Abstract
AIM: To investigate the role of second-look laparoscopy in patients with acute mesenteric ischemia (AMI).
METHODS: Between January 2000 and November 2005, 71 patients were operated for the treatment of AMI. The indications for a second-look were low flow state, bowel resection and anastomosis or mesenteric thromboembolectomy performed during the first operation. Regardless of the clinical course of patients, the second-look laparoscopic examination was performed 72 h post-operatively at the bed side in the ICU or operating room.
RESULTS: The average time of admission to the hospital after the initiation of symptoms was 3 d (range, 5 h-9 d). In 14 patients, laparotomy was performed. In 11 patients, small and/or large bowel necrosis was detected and initial resection and anastomosis were conducted. A low flow state was observed in two patients and superior mesenteric artery thromboembolectomy with small bowel resection was performed in one patient. In 13 patients, a second-look laparoscopic examination revealed normal bowel viability, but in one patient, intestinal necrosis was detected. In two of the patients, a third operation was necessary to correct anastomotic leakage. The overall complication rate was 42.8%, and in-hospital mortality rate was 57.1% (n = 6).
CONCLUSION: Second-look laparoscopy is a minimally invasive, technically simple procedure that is performed for diagnostic as well as therapeutic purposes. The simplicity and ease of this method may encourage wider application to benefit more patients. However, the timing of a second-look procedure is unclear particularly in a patient with anastomosis.
Keywords: Acute mesenteric ischemia, Second-look laparoscopy, Minimally invasive, Planned, Low flow state
INTRODUCTION
Acute mesenteric ischemia (AMI) resulting in intestinal ischemia or infarction is associated with an extremely serious prognosis and mortality rate ranging from 40%-100%[1-3]. Acute mesenteric vascular ischemic diseases are diagnosed more commonly as a consequence of the aging population and often result in emergency bowel resection. Abdominal second-look may occasionally be necessary in cases of doubtful bowel viability or intra-abdominal sepsis after primary anastomosis[4,5]. In 1965, Shaw[6] introduced the “second-look laparotomy” to overcome the difficulty in assessing the adequacy of bowel resection during surgery.
Second-look entails early surgical re-exploration to check the viability of intestinal loops and is the mainstay of AMI surgical treatment[7,8]. When a second-look surgery is indicated, second-look laparoscopy may be a useful alternative to conventional surgery, because it prevents critically ill patients from the trauma and risks of re-laparotomy and can be performed as a bed-side operation in the intensive care unit[4]. In this study, we aimed to determine the outcome of patients with AMI with or without bowel necrosis, who were subjected to a second-look laparoscopy.
MATERIALS AND METHODS
Between January 2000 and November 2005, 71 patients were operated to treat acute mesenteric ischemia (AMI) at Istanbul University, Istanbul Faculty of Medicine, Trauma and Emergency Surgery Service. Triple-contrast computed tomography (CT) scanning or CT angiography was used to confirm either arterial occlusion or bowel changes compatible with AMI. Fifty-seven patients were excluded and did not undergo a second-look laparoscopy because the bowel resection required an ostomy during the first procedure. The remaining 14 patients underwent a second-look laparoscopic examination. In this study, we only discuss those 14 patients, who underwent a second-look laparoscopy.
In our clinic, our policy is to perform a second-look laparoscopy for all patients operated on for AMI. Regardless of the clinical course of patients during the first operation when bowel viability was suspected and a low flow state was detected or bowel resection and anastomosis were performed, we performed a second-look laparoscopy within 72 h following the first operation at the bed side in the ICU or operating room. At the end of the operation, a 10-mm laparoscopic trocar was inserted into the left lower quadrant of the abdomen prior to closing the abdominal wall. Data were collected on patients’ demographics, co-morbid diseases, clinical signs and symptoms, intra-operative findings and hospital course.
All patients were given a low molecular weight heparin (Enoxaparin sodium-Clexane®, 1 mg/kg per day) treatment once AMI was diagnosed and continued on enoxaparin until the patient received an oral anticoagulant (Warfarin-Na), if indicated. When the patient was stabilized, an echocardiography was performed. Mortality was defined as in-hospital death. The study was approved by the Institutional Review Board.
RESULTS
There were nine men and five women with a median age of 68 years (range, 45-76 years). The median hospital stay was 16 d (range, 1-52 d). The most common co-morbid diseases were hypertension (HTN) in 7 (50%) patients (Table 1). Abdominal pain was present in all of the patients. Nausea was the second most frequent symptom and observed in 10 (71.4%) patients, followed by vomiting in 7 (50%) patients, and bloody diarrhea in 3 (21.4%) patients. The median time of admission after the onset of symptoms was 3 d (range, 5 h-9 d).
Table 1.
