Abstract
AIM: To study at transcriptional level the similarities and differences of the physiological and biochemical activities between liver tumor (LT) and regenerating liver cells.
METHODS: LT-associated genes and their expression changes in LT were obtained from databases and scientific articles, and their expression profiles in rat liver regeneration (LR) were detected using Rat Genome 230 2.0 array. Subsequently their expression changes in LT and LR were compared and analyzed.
RESULTS: One hundred and twenty one LT-associated genes were found to be LR-associated. Thirty four genes were up-regulated, and 14 genes were down-regulated in both LT and regenerating liver; 20 genes up-regulated in LT were down-regulated in regenerating liver; 21 up-regulated genes and 16 down-regulated genes in LT were up-regulated at some time points and down-regulated at others during LR.
CONCLUSION: Results suggested that apoptosis activity suppressed in LT was still active in regenerating liver, and there are lots of similarities and differences between the LT and regenerating liver at the aspects of cell growth, proliferation, differentiation, migration and angiogenesis.
Keywords: Partial hepatectomy, Rat Genome 230 2.0 Array, Apoptosis, Liver regeneration-associated gene, Liver tumor-associated gene
INTRODUCTION
The liver is an organ with considerable regenerative capacity[1]. After partial hepatectomy (PH)[2], about 95% of quiescent hepatocytes re-enter synchronously into the cell cycle to replenish the missing hepatocytes[3,4]. Whereas excessive liver mass is regulated by apoptosis[5], this process is called liver regeneration (LR)[3]. The regeneration process, which according to cellular physiological and biochemical activities is divided into the following parts: initiation (0.5-4 h after PH), transition from G0 to G1 (4-6 h after PH), cell proliferation (6-66 h after PH), and cell differentiation and reorganization of the structure-function (72-168 h after PH)[6], or according to time course, into forepart (0.5-4 h after PH), prophase (6-12 h after PH), metaphase (16-66 h after PH) and anaphase (72-168 h after PH), involves various physiological and biochemical activities such as cell activation, de-differentiation, proliferation and its regulation, re-differentiation, and rebuilding of the structure and function[7,8]. Actually, some biological activities in LR including cell proliferation and growth are also observed in liver tumor (LT). It is usually thought that tumorigenesis is mainly ascribed to the anomalous activation of the genes having positive effects on LT cell proliferation, growth, invasion and LT angiogenesis, as well as the genes suppressing LT cell apoptosis, and/or inactivation of the inhibitory genes related to LT cell proliferation, growth, invasion and LT angiogenesis[9], and the promotive genes of LT cell differentiation and apoptosis. To elucidate the intrinsic differences between the two events at transcriptional level, we checked the expression profiles of above genes in regenerating livers following 2/3 hepatectomy utilizing the Rat Genome 230 2.0 Array containing 249 LT-associated genes, and primarily analyzed their expression changes and actions in LR, as well as their relevance with LR.
MATERIALS AND METHODS
Regenerating liver preparation
Healthy Sprague-Dawley rats weighing 200-250 g were obtained from the Animal Center of Henan Normal University. The 276 rats were separated into 46 groups randomly, 23 hepatectomized groups and 23 sham-operation (SO) groups,and each group included 6 rats. PH was performed according to Higgins and Anderson[2], by which the left and middle lobes of liver were removed. Rats were killed by cervical vertebra dislocation at 0, 0.5, 1, 2, 4, 6, 8, 12, 16, 18, 24, 30, 36, 42, 48, 54, 60, 66, 72, 96, 120, 144 and 168 h after PH and the regenerating livers were observed at corresponding time point. The livers were rinsed three times in PBS at 4°C, and then total 1-2 g livers (100-200 mg livers from middle parts of right lobe of each sample, 6 samples per group) were gathered and mixed together, then stored at -80°C. The SO group was the same as hepatectomized group except the liver lobes were not removed. The laws of animal protection of China were enforced strictly.
RNA isolation and purification
Total RNA was isolated from frozen livers according to the manual of Trizol reagent (Invitrogen Corporation, Carlsbad, California, USA)[10] and then purified base on the guide of RNeasy mini kit (Qiagen, Inc, Valencia, CA, USA)[11]. Total RNA samples were checked to exhibit a 2:1 ratio of 28S rRNA to 18S rRNA intensities by agarose electrophoresis (180 V, 0.5 h). Total RNA concentration and purity were estimated by optical density measurements at 260/280 nm[12].
cDNA, cRNA synthesis and purification
One to eight gram total RNA as template was used for cDNA synthesis. cDNA purification was based on the way established by Affymetrix[13]. cRNA labeled with biotin was synthesized using cDNA as the template, and cDNA and cRNA were purified according to the purification procedure of GeneChip Analysis[13]. Measurement of cDNA, cRNA concentration and purity were the same as above.
cRNA fragmentation and microarray detection
Fifty μL (1 μg/μL) cRNA incubated with 5 × fragmentation buffer at 94°C for 35 min was digested into 35-200 bp fragments. The hybridization buffer prepared according to the way Affymetrix provided was added to the prehybridized Rat Genome 230 2.0 array produced by Affymetrix, then hybridization was carried out at 45°C for 16 h on a rotary mixer at 60 rpm. The microarray was washed and stained by GeneChip fluidics station 450 (Affymetrix Inc., Santa Clara, CA , USA). The chips were scanned by GeneChip Scan 3000 (Affymetrix Inc., Santa Clara, CA, USA), and the signal values of gene expression were observed[14].
Microarray data analysis
The normalized signal values, signal detections (P, A, M) and experiment/control (Ri) were obtained by quantifying and normalizing the signal values using GCOS (GeneChip operating software) 1.2[14].
Normalization of the microarray data
To minimize the technical error from the microarray analysis, each sample was hybridized three times to the gene chips. The average value of three measurements was normalized, and statistics and cluster analyses were conducted on these values with GeneMath, GeneSpring (Silicon Genetics, San Carlos, CA) and Microsoft Excel Software (Microsoft, Redmond, WA)[14-16].
Verification of array results by RT-PCR
Primer and probe sequences were designed by primer express 2.0 software according to mRNA sequences of three target genes jun, myc, tp53 and internal control ®-actin gene (GenBank number: BC078738, NM_012603, AY009504 and NM_031144) and synthesized by Shanghai GeneCore BioTechnologies Co. Ltd (Table 1).
Table 1.
Primer and probe sequences used to validate the microarray analysis by quantitative RT-PCR
| Genes | Primer sequences | Tm | Amplified products |
| β-actin | FP: CCTGGCACCCAGCACAAT | 58°C | 221 bp |
| RP: GCTGATCCACATCTGCTGGAA | 58°C | ||
| Probe: ATCAAGATCATTGCTCCTCCTGAGCGC | 68°C | ||
| jun | FP: TGCAAAGATGGAAACGACCTT | 58°C | 76 bp |
| RP: GCCGTAGGCGCCACTCT | 59°C | ||
| Probe: TACGACGATGCCCTCAACGCCTC | 68°C | ||
| myc | FP: CCCCTAGTGCTGCATGAAGAG | 59°C | 95 bp |
| RP: TCCACAGACACCACATCAATTTC | 58°C | ||
| Probe: CACCAGCAGCGACTCTGAAGAAGAACA | 68°C | ||
| tp53 | FP: ATGAGGCCTTGGAATTAAAGGAT | 58°C | 98 bp |
| RP: CGTAGACTGGCCCTTCTTGGT | 59°C | ||
| Probe: CAGGGCTCACTCCAGCTACCCGAA | 68°C |
FP: forward primer; RP: reverse primer.
