a review of literature. Dig Surg. 2002;19:418–420. doi: 10.1159/000065825. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez MJ, Davis RP, Nora PF. Gastrointestinal lipomas. Arch Surg. 1983;118:1081–1083. doi: 10.1001/archsurg.1983.01390090065015. [DOI] [PubMed] [Google Scholar]
- 25.Agha FP, Dent TL, Fiddian-Green RG, Braunstein AH, Nostrant TT. Bleeding lipomas of the upper gastrointestinal tract. A diagnostic challenge. Am Surg. 1985;51:279–285. [PubMed] [Google Scholar]
- 26.Maderal F, Hunter F, Fuselier G, Gonzales-Rogue P, Torres O. Gastric lipomas--an update of clinical presentation, diagnosis, and treatment. Am J Gastroenterol. 1984;79:964–967. [PubMed] [Google Scholar]
- 27.Levy AD, Patel N, Dow N, Abbott RM, Miettinen M, Sobin LH. From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation. Radiographics. 2005;25:455–480. doi: 10.1148/rg.252045176. [DOI] [PubMed] [Google Scholar]
- 28.Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13:237–240. [PubMed] [Google Scholar]
- 29.Chan AO, Lai KC. A patient with long-standing iron-deficient anemia. Nat Clin Pract Gastroenterol Hepatol. 2006;3:112–116; quiz 117. doi: 10.1038/ncpgasthep0413. [DOI] [PubMed] [Google Scholar]
- 30.Hatch KF, Blanchard DK, Hatch GF, Wertheimer-Hatch L, Davis GB, Foster RS, Skandalakis JE. Tumors of the rectum and anal canal. World J Surg. 2000;24:437–443. doi: 10.1007/s002689910069. [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick TB, Johnson RA, Wolff K, Polano MK, Suurmond D. Atlas und Synopsis der klinischen Dermatologie -- Häufige und bedrohliche Krankheiten (Color Atlas and Synopsis of Clinical Dermatology. Common and Serious Diseases) London: McGraw-Hill; 1998. [Google Scholar]
- 32.Odze RD, Antonioli DA, Wallace MB. Gastrointestinal Cancers-A comparison to Sleisenger and Fordtran's Gastrointestinal and Liver Disease. Spain: Elsevier Science Limited; 2003. [Google Scholar]
- 33.Dezube BJ. Acquired immunodeficiency syndrome-related Kaposi's sarcoma: clinical features, staging, and treatment. Semin Oncol. 2000;27:424–430. [PubMed] [Google Scholar]
- 34.Hsu CC, Chen JJ, Changchien CS. Endoscopic features of metastatic tumors in the upper gastrointestinal tract. Endoscopy. 1996;28:249–253. doi: 10.1055/s-2007-1005437. [DOI] [PubMed] [Google Scholar]
- 35.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–645. doi: 10.1055/s-2005-861422. [DOI] [PubMed] [Google Scholar]
- 36.El-Zohairy M, Khalil el-SA, Fakhr I, El-Shahawy M, Gouda I. Gastrointestinal stromal tumor (GIST)'s surgical treatment, NCI experience. J Egypt Natl Canc Inst. 2005;17:56–66. [PubMed] [Google Scholar]
- 37.Lai IR, Lee WJ, Yu SC. Minimally invasive surgery for gastric stromal cell tumors: intermediate follow-up results. J Gastrointest Surg. 2006;10:563–566. doi: 10.1016/j.gassur.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Bucher P, Taylor S, Villiger P, Morel P, Brundler MA. Are there any prognostic factors for small intestinal stromal tumors? Am J Surg. 2004;187:761–766. doi: 10.1016/j.amjsurg.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Knoop M, St Friedrichs K, Dierschke J. Surgical management of gastrointestinal stromal tumors of the stomach. Langenbecks Arch Surg. 2000;385:194–198. doi: 10.1007/s004230050264. [DOI] [PubMed] [Google Scholar]
- 40.Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, Kubota T, Mukai M, Kameyama K, Sugino Y, et al. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006;139:484–492. doi: 10.1016/j.surg.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Nunobe S, Sano T, Shimada K, Sakamoto Y, Kosuge T. Surgery including liver resection for metastatic gastrointestinal stromal tumors or gastrointestinal leiomyosarcomas. Jpn J Clin Oncol. 2005;35:338–341. doi: 10.1093/jjco/hyi091. [DOI] [PubMed] [Google Scholar]