Clinical characteristics and outcome of the fourteen patients
| No. | Comorbid disease | Duration of symptom onset before admission to hospital | Duration of hospital stay (d) | Results of second-look | Result |
| 1 | - | 4 d | 13 | Normal | |
| 2 | IHD | 7 d | 13 | Normal | |
| 3 | IHD + HT | 5 h | 52 | Normal | Died |
| 4 | - | 1 h | 23 | Normal | Died |
| 5 | Acute pancreatitis, IHD, HTN | 1 h | 18 | Normal | Died |
| 6 | IHD | 8 h | 10 | Normal | |
| 7 | Epilepsy | 3 d | 10 | Normal | |
| 8 | DM + HTN + AF | 10 h | 10 | Normal | |
| 9 | HTN+AF | 2 d | 10 | Normal | |
| 10 | DM + HTN + IHD | 7 d | 24 | Normal | Died |
| 11 | DM | 7 d | 8 | Normal | |
| 12 | - | 9 d | 38 | Partial small intestine resection | Died |
| 13 | DM + IHD + HTN | 2 d | 16 | Normal | |
| 14 | DM + HTN + AF + Toxic goiter + Asthma | 3 d | 17 | Normal | Died |
IHD: Ischemic heart disease; HTN: Hypertension; DM: Diabetes mellitus; AF: Atrial fibrilation.
In 11 patients, small and/or large bowel necrosis was detected. Bowel resection and primary anastomosis were performed during the first procedure. In two patients, non-occlusive mesenteric ischemia (NOMI) without bowel necrosis was detected. In one patient, who was admitted to the emergency service within 3 h following abdominal pain, superior mesenteric artery (SMA) thromboembolectomy was performed because of an embolism in the SMA.
In 13 patients, a second-look laparoscopic examination showed normal viable intestinal loops and a normal healing anastomosis (Figure 1). In one patient, who previously underwent SMA thromboembolectomy, intestinal necrosis was found and spanned the distance from 70 cm distal to the ligament of Treitz to 10 cm proximal to the ileocecal valve, and therefore a partial small bowel resection with end jejunostomy and end ileostomy were performed. In two of the patients, the third operation was required due to peritonitis and leucocytosis, an anastomotic leakage was found in both patients on 6th post-operative day; so ileostomy or colostomy was performed.
Figure 1.
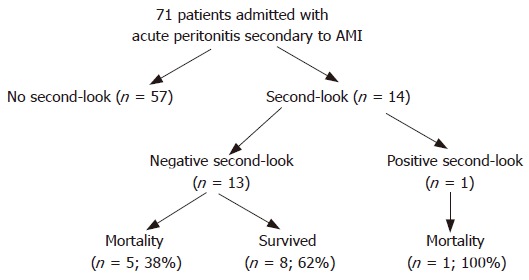
Outcome of patients with acute mesenteric ischemia treated with a second-look procedure.
Overall in-hospital mortality was reported in 6 (57.1%) patients. Multiorgan failure caused death in 4 (66.6%) patients, being the most common cause of mortality. One patient died secondary to myocardial infarction and one died from sepsis. The overall complication rate was 42.8%. Peptic ulcer perforation occurred in one patient, who had previously suffered from acute pancreatitis, despite H2 blocker prophylaxis. This patient was re-operated for pancreatic necrosis. An another patient had wound dehiscence.
DISCUSSION
The mortality associated with AMI decreased from 80%-90% in the 1970’s to 60%-70% in the 1980’s and 1990’s[2,3]. This has been attributed to earlier diagnosis secondary to increased awareness, aggressive angiography, surgical and non-surgical blood flow restoration, resection of all necrotic bowel, second-look laparotomy or second-look laparoscopy and supportive intensive care[8,9].
Second-look laparotomy remains the gold standard for determination of further bowel viability and an operation is the only way to remove dead bowel. During the operation, bowel viability can be assessed by physical examination (inspection of bowel and palpation of vessels), hand-held Doppler ultrasound examination and intravenous injection of fluorescein[9-11]. These techniques are helpful but far from being sensitive and specific enough to allow omitting the second-look procedure[10-13]. Indications for the second-look procedure remained viable even when more objective methods such as Doppler ultrasonography and fluorescein testing became available. We use neither Doppler nor flurescein testing pre-operatively. We believe that if the bleeding is enough on the cutting end and the arterial pulse is palpable on the mesenteric side of the bowel in a normotensive patient, the patient is amenable to anastomosis, unless intra-abdominal sepsis or peritonitis is present.
In critically ill patients, conventional laparotomy is associated with certain general and access-related risks[5]. Second-look laparoscopy has become a diagnostic technique with potential therapeutic options. Second-look procedure has become more common in mesenteric vessel occlusion with uncertain intestinal viability observed during the primary surgery[1-3]. This procedure can also be applied under local anesthesia and be performed in the intensive care unit under sedation or analgesia. We prefer to perform the second-look procedure in the operating room, unless the patient is hemodynamically unstable.
In a large French study, although the overall survival of patients with AMI improved from the early 1980’s to early 1990’s, the percentage of second-look procedures remained unchanged[1]. Endean et al[16] stated that 15 of 43 (35%) patients with AMI with either thrombosis or embolism underwent a second-look procedure. In our clinic, a second-look laparoscopy is warranted in patients with AMI, if they exhibit either a low flow state, or have had a bowel resection with anastomosis during the first operation. However, among 71 patients, only 14 patients underwent a second-look laparoscopy. The reason for the lower incidence of a second-look laparoscopy was that most patients were transferred to our clinic from other hospitals. This delay in diagnosis led to bowel perforation and peritonitis resulting in the creation of a stoma and the abdomen was closed by using a Bogota bag.