Identification of genes associated with LR
Nomenclatures such as LT, hepatoma, hepatocellular carcinoma, hepatocarcinogenesis, cholangiocarcinoma and so on were input into the databases at NCBI (www. ncbi.nlm.nih.gov) and RGD (rgd. mcw.edu) to identify rat, mouse and human genes associated with LT. Then these LT-associated genes were reconfirmed through literature searches of the pertinent articles. Besides the rat genes, other genes, that are now thought existing in mouse and/or human and showed a greater than two-fold change in the rat regenerating livers, were referred to as rat homologous genes. Genes that displayed reproducible results with three independent analyses using Rat Genome 230 2.0 array and that showed a greater than two-fold change in expression at least at one time point as a significant difference (P ≤ 0.05) or an extremely significant difference (P ≤ 0.01) between PH and SO, were included as being associated with LR.
RESULTS
Comparison between the quantitative RT-PCR results and the microarray results
The quantitative RT-PCR results of three chosen genes jun, myc and tp53 at 0, 0.5, 2, 4, 6, 12, 24, 30, 36 and 96 h after partial hepatectemy (PH) were compared with Rat Genome 230 2.0 Array results (Figure 1) in order to verify validity of this chip. According to quantitative RT-PCR results, myc was up-regulated at 0.5-12 and 30-96 h after PH with the highest point of 10.33 folds higher than control at 4 h; jun expression was significantly up-regulated at 0.5-4 h after PH, showing the greatest abundance of 4.28-fold of control at 0.5 h; and tp53 was up-regulated at 96 h after PH. The result of RT-PCR suggested that expression profiles of these three genes were basically similar to that of array, which indicated that Rat Genome 230 2.0 Array had great reliability.
Figure 1.
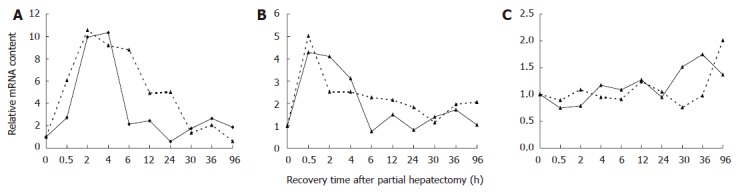
Comparison of relative mRNA levels in regenerating liver detected by Affymetrix Rat Genome 230 2.0 microarray and real-time PCR analysis A: myc; B: jun; C: tp53; Real line represents quantitative real time PCR results; broken line indicates Rat Genome 230 2.0 microarray results.
Expression changes of the associated-genes in LT and LR
Among 252 genes associated with LT obtained by searching the related data in databases such as NCBI, RGD etc., 249 genes were contained in the Rat Genome 230 2.0 Array. 121 of 249 genes yielded meaningful expression changes on at least single time point after PH, showed significant or extremely significant difference between PH and SO, and displayed reproducible results with three independent analyses with Rat Genome 230 2.0 Array, suggesting that these genes were associated with LR. The data listed below indicated that expression trends of 48 genes in LT was similar to that in LR, whereas expression of 34 genes in the former underwent opposite trend comparing with in the latter, and expression changes of 39 genes in LT were similar to that in some time point of LR. Specifically, the same trend towards up regulation of 34 genes and down regulation of 14 genes were exhibited in both LT and LR; 20 up-regulated genes in LT showed down-regulation during LR, and 14 down-regulated genes in LT revealed up-regulation during LR; 23 up-regulated genes and 16 down-regulated genes in LT were up-regulated at some time points and down-regulated at others during LR (Table 2).
Table 2.
Expression abundance of 121 liver tumor-associated genes during liver regeneration
| Name | Gene | Associated | Fold |
Comparison |
|
| Abbr. | to | difference | LT. | RRL. | |
| The same in gene expression trend | |||||
| Cyclin A2 | *Ccna2 | 2 | 45.1 | ↑ | ↑ |
| WEE1 homolog | Wee1 | 2 | 20.9 | ↑ | ↑ |
| Cyclin E1 | Ccne1 | 2 | 18.5 | ↑ | ↑ |
| Proliferating cell nuclear antigen | Pcna | 2 | 10.6 | ↑ | ↑ |
| Smoothened homolog | Smo | 1,2 | 3 | ↑ | ↑ |
| Protein NIMA-interacting 1 | Pin1 | 2 | 2.5 | ↑ | ↑ |
| Cyclin-dependent kinase 4 | Cdk4 | 2 | 2.5 | ↑ | ↑ |
| NIMA (never in mitosis gene a)-related kinase 6 | Nek6 | 2 | 2.3 | ↑ | ↑ |
| Aryl-hydrocarbon receptor | Ahr | 1 | 2.2 | ↑ | ↑ |
| Serpin peptidase inhibitor, clade E, member 1 | Serpine1 | 2 | 16.7 | ↑ | ↑ |
| Heat shock 27 kDa protein 1 | Hspb1 | 2 | 11 | ↑ | ↑ |
| Transforming growth factor, beta 1 | Tgfb1 | 2 | 4 | ↑ | ↑ |
| Granulin | Grn | 2 | 2.3 | ↑ | ↑ |
| Myeloid cell leukemia sequence 1 | Mcl1 | 2,3 | 4.3 | ↑ | ↑ |
| WNT1 inducible signaling pathway protein 1 | Wisp1 | 2,3 | 14.9 | ↑ | ↑ |
| Selectin E | Sele | 2 | 12.9 | ↑ | ↑ |
| Metastasis associated 1 | Mta1 | 2 | 9.6 | ↑ | ↑ |
| TIMP metallopeptidase inhibitor 1 | Timp1 | 2 | 8.6 | ↑ | ↑ |
| Integrin, alpha V | Itgav | 2 | 5.2 | ↑ | ↑ |
| Discs, large homolog 7 | Dlg7 | 2 | 4.3 | ↑ | ↑ |
| Lectin, galactoside-binding, soluble, 1 | Lgals1 | 2 | 3.7 | ↑ | ↑ |
| ADAM metallopeptidase domain 17 | Adam17 | 2 | 2.7 | ↑ | ↑ |
| Integrin, beta 1 | Itgb1 | 2 | 2.6 | ↑ | ↑ |
| Calponin 1, basic, smooth muscle | Cnn1 | 2 | 7 | ↑ | ↑ |
| Macrophage migration inhibitory factor | Mif | 2 | 3.2 | ↑ | ↑ |
| Collagen, type XVIII, alpha 1 | Col18a1 | 2 | 3.1 | ↑ | ↑ |
| Connective tissue growth factor | *Ctgf | 2 | 13.9 | ↑ | ↑ |
| Hexokinase 2 | Hk2 | 2 | 8.9 | ↑ | ↑ |
| Chemokine (C-C motif) ligand 20 | Ccl20 | 2 | 8 | ↑ | ↑ |
| v-jun sarcoma virus 17 oncogene homolog | *Jun | 2 | 6.9 | ↑ | ↑ |
| Methyl-CpG binding domain protein 2 | Mbd2 | 2 | 3 | ↑ | ↑ |
| TERF1 (TRF1)-interacting nuclear factor 2 | Tinf2 | 1 | 2.8 | ↑ | ↑ |
| FMS-like tyrosine kinase 1 | *Flt1 | 2 | 2.3 | ↑ | ↑ |
| Proteasome 26S subunit, non-ATPase, 10 | Psmd10 | 2 | 2 | ↑ | ↑ |
| Phosphatase and tensin homolog | Pten | 1,2 | 0.5 | ↓ | ↓ |
| Cold shock domain protein A | Csda | 1 | 0.5 | ↓ | ↓ |
| cAMP responsive element binding protein 3-like 3 | Creb3l3 | 1 | 0.4 | ↓ | ↓ |