- 42.Rubin B, Demetri G. Gastrointestinal Oncology -- principles and practice. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 43.Rossi G, Valli R, Bertolini F, Marchioni A, Cavazza A, Mucciarini C, Migaldi M, Federico M, Trentini GP, Sgambato A. PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours of the gastrointestinal tract. Histopathology. 2005;46:522–531. doi: 10.1111/j.1365-2559.2005.02128.x. [DOI] [PubMed] [Google Scholar]
- 44.Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: best monitored with FDG PET. Nucl Med Commun. 2004;25:433–438. doi: 10.1097/00006231-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 46.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 47.Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, Dimitrijevic S, Sciot R, Stul M, Vranck H, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40:689–695. doi: 10.1016/j.ejca.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Buck L, Perry WB, Richards ML. Periampullary carcinoid tumor in a woman with neurofibromatosis. Curr Surg. 2006;63:252–254. doi: 10.1016/j.cursur.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Blasberg RG, Tjuvajev JG. Molecular-genetic imaging: current and future perspectives. J Clin Invest. 2003;111:1620–1629. doi: 10.1172/JCI18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reichardt P, Schneider U, Stroszczynski C, Pink D, Hohenberger P. Molecular response of gastrointestinal stromal tumour after treatment with tyrosine kinase inhibitor imatinib mesylate. J Clin Pathol. 2004;57:215–217. doi: 10.1136/jcp.2004.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucher P, Villiger P, Egger JF, Buhler LH, Morel P. Management of gastrointestinal stromal tumors: from diagnosis to treatment. Swiss Med Wkly. 2004;134:145–153. doi: 10.4414/smw.2004.10530. [DOI] [PubMed] [Google Scholar]
- 52.Hansen MS, Kampmann JP. Lægeforeningens Medicinfortegnelse (The Drug Register of the Danish Medical Society) Copenhagen: Lægeforeningens Forlag; 2005. [Google Scholar]
- 53.Fockens P. Current endosonographic possibilities in the upper gastrointestinal tract. Baillieres Clin Gastroenterol. 1994;8:603–619. doi: 10.1016/0950-3528(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 54.Kojima T, Takahashi H, Parra-Blanco A, Kohsen K, Fujita R. Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection. Gastrointest Endosc. 1999;50:516–522. doi: 10.1016/s0016-5107(99)70075-1. [DOI] [PubMed] [Google Scholar]
- 55.Hatch KF, Blanchard DK, Hatch GF, Wertheimer-Hatch L, Davis GB, Foster RS, Skandalakis JE. Tumors of the appendix and colon. World J Surg. 2000;24:430–436. doi: 10.1007/s002689910068. [DOI] [PubMed] [Google Scholar]
- 56.Wei SC, Wong JM, Shieh MJ, Sun CT, Wang CY, Wang TH. Endoscopic resection of gastrointestinal submucosal tumors. Hepatogastroenterology. 1998;45:114–118. [PubMed] [Google Scholar]
- 57.Kawamoto K, Yamada Y, Furukawa N, Utsunomiya T, Haraguchi Y, Mizuguchi M, Oiwa T, Takano H, Masuda K. Endoscopic submucosal tumorectomy for gastrointestinal submucosal tumors restricted to the submucosa: a new form of endoscopic minimal surgery. Gastrointest Endosc. 1997;46:311–317. doi: 10.1016/s0016-5107(97)70116-0. [DOI] [PubMed] [Google Scholar]
- 58.Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712–714. doi: 10.1055/s-2000-9025. [DOI] [PubMed] [Google Scholar]