| v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | Kit | 2 | 0.4 | ↓ | ↓ |
| Gap junction protein, beta 1, 32 kDa | Gjb1 | 1,2 | 0.2 | ↓ | ↓ |
| Trefoil factor 1 | Tff1 | 3 | 0.1 | ↓ | ↓ |
| Caspase 9, apoptosis-related cysteine peptidase | Casp9 | 2 | 0.5 | ↓ | ↓ |
| Deleted in liver cancer 1 | Dlc1 | 1,2 | 0.5 | ↓ | ↓ |
| B-cell CLL/lymphoma 2 | Bcl2 | 2 | 0.3 | ↓ | ↓ |
| Inhibitor of DNA binding 1 | Id1 | 1,2 | 0.3 | ↓ | ↓ |
| Protein tyrosine phosphatase, receptor type, H | Ptprh | 2 | 0.2 | ↓ | ↓ |
| CD74 molecule, major histocompatibility complex, class II invariant chain | Cd74 | 2 | 0.4 | ↓ | ↓ |
| Hepatocyte growth factor | *Hgf | 1,2 | 0.4 | ↓ | ↓ |
| Mannose-binding lectin (protein C) 2, soluble | Mbl2 | 2 | 0.2 | ↓ | ↓ |
| The contrary in gene expression trend | |||||
| Myelocytomatosis oncogene | Myc | 1,2 | 19.7 | ↓ | ↑ |
| Sprouty homolog 2 | Spry2 | 2 | 8.1 | ↓ | ↑ |
| Growth arrest and DNA-damage-inducible, beta | Gadd45b | 2 | 55.7 | ↓ | ↑ |
| Serine peptidase inhibitor, Kunitz type, 2 | Spint2 | 2 | 7.2 | ↓ | ↑ |
| MAD homolog 4 | Smad4 | 1,2 | 3 | ↓ | ↑ |
| Fibrinogen-like 1 | Fgl1 | 2 | 2.2 | ↓ | ↑ |
| Caspase 8, apoptosis-related cysteine peptidase | Casp8 | 2 | 10.6 | ↓ | ↑ |
| Interferon gamma | Ifng | 1,2 | 6.5 | ↓ | ↑ |
| Tumor protein p53 | Tp53 | 1,2,3 | 2.9 | ↓ | ↑ |
| Early growth response 1 | *Egr1 | 2 | 18.6 | ↓ | ↑ |
| Transcription factor 1, hepatic | Tcf1 | 2 | 6.8 | ↓ | ↑ |
| O-6-methylguanine-DNA methyltransferase | Mgmt | 2 | 4.3 | ↓ | ↑ |
| Glutathione S-transferase theta 1 | Gstt1 | 2 | 3.2 | ↓ | ↑ |
| Glutathione S-transferase M1 | Gstm1 | 2 | 2.2 | ↓ | ↑ |
| Wingless-type MMTV integration site family, member 1 | Wnt1 | 2 | 0.5 | ↑ | ↓ |
| SHC (Src homology 2 domain containing) | Shc1 | 2 | 0.5 | ↑ | ↓ |
| Transforming protein 1 | |||||
| Inhibitor of kappaB kinase beta | Ikbkb | 1,2 | 0.3 | ↑ | ↓ |
| FK506 binding protein 4, 59 kDa | Fkbp4 | 2 | 0.3 | ↑ | ↓ |
| Transcription factor 7-like 2 | Tcf7l2 | 2 | 0.2 | ↑ | ↓ |
| v-erb-b2 erythroblastic leukemia viral oncogene | Erbb2 | 3 | 0.1 | ↑ | ↓ |
| Homolog 2 | |||||
| Heat shock 70kDa protein 1A | Hspa1a | 2 | 0.2 | ↑ | ↓ |
| Heat shock 70kDa protein 5 | Hspa5 | 1,2 | 0.1 | ↑ | ↓ |
| High mobility group AT-hook 1 | Hmga1 | 2 | 0.4 | ↑ | ↓ |
| Ras homolog gene family, member C | Rhoc | 2 | 0.3 | ↑ | ↓ |
| Cortactin | Cttn | 2 | 0.1 | ↑ | ↓ |
| Serpin peptidase inhibitor, clade B, member 3 | Serpinb3 | 2 | 0.1 | ↑ | ↓ |
| Ephrin-B1 | Efnb1 | 2 | 0.4 | ↑ | ↓ |
| Coagulation factor II | F2 | 1,2 | 0.3 | ↑ | ↓ |
| Trefoil factor 3 | Tff3 | 2 | 0.3 | ↑ | ↓ |
| Forkhead box A2 | Foxa2 | 2 | 0.4 | ↑ | ↓ |
| Glycogen synthase kinase 3 beta | Gsk3b | 1,2 | 0.4 | ↑ | ↓ |
| ATP-binding cassette, sub-family B, member 1A | *Abcb1a | 1 | 0.2 | ↑ | ↓ |
| Solute carrier family 2 , member 1 | *Slc2a1 | 2 | 0.2 | ↑ | ↓ |
| Apolipoprotein E | *Apoe | 2 | 0.1 | ↑ | ↓ |
| The comparable in gene expression trend | |||||
| Lysosomal-associated protein transmembrane 4B | Laptm4b | 2 | 2.3,0.5 | ↑ | ↑↓ |
| Met proto-oncogene | *Met | 1,2,3 | 2.3,0.4 | ↑ | ↑↓ |
| Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (p105) | Nfkb1 | 2 | 2.3,0.4 | ↑ | ↑↓ |
| Acyl-CoA synthetase long-chain family member 4 | Acsl4 | 2 | 2.1,0.4 | ↑ | ↑↓ |
| X-box binding protein 1 | Xbp1 | 1 | 4.3,0.3 | ↑ | ↑↓ |
| Nerve growth factor, beta polypeptide | Ngfb | 2 | 3.7,0.5 | ↑ | ↑↓ |
| Stearoyl-Coenzyme A desaturase 1 | Scd1 | 1,2 | 3.5,0.3 | ↑ | ↑↓ |
| Telomerase reverse transcriptase | Tert | 1,2,3 | 5.3,0.3 | ↑ | ↑↓ |
| Telomeric repeat binding factor (NIMA-interacting) 1 | Terf1 | 1 | 2.2,0.4 | ↑ | ↑↓ |
| Cadherin 17 | Cdh17 | 2 | 26.1,0.2 | ↑ | ↑↓ |
| Glycoprotein (transmembrane) nmb | Gpnmb | 2 | 9.2,0.3 | ↑ | ↑↓ |
| Claudin 10 | Cldn10 | 2 | 6.5,0.3 | ↑ | ↑↓ |
| Plasminogen activator, urokinase | Plau | 2 | 3,0.4 | ↑ | ↑↓ |
| Secreted phosphoprotein 1 | Spp1 | 2,3 | 2.7,0.5 | ↑ | ↑↓ |
| Alpha-2-macroglobulin | *A2m | 2 | 46.2,0.4 | ↑ | ↑↓ |
| Chemokine (C-C motif) receptor 1 | Ccr1 | 2 | 27.9,0.4 | ↑ | ↑↓ |
| Matrix metallopeptidase 9 | Mmp9 | 1,2 | 9.5,0.5 | ↑ | ↑↓ |
| Angiopoietin 1 | *Angpt1 | 2 | 9.2,0.2 | ↑ | ↑↓ |
| Mucin 1, cell surface associated | Muc1 | 2,3 | 6.8,0.2 | ↑ | ↑↓ |
| Heparanase | *Hpse | 2 | 6.3,0.3 | ↑ | ↑↓ |
| Megalencephalic leukoencephalopathy with subcortical cysts 1 | Mlc1 | 2 | 4.3,0.4 | ↑ | ↑↓ |
| Kinase insert domain protein receptor | *Kdr | 2 | 2.4,0.4 | ↑ | ↑↓ |
| Prostaglandin-endoperoxide synthase 2 | Ptgs2 | 1,2,3 | 2.1,0.1 | ↑ | ↑↓ |
| Dual specificity phosphatase 1 | Dusp1 | 2 | 6,0.4 | ↓ | ↑↓ |
| Cyclin-dependent kinase inhibitor 1C | Cdkn1c | 2 | 2.8,0.1 | ↓ | ↑↓ |
| Growth arrest and DNA-damage-inducible, gamma | Gadd45g | 2 | 8,0.4 | ↓ | ↑↓ |
| Hepatic nuclear factor 4, alpha | Hnf4a | 2 | 4.5,0.1 | ↓ | ↑↓ |
| Runt-related transcription factor 3 | Runx3 | 1,2 | 4.3,0.5 | ↓ | ↑↓ |
| Insulin-like growth factor binding protein 3 | Igfbp3 | 1,2 | 2.7,0.4 | ↓ | ↑↓ |
| Suppressor of cytokine signaling 3 | *Socs3 | 2 | 2.5,0.1 | ↓ | ↑↓ |
| Suppressor of cytokine signaling 1 | *Socs1 | 1,2 | 2.4,0.5 | ↓ | ↑↓ |
| Fibroblast growth factor 2 | Fgf2 | 1,2 | 2.1,0.5 | ↓ | ↑↓ |
| Fragile histidine triad gene | Fhit | 1,2 | 7.8,0.1 | ↓ | ↑↓ |
| Gamma-glutamyltransferase 1 | Ggt1 | 2 | 3.4,0.2 | ↓ | ↑↓ |
| Bone morphogenetic protein 7 | Bmp7 | 2 | 3,0.4 | ↓ | ↑↓ |
| E74-like factor 1 (ets domain transcription factor) | Elf1 | 2 | 3,0.4 | ↓ | ↑↓ |
| CD80 molecule | Cd80 | 2 | 3,0.3 | ↓ | ↑↓ |
| Glycine N-methyltransferase | Gnmt | 2 | 2.5,0.4 | ↓ | ↑↓ |
| Acyl-Coenzyme A oxidase 1, palmitoyl | Acox1 | 2 | 2.3,0.5 | ↓ | ↑↓ |
Asterisks represent the reported genes associated with liver regeneration; LT: liver tumor; RRL: rat regenerating liver; 1: hepatocarcinogenesis; 2: hepatocellular carcinoma; 3: cholangiocarcinoma. ↑ represents genes up-regulated, ↓ down-regulated, and ↑↓ up-regulated at some time points and down-regulated at others during liver regeneration. Gene expression changes in liver tumors were obtained from scientific articles, and expression changes during liver regeneration were the result of microarray detection.
The relationship of LT-associated genes with LR
According to function feature and expression profiles of total 121 LT-associated genes in LR, they were divided into six classes and twenty-nine subclasses (Figure 2), and their expression changes in LR were present. Genes up-regulated in both LT and regenerating liver include nine cell proliferation-associated genes (1), four cell growth-associated genes (2), one apoptosis-associated gene (3), nine cell migration-associated genes (4), three angiogenesis-associated genes (5), and eight genes involved in other biological processes (6); Genes down-regulated in both LT and regenerating liver include six cell proliferation-associated genes (7), three apoptosis-associated genes (8), two differentiation-associated genes (9), and three genes with other functions (10); Genes down-regulated in LT but up-regulated in LR include two cell proliferation-associated genes (11), four cell growth-associated genes (12), three apoptosis-associated gene (13), and another five genes having other biological activities (14); Genes up-regulated in LT but down-regulated in LR include six cell proliferation-associated genes (15), two cell growth-associated genes (16), one apoptosis-associated gene (17), three cell migration-associated gene (18), three angiogenesis-associated genes (19), and five genes with other functions (20); Genes up-regulated in LT but up-regulated at some time points and down-regulated at others in LR include four cell proliferation-associated genes (21), three cell growth-associated genes (22), two telomerase-associated genes (23), five cell migration-associated genes (24), and nine genes participating in other actions (25). Genes down-regulated in LT but up-regulated at some time points and down-regulated at others during LR include two cell proliferation-associated genes (26), six cell growth-associated genes (27), one apoptosis-associated gene (28), and seven genes related to biological events differed from the above-mentioned actions (29).
Figure 2.

Correlation analysis of 121 liver tumor-associated genes with liver regeneration. Twenty-nine subcategories were obtained by the analysis for detection data of Rat Genome 230 2.0 array with Microsoft Excel. 1-6: 34 genes up-regulated in both liver tumor (LT) and rat regenerating liver (RRL); 7-10: 14 genes down-regulated in both LT and RRL; 11-14: 14 genes down-regulated in LT but up-regulated in RRL; 15-20: 20 genes up-regulated in LT but down-regulated in RRL; 21-25: 23 up-regulated genes in LT were up-regulated at some time points and down-regulated at others in RRL; 26-29: 16 down-regulated genes in LT were up-regulated at some time points and down-regulated at others in RRL. X-axis represents recovery time after PH (h); Y-axis shows logarithm ratio of the signal values of genes at each time point to control.
DISCUSSION
Generally, cell proliferation and growth was done in both LT and LR, but the former are malignant, and the latter are controlled stringently. According to our data, proliferation-promoting genes pcna, ccne1, cdk4, ahr, wee1, ccna2, pin1, nek6 and smo[17-22] were up-regulated in both LT and LR, and proliferation-inhibiting genes creb3l3, pten, kit, gjb1, tff1 and csda[23-28], were down-regulated in both, indicating that these genes promote cell proliferation in the two events. Notably, the abundance of CCNA2 mRNA in LT was approximately five-fold higher than that in normal liver[17], and it reached its peak with 45 folds of control at 66 h after PH, which might be associated with an increased proportion of regenerated hepatocytes. Growth-promoting genes hspb1, grn, tgfb1 and serpine1[29-31], whose expression levels were elevated in LT, were up-regulated at metaphase of LR. Among these four genes, serpine1 having the highest expression (16.7 folds higher than control) at 6 h following PH might explain why it played an important role in growth of the regenerated hepatocytes. Dysregulated expression of anti-apoptosis gene bcl-2 was present in LT as well as at metaphase and anaphase of LR, and another anti-apoptotic gene mcl1[32], whose change trend toward up-regulation in LT was identical to that in LR; and down-regulation of pro-apoptosis genes casp9 and dlc1[33,34] occurred in LT and the metaphase of LR, which supported the idea that mcl1, casp9 and dlc1 might are related with cell survival in the two events. The differentiation-related genes id1 and ptprh[35,36] down-regulation in LT and at forepart, metaphase and anaphase of LR suggested that they failed to promote cell differentiation in both events. Up-regulation of enhancement of hepatoma cell migration-related genes itgav, itgb1, adam17, dlg7, sele, mta1, wisp1 and lgals1[37-43] in LT and the entire LR, especially a sustained high-level expression (12-fold higher than control) of wisp1 at 48-60 h post-PH might imply the active cell migration in both LT and LR. According to up-regulated expression pattern in LT and almost the whole LR, metallopeptidase inhibitor timp1 was supposed to perform other biological functions except cell migration in the two events. Mif inducing angiogenesis of LT[44], cnn1 enhancing differentiation of vascular smooth muscle cells[45] and apoptosis-inhibiting gene col18a1 encoding endostatin[46] were up-regulated both in LT and in forepart of LR, which was presumably that the three genes might co-regulate angiogenesis in LT and LR.
Study demonstrated that six pro-proliferation genes ikbkb, shc1, erbb2, fkbp4, wnt1, tcf7l2[47-51] up-regulated in LT were down-regulated during LR, at the same time, the down-regulated genes myc and spry2 possessing anti-proliferation effect[52,53] in LT were up-regulated almost during the whole LR; two growth-promoting genes hspa5 and hspa1a[29,54] and four growth inhibitory genes gadd45b, fgl1, spint2 and smad4[55-58] were respectively up-regulated and down-regulated in LT, whose expression correspondingly underwent opposite trend at metaphase of LR comparing with LT, which was supposed to be closely associated with the differences in proliferation and growth between hepatoma cells and regenerating hepatocytes. Particularly, the expression abundance of gadd45b in human hepatocellular carcinoma was fifteen-fold lower than control[55], just the contrary, its expression reached climax (nearly 56-fold over the control) at 2 h in rat LR, demonstrating there was a significant distinction in gadd45b expression change between normal and transforming liver cells. The down-regulated pro-apoptotic genes tp53, ifng and casp8[59-61] and the up-regulated apoptosis-inhibitory gene serpinb3[62] in LT were respectively up-regulated and down-regulated at metaphase and anaphase of LR might account for the suppression of apoptosis in LT and enhancement of apoptosis at metaphase and anaphase of LR. In addition, casp8, inactivated caused by frame-shift mutation in hepatocellular carcinoma[61], was up-regulated to its highest levels (10.6 folds higher than control) at 48 h post-PH, and expression of serpinb3 declined to the lowest point (11.4 folds lower than control) at 48 h, signifying the important regulatory effect of the two genes on liver mass. Contribution of efnb1 and tff3 in neovasculargenesis activity[63,64] and the crucial role for f2 in maintenance of vascular integrity[65] were helpful for understanding the hypothesis that the three up-regulated genes in LT down-regulated at metaphase and anaphase of LR implied the control of blood-vessel growth serving as one of modulation pathways of regenerated liver mass. Three hepatoma cell migration and invasion-associated genes hmga1, cttn and rhoc up-regulated in LT[66,67] and down-regulated in LR possibly showed the stronger migration ability of hepatoma cells.
Four up-regulated in LT genes promoting hepatoma cell proliferation including met, laptm4b, nfkb1 and acsl4[68,69], revealed down-regulation at metaphase and up-regulation at anaphase of LR, and another two inhibitory genes dusp1 and cdkn1c[70] were up/down-regulated in LR, i.e. the former was up-regulated at 0.5-12 and 24 h, and down-regulated at 54-60 h, while the latter was down-regulated at 6-18 h and up-regulated at 30 and 42 h; Three up-regulated scd1, xbp1 and ngfb genes involved in hepatoma cell growth in LT[54,71,72] were up-regulated at forepart, prophase and metaphase, and down-regulated at some time points in the late phase of LR, while another six negative regulatory genes including socs1, socs3, gadd45g, igfbp3, runx3 and hnf4a[73-77] down-regulated in LT had a significant increase in expression at some time points and significant decrease at others during LR. The more complicated expression of these genes during the proliferation and growth of regenerating liver cells comparing with that of hepatoma cells concluded from the above results was presumably consistent with the further improved control mechanism upon proliferation and growth of regenerating liver cells than that of hepatoma cells. Telomerase activity of TERT was interfered by terf1 expression product[78], and the two were up-regulated in both LT and the metaphase of LR, while down-regulated at anaphase, indicating that the balance of quantity of the two gene products was essential for maintaining telomere stability. The up-regulated genes spp1, plau and gpnmb promoting hepatoma cell migration and invasion[79,80] as well as the up-regulated intercellular junction-involved cdh17 and cldn10 genes[47,81] were up-regulated at forepart, prophase and some time points after 16h, and down-regulated at other points after 16 h, possibly illustrating that the similar cell migration and interactions in LT occurred at forepart and prophase of LR, however the difference emerged when entering the metaphase of LR. The dysregulated gene fgf2[82] promoting hepatoma cell apoptosis in LT was up-regulated at 4 h post-PH, consistent with the enhanced apoptotic action of regenerating liver cell at the forepart of LR, demonstrating that it acted as a key gene involved in the regulation of apoptosis.
In conclusion, at transcriptional level, while decrease of apoptosis occurring in liver tumors, the process was still going on in LR; as far as cell growth, proliferation, differentiation, migration and angiogenesis are concerned, not only resemblances but differences exist between LT and LR. Especially, expressions of the genes, such as ccna2, serpine1, wisp1, gadd45b, casp8 and serpinb3, display marked changes in two events, so their actions deserve the further study. Of course, the process of DNA→mRNA→protein→function could be influenced by many factors including gene mutation, protein interaction etc. Therefore, the further analyses are required for confirming the above results using techniques such as gene addition, knock-out, RNAi, etc.
COMMENTS
Background
The liver is susceptible to tumorigenesis, and it’s also an organ with strong regenerating capacity. Both hepatocarcinogenesis and LR are associated with cell proliferation and growth.
Research frontiers
Most research works had been done on the mechanism of hepatocarcinogenesis and LR, but heretofore no research report was found in investigating the correlation between them.
Innovations and breakthroughs
121 genes associated with both hepatocarcinogenesis and LR were found. Their expression changes in LR were analyzed by Rat Genome 230 2.0 Array, and their expression similarities and differences in hepatocarcinogenesis and LR were compared.
Applications
Our results may provide basic and useful data for further research on both LT therapy and LR mechanism.
Terminology
PH model, an effective surgical operation to trigger LR, was established by Higgins and Anderson in 1931. The hepatectomized rat, whose left and middle lobes of liver were removed, can recover its liver mass after about a week, so it’s widely used to investigate LR mechanism.
Peer review
This is an important work examining common genes associated with HCC and LR.
Footnotes
Supported by the National Basic Research 973 Pre-research Program of China, No. 2006CB708506
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
References
- 1.Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- 2.Higgins GM, Anderson RM. Experimental pathology of the liver: restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 3.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T, Tsukamoto I. Apoptosis induced by 5-(N,N-hexamethylene)-amiloride in regenerating liver after partial hepatectomy. Eur J Pharmacol. 2004;503:1–7. doi: 10.1016/j.ejphar.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Lai HS, Chen Y, Lin WH, Chen CN, Wu HC, Chang CJ, Lee PH, Chang KJ, Chen WJ. Quantitative gene expression analysis by cDNA microarray during liver regeneration after partial hepatectomy in rats. Surg Today. 2005;35:396–403. doi: 10.1007/s00595-004-2962-7. [DOI] [PubMed] [Google Scholar]
- 6.Xu CS, Chang CF, Yuan JY, Li WQ, Han HP, Yang KJ, Zhao LF, Li YC, Zhang HY, Rahman S, et al. Expressed genes in regenerating rat liver after partial hepatectomy. World J Gastroenterol. 2005;11:2932–2940. doi: 10.3748/wjg.v11.i19.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 8.Xu CS, Zhao LF, Yang KJ, Zhang JB. The origination and action of the hepatic stems cells. Shi Yan Sheng Wu Xue Bao. 2004;37:72–77. [PubMed] [Google Scholar]
- 9.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- 10.Knepp JH, Geahr MA, Forman MS, Valsamakis A. Comparison of automated and manual nucleic acid extraction methods for detection of enterovirus RNA. J Clin Microbiol. 2003;41:3532–3536. doi: 10.1128/JCM.41.8.3532-3536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuyts S, Van Mellaert L, Lambin P, Anné J. Efficient isolation of total RNA from Clostridium without DNA contamination. J Microbiol Methods. 2001;44:235–238. doi: 10.1016/s0167-7012(01)00219-6. [DOI] [PubMed] [Google Scholar]
- 12.Arkin A, Ross J, McAdams HH. Stochastic kinetic analysis of developmental pathway bifurcation in phage lambda-infected Escherichia coli cells. Genetics. 1998;149:1633–1648. doi: 10.1093/genetics/149.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Roden J, Shapiro BE, Wold BJ, Bhatia S, Forman SJ, Bhatia R. Reproducibility, fidelity, and discriminant validity of mRNA amplification for microarray analysis from primary hematopoietic cells. J Mol Diagn. 2005;7:48–56. doi: 10.1016/S1525-1578(10)60008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins JF. Gene chip analyses reveal differential genetic responses to iron deficiency in rat duodenum and jejunum. Biol Res. 2006;39:25–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner T. Cluster analysis and promoter modelling as bioinformatics tools for the identification of target genes from expression array data. Pharmacogenomics. 2001;2:25–36. doi: 10.1517/14622416.2.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Masaki T, Shiratori Y, Rengifo W, Igarashi K, Yamagata M, Kurokohchi K, Uchida N, Miyauchi Y, Yoshiji H, Watanabe S, et al. Cyclins and cyclin-dependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology. 2003;37:534–543. doi: 10.1053/jhep.2003.50112. [DOI] [PubMed] [Google Scholar]
- 18.Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L, Schwarz M. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64:4707–4710. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 19.Payraudeau V, Sarsat JP, Sobczak J, Bréchot C, Albaladéjo V. Cyclin A2 and c-myc mRNA expression in ethinyl estradiol induced liver proliferation. Mol Cell Endocrinol. 1998;143:107–116. doi: 10.1016/s0303-7207(98)00136-1. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Li L, Zhang Y, Yang H, Wei Y, Zhang L, Liu X, Yu L. Interaction of Pin1 with Nek6 and characterization of their expression correlation in Chinese hepatocellular carcinoma patients. Biochem Biophys Res Commun. 2006;341:1059–1065. doi: 10.1016/j.bbrc.2005.12.228. [DOI] [PubMed] [Google Scholar]
- 21.Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem. 2003;278:52454–52460. doi: 10.1074/jbc.M308080200. [DOI] [PubMed] [Google Scholar]
- 22.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS, et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–757. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 23.Chin KT, Zhou HJ, Wong CM, Lee JM, Chan CP, Qiang BQ, Yuan JG, Ng IO, Jin DY. The liver-enriched transcription factor CREB-H is a growth suppressor protein underexpressed in hepatocellular carcinoma. Nucleic Acids Res. 2005;33:1859–1873. doi: 10.1093/nar/gki332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman MA, Kyriazanos ID, Ono T, Yamanoi A, Kohno H, Tsuchiya M, Nagasue N. Impact of PTEN expression on the outcome of hepatitis C virus-positive cirrhotic hepatocellular carcinoma patients: possible relationship with COX II and inducible nitric oxide synthase. Int J Cancer. 2002;100:152–157. doi: 10.1002/ijc.10458. [DOI] [PubMed] [Google Scholar]
- 25.Chung CY, Yeh KT, Hsu NC, Chang JH, Lin JT, Horng HC, Chang CS. Expression of c-kit protooncogene in human hepatocellular carcinoma. Cancer Lett. 2005;217:231–236. doi: 10.1016/j.canlet.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 26.Dagli ML, Yamasaki H, Krutovskikh V, Omori Y. Delayed liver regeneration and increased susceptibility to chemical hepatocarcinogenesis in transgenic mice expressing a dominant-negative mutant of connexin32 only in the liver. Carcinogenesis. 2004;25:483–492. doi: 10.1093/carcin/bgh050. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki M, Tsuneyama K, Nakanuma Y. Aberrant expression of trefoil factor family 1 in biliary epithelium in hepatolithiasis and cholangiocarcinoma. Lab Invest. 2003;83:1403–1413. doi: 10.1097/01.lab.0000092230.59485.9e. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi J, Kajino K, Umeda T, Takano S, Arakawa Y, Kudo M, Hino O. Somatic mutation and SNP in the promoter of dbpA and human hepatocarcinogenesis. Int J Oncol. 2002;21:847–850. [PubMed] [Google Scholar]
- 29.Luk JM, Lam CT, Siu AF, Lam BY, Ng IO, Hu MY, Che CM, Fan ST. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006;6:1049–1057. doi: 10.1002/pmic.200500306. [DOI] [PubMed] [Google Scholar]
- 30.Cheung ST, Wong SY, Leung KL, Chen X, So S, Ng IO, Fan ST. Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma. Clin Cancer Res. 2004;10:7629–7636. doi: 10.1158/1078-0432.CCR-04-0960. [DOI] [PubMed] [Google Scholar]
- 31.Sugano Y, Matsuzaki K, Tahashi Y, Furukawa F, Mori S, Yamagata H, Yoshida K, Matsushita M, Nishizawa M, Fujisawa J, et al. Distortion of autocrine transforming growth factor beta signal accelerates malignant potential by enhancing cell growth as well as PAI-1 and VEGF production in human hepatocellular carcinoma cells. Oncogene. 2003;22:2309–2321. doi: 10.1038/sj.onc.1206305. [DOI] [PubMed] [Google Scholar]
- 32.Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, Bodingbauer M, Crevenna R, Monia BP, Peck-Radosavljevic M, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Poole BD, Karetnyi YV, Naides SJ. Parvovirus B19-induced apoptosis of hepatocytes. J Virol. 2004;78:7775–7783. doi: 10.1128/JVI.78.14.7775-7783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Thorgeirsson SS, Popescu NC. Restoration of DLC-1 gene expression induces apoptosis and inhibits both cell growth and tumorigenicity in human hepatocellular carcinoma cells. Oncogene. 2004;23:1308–1313. doi: 10.1038/sj.onc.1207246. [DOI] [PubMed] [Google Scholar]
- 35.Damdinsuren B, Nagano H, Kondo M, Yamamoto H, Hiraoka N, Yamamoto T, Marubashi S, Miyamoto A, Umeshita K, Dono K, et al. Expression of Id proteins in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Int J Oncol. 2005;26:319–327. [PubMed] [Google Scholar]
- 36.Nagano H, Noguchi T, Inagaki K, Yoon S, Matozaki T, Itoh H, Kasuga M, Hayashi Y. Downregulation of stomach cancer-associated protein tyrosine phosphatase-1 (SAP-1) in advanced human hepatocellular carcinoma. Oncogene. 2003;22:4656–4663. doi: 10.1038/sj.onc.1206588. [DOI] [PubMed] [Google Scholar]
- 37.Nejjari M, Hafdi Z, Gouysse G, Fiorentino M, Béatrix O, Dumortier J, Pourreyron C, Barozzi C, D'errico A, Grigioni WF, et al. Expression, regulation, and function of alpha V integrins in hepatocellular carcinoma: an in vivo and in vitro study. Hepatology. 2002;36:418–426. doi: 10.1053/jhep.2002.34611. [DOI] [PubMed] [Google Scholar]
- 38.Fu BH, Wu ZZ, Dong C. Integrin beta1 mediates hepatocellular carcinoma cells chemotaxis to laminin. Hepatobiliary Pancreat Dis Int. 2004;3:548–551. [PubMed] [Google Scholar]
- 39.Zhao L, Qin LX, Ye QH, Zhu XQ, Zhang H, Wu X, Chen J, Liu YK, Tang ZY. KIAA0008 gene is associated with invasive phenotype of human hepatocellular carcinoma--a functional analysis. J Cancer Res Clin Oncol. 2004;130:719–727. doi: 10.1007/s00432-004-0595-2. [DOI] [PubMed] [Google Scholar]
- 40.Zhang BH, Chen H, Yao XP, Cong WM, Wu MC. E-selectin and its ligand-sLeX in the metastasis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:80–82. [PubMed] [Google Scholar]
- 41.Hamatsu T, Rikimaru T, Yamashita Y, Aishima S, Tanaka S, Shirabe K, Shimada M, Toh Y, Sugimachi K. The role of MTA1 gene expression in human hepatocellular carcinoma. Oncol Rep. 2003;10:599–604. [PubMed] [Google Scholar]
- 42.Cervello M, Giannitrapani L, Labbozzetta M, Notarbartolo M, D'Alessandro N, Lampiasi N, Azzolina A, Montalto G. Expression of WISPs and of their novel alternative variants in human hepatocellular carcinoma cells. Ann N Y Acad Sci. 2004;1028:432–439. doi: 10.1196/annals.1322.051. [DOI] [PubMed] [Google Scholar]
- 43.Kondoh N, Hada A, Ryo A, Shuda M, Arai M, Matsubara O, Kimura F, Wakatsuki T, Yamamoto M. Activation of Galectin-1 gene in human hepatocellular carcinoma involves methylation-sensitive complex formations at the transcriptional upstream and downstream elements. Int J Oncol. 2003;23:1575–1583. [PubMed] [Google Scholar]
- 44.Hira E, Ono T, Dhar DK, El-Assal ON, Hishikawa Y, Yamanoi A, Nagasue N. Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer. 2005;103:588–598. doi: 10.1002/cncr.20818. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki Y, Yamamura H, Kawakami Y, Yamada T, Hiratsuka M, Kameyama M, Ohigashi H, Ishikawa O, Imaoka S, Ishiguro S, et al. Expression of smooth muscle calponin in tumor vessels of human hepatocellular carcinoma and its possible association with prognosis. Cancer. 2002;94:1777–1786. doi: 10.1002/cncr.10402. [DOI] [PubMed] [Google Scholar]
- 46.Hu TH, Huang CC, Wu CL, Lin PR, Liu SY, Lin JW, Chuang JH, Tai MH. Increased endostatin/collagen XVIII expression correlates with elevated VEGF level and poor prognosis in hepatocellular carcinoma. Mod Pathol. 2005;18:663–672. doi: 10.1038/modpathol.3800336. [DOI] [PubMed] [Google Scholar]
- 47.Wang XQ, Luk JM, Leung PP, Wong BW, Stanbridge EJ, Fan ST. Alternative mRNA splicing of liver intestine-cadherin in hepatocellular carcinoma. Clin Cancer Res. 2005;11:483–489. [PubMed] [Google Scholar]
- 48.Wong N, Chan A, Lee SW, Lam E, To KF, Lai PB, Li XN, Liew CT, Johnson PJ. Positional mapping for amplified DNA sequences on 1q21-q22 in hepatocellular carcinoma indicates candidate genes over-expression. J Hepatol. 2003;38:298–306. doi: 10.1016/s0168-8278(02)00412-9. [DOI] [PubMed] [Google Scholar]
- 49.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 50.Cui J, Zhou X, Liu Y, Tang Z, Romeih M. Wnt signaling in hepatocellular carcinoma: analysis of mutation and expression of beta-catenin, T-cell factor-4 and glycogen synthase kinase 3-beta genes. J Gastroenterol Hepatol. 2003;18:280–287. doi: 10.1046/j.1440-1746.2003.02973.x. [DOI] [PubMed] [Google Scholar]
- 51.Yau TO, Chan CY, Chan KL, Lee MF, Wong CM, Fan ST, Ng IO. HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene. 2005;24:1607–1614. doi: 10.1038/sj.onc.1208340. [DOI] [PubMed] [Google Scholar]
- 52.Ikeguchi M, Hirooka Y. Expression of c-myc mRNA in hepatocellular carcinomas, noncancerous livers, and normal livers. Pathobiology. 2004;71:281–286. doi: 10.1159/000080063. [DOI] [PubMed] [Google Scholar]
- 53.Fong CW, Chua MS, McKie AB, Ling SH, Mason V, Li R, Yusoff P, Lo TL, Leung HY, So SK, et al. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res. 2006;66:2048–2058. doi: 10.1158/0008-5472.CAN-05-1072. [DOI] [PubMed] [Google Scholar]
- 54.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 55.Qiu W, David D, Zhou B, Chu PG, Zhang B, Wu M, Xiao J, Han T, Zhu Z, Wang T, et al. Down-regulation of growth arrest DNA damage-inducible gene 45beta expression is associated with human hepatocellular carcinoma. Am J Pathol. 2003;162:1961–1974. doi: 10.1016/s0002-9440(10)64329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan J, Yu Y, Wang N, Chang Y, Ying H, Liu W, He J, Li S, Jiang W, Li Y, et al. LFIRE-1/HFREP-1, a liver-specific gene, is frequently downregulated and has growth suppressor activity in hepatocellular carcinoma. Oncogene. 2004;23:1939–1949. doi: 10.1038/sj.onc.1207306. [DOI] [PubMed] [Google Scholar]
- 57.Fukai K, Yokosuka O, Chiba T, Hirasawa Y, Tada M, Imazeki F, Kataoka H, Saisho H. Hepatocyte growth factor activator inhibitor 2/placental bikunin (HAI-2/PB) gene is frequently hypermethylated in human hepatocellular carcinoma. Cancer Res. 2003;63:8674–8679. [PubMed] [Google Scholar]
- 58.Park DY, Lee CH, Sol MY, Suh KS, Yoon SY, Kim JW. Expression and localization of the transforming growth factor-beta type I receptor and Smads in preneoplastic lesions during chemical hepatocarcinogenesis in rats. J Korean Med Sci. 2003;18:510–519. doi: 10.3346/jkms.2003.18.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu Y, Deng W, Kawarada Y, Kawagoe M, Ma YZ, Li X, Guo N, Kameda T, Terada K, Sugiyama T. Mutation and expression of the p53 gene during chemical hepatocarcinogenesis in F344 rats. Biochim Biophys Acta. 2003;1628:40–49. doi: 10.1016/s0167-4781(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 60.Detjen KM, Murphy D, Welzel M, Farwig K, Wiedenmann B, Rosewicz S. Downregulation of p21(waf/cip-1) mediates apoptosis of human hepatocellular carcinoma cells in response to interferon-gamma. Exp Cell Res. 2003;282:78–89. doi: 10.1016/s0014-4827(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 61.Soung YH, Lee JW, Kim SY, Sung YJ, Park WS, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene. 2005;24:141–147. doi: 10.1038/sj.onc.1208244. [DOI] [PubMed] [Google Scholar]
- 62.Pontisso P, Calabrese F, Benvegnù L, Lise M, Belluco C, Ruvoletto MG, Marino M, Valente M, Nitti D, Gatta A, et al. Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br J Cancer. 2004;90:833–837. doi: 10.1038/sj.bjc.6601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawai Y, Tamura S, Fukui K, Ito N, Imanaka K, Saeki A, Sakuda S, Kiso S, Matsuzawa Y. Expression of ephrin-B1 in hepatocellular carcinoma: possible involvement in neovascularization. J Hepatol. 2003;39:991–996. doi: 10.1016/s0168-8278(03)00498-7. [DOI] [PubMed] [Google Scholar]
- 64.Khoury T, Chadha K, Javle M, Donohue K, Levea C, Iyer R, Okada H, Nagase H, Tan D. Expression of intestinal trefoil factor (TFF-3) in hepatocellular carcinoma. Int J Gastrointest Cancer. 2005;35:171–177. doi: 10.1385/IJGC:35:3:171. [DOI] [PubMed] [Google Scholar]
- 65.Tang W, Miki K, Kokudo N, Sugawara Y, Imamura H, Minagawa M, Yuan LW, Ohnishi S, Makuuchi M. Des-gamma-carboxy prothrombin in cancer and non-cancer liver tissue of patients with hepatocellular carcinoma. Int J Oncol. 2003;22:969–975. [PubMed] [Google Scholar]
- 66.Chuma M, Saeki N, Yamamoto Y, Ohta T, Asaka M, Hirohashi S, Sakamoto M. Expression profiling in hepatocellular carcinoma with intrahepatic metastasis: identification of high-mobility group I(Y) protein as a molecular marker of hepatocellular carcinoma metastasis. Keio J Med. 2004;53:90–97. doi: 10.2302/kjm.53.90. [DOI] [PubMed] [Google Scholar]
- 67.Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, Ohta T, Asaka M, Hirohashi S. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–636. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 68.Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G, Sottile A, Naldini L, Comoglio PM. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434:396–400. doi: 10.1038/nature03357. [DOI] [PubMed] [Google Scholar]
- 69.Kasper G, Vogel A, Klaman I, Gröne J, Petersen I, Weber B, Castaños-Vélez E, Staub E, Mennerich D. The human LAPTM4b transcript is upregulated in various types of solid tumours and seems to play a dual functional role during tumour progression. Cancer Lett. 2005;224:93–103. doi: 10.1016/j.canlet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Tsujita E, Taketomi A, Gion T, Kuroda Y, Endo K, Watanabe A, Nakashima H, Aishima S, Kohnoe S, Maehara Y. Suppressed MKP-1 is an independent predictor of outcome in patients with hepatocellular carcinoma. Oncology. 2005;69:342–347. doi: 10.1159/000089766. [DOI] [PubMed] [Google Scholar]
- 71.Falvella FS, Pascale RM, Gariboldi M, Manenti G, De Miglio MR, Simile MM, Dragani TA, Feo F. Stearoyl-CoA desaturase 1 (Scd1) gene overexpression is associated with genetic predisposition to hepatocarcinogenesis in mice and rats. Carcinogenesis. 2002;23:1933–1936. doi: 10.1093/carcin/23.11.1933. [DOI] [PubMed] [Google Scholar]
- 72.Tokusashi Y, Asai K, Tamakawa S, Yamamoto M, Yoshie M, Yaginuma Y, Miyokawa N, Aoki T, Kino S, Kasai S, et al. Expression of NGF in hepatocellular carcinoma cells with its receptors in non-tumor cell components. Int J Cancer. 2005;114:39–45. doi: 10.1002/ijc.20685. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y, Sata M, Nagai H, Yoshimura A. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J Exp Med. 2004;199:1701–1707. doi: 10.1084/jem.20031675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 75.Sun L, Gong R, Wan B, Huang X, Wu C, Zhang X, Zhao S, Yu L. GADD45gamma, down-regulated in 65% hepatocellular carcinoma (HCC) from 23 chinese patients, inhibits cell growth and induces cell cycle G2/M arrest for hepatoma Hep-G2 cell lines. Mol Biol Rep. 2003;30:249–253. doi: 10.1023/a:1026370726763. [DOI] [PubMed] [Google Scholar]
- 76.Mori T, Nomoto S, Koshikawa K, Fujii T, Sakai M, Nishikawa Y, Inoue S, Takeda S, Kaneko T, Nakao A. Decreased expression and frequent allelic inactivation of the RUNX3 gene at 1p36 in human hepatocellular carcinoma. Liver Int. 2005;25:380–388. doi: 10.1111/j.1478-3231.2005.1059.x. [DOI] [PubMed] [Google Scholar]
- 77.Lazarevich NL, Cheremnova OA, Varga EV, Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI, Engelhardt NV, Duncan SA. Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors. Hepatology. 2004;39:1038–1047. doi: 10.1002/hep.20155. [DOI] [PubMed] [Google Scholar]
- 78.Oh BK, Kim YJ, Park C, Park YN. Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is related to telomere shortening during human multistep hepatocarcinogenesis. Am J Pathol. 2005;166:73–80. doi: 10.1016/S0002-9440(10)62233-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salvi A, Arici B, De Petro G, Barlati S. Small interfering RNA urokinase silencing inhibits invasion and migration of human hepatocellular carcinoma cells. Mol Cancer Ther. 2004;3:671–678. [PubMed] [Google Scholar]
- 80.Onaga M, Ido A, Hasuike S, Uto H, Moriuchi A, Nagata K, Hori T, Hayash K, Tsubouchi H. Osteoactivin expressed during cirrhosis development in rats fed a choline-deficient, L-amino acid-defined diet, accelerates motility of hepatoma cells. J Hepatol. 2003;39:779–785. doi: 10.1016/s0168-8278(03)00361-1. [DOI] [PubMed] [Google Scholar]
- 81.Cheung ST, Leung KL, Ip YC, Chen X, Fong DY, Ng IO, Fan ST, So S. Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clin Cancer Res. 2005;11:551–556. [PubMed] [Google Scholar]
- 82.Lai JP, Chien JR, Moser DR, Staub JK, Aderca I, Montoya DP, Matthews TA, Nagorney DM, Cunningham JM, Smith DI, et al. hSulf1 Sulfatase promotes apoptosis of hepatocellular cancer cells by decreasing heparin-binding growth factor signaling. Gastroenterology. 2004;126:231–248. doi: 10.1053/j.gastro.2003.09.043. [DOI] [PubMed] [Google Scholar]