Second-look laparoscopy is a safe method that decreases the negative second-look laparotomy risk in critically ill patients[4]. In reviewing results on 92 patients, Levy et al[17] stressed the beneficial role of a second-look on patient survival, although only 14% of their patients were exposed to this procedure. Since there are no predictive criteria for the progression of ischemia and not all patients undergo second-look procedures, some patients will undergo unnecessary surgical and anesthesiological procedures (negative second-look), and others in whom a surgical second-look might be beneficial, will not be operated on[7]. At this critical point when bowel ischemia is suspected, a laparoscopic second-look is important, because it can reduce severe unnecessary anesthesiological and surgical trauma to the patients by easily replacing the open surgical procedure for permanent treatment.
Although studies have advocated that laparoscopic second-look is a routine substitute of surgical second-look, there exist controversies regarding the timing of second-look operations. Practically, re-operation may be performed within 24 h. However, we prefer to perform the second-look operation within 72 h, which promotes bowel viability and anastomotic healing. We performed a second-look laparoscopy for our 14 patients during the ensuing 72 h. To our knowledge, the majority of anastomotic leakage occurs at 3rd to 5th post-operative days. We believe this contributes to early detection of leakage and prevent peritonitis. Although the laparoscopic findings were normal, we found anastomostic leakages in two patients at 6th post-operative day.
Denecke and Stiegler[18] stated that they performed second-look to control viability or lavage in 36 of 87 AMI patients. In 10 of 87 patients, a resection was performed. Unfortunately, only five of these patients survived. By the second-look procedure, 5 of 87 patients could be saved[20]. However, even in earlier studies that reported an advantage of the second-look procedure, the best survival rates were not much higher than 65%[16,19].
Anadol et al[20] compared open and laparoscopic second-look procedures in AMI patients. In the first group of 41 patients, the abdomen was closed after the first procedure. In the second group of 36 patients, a 10-mm trocar was inserted before closing the abdomen and a second-look intervention was performed by telescope in 23 patients. Seventy percent of re-laparotomies revealed nothing and were unnecessary. Eight percent of the re-laparoscopy group required re-resection while 87% of patients were rescued from unnecessary laparotomies[20].
Finally, a second-look laparoscopy is minimally invasive and technically simple. Laparoscopy has a shorter operative time compared to conventional laparotomy. It can not only be performed as a bedside procedure and sometimes without anesthesia, but also minimizes the risks of a redo-laparotomy.
The simplicity and ease of this method may encourage wider application to benefit more patients. However, prospective randomized studies are required for clarification if second-look procedures make a difference in outcomes.
Footnotes
S- Editor Liu Y L- Editor Kumar M E- Editor Lu W
References
- 1.Duron JJ, Peyrard P, Boukhtouche S, Farah A, Suc B. Acute mesenteric ischemia: changes in 1985-1995. Surgical Research Associations. Chirurgie. 1998;123:335–342. doi: 10.1016/s0001-4001(98)80002-0. [DOI] [PubMed] [Google Scholar]
- 2.Sachs SM, Morton JH, Schwartz SI. Acute mesenteric ischemia. Surgery. 1982;92:646–653. [PubMed] [Google Scholar]
- 3.Mamode N, Pickford I, Leiberman P. Failure to improve outcome in acute mesenteric ischaemia: seven-year review. Eur J Surg. 1999;165:203–208. doi: 10.1080/110241599750007054. [DOI] [PubMed] [Google Scholar]
- 4.Seshadri PA, Poulin EC, Mamazza J, Schlachta CM. Simplified laparoscopic approach to "second-look" laparotomy: a review. Surg Laparosc Endosc Percutan Tech. 1999;9:286–289. [PubMed] [Google Scholar]
- 5.Nassar AH, Htwe T, Hefny H, Kholeif Y. The abdominal drain. A convenient port for second-look laparoscopy. Surg Endosc. 1996;10:1114–1115. doi: 10.1007/s004649900255. [DOI] [PubMed] [Google Scholar]
- 6.Shaw RS. The “second look” after superior mesenterial embolectomy or reconstruction for mesenteric infarction. Current Surgical Management. Philadelphia: W. B. Sounders Company; 1965. p. 509. [Google Scholar]
- 7.Tola M, Portoghese A, Maniga AM. Laparoscopic second-look in acute intestinal ischemia. Minerva Chir. 1997;52:527–530. [PubMed] [Google Scholar]
- 8.Kaminsky O, Yampolski I, Aranovich D, Gnessin E, Greif F. Does a second-look operation improve survival in patients with peritonitis due to acute mesenteric ischemia? A five-year retrospective experience. World J Surg. 2005;29:645–648. doi: 10.1007/s00268-005-7380-5. [DOI] [PubMed] [Google Scholar]
- 9.Horgan PG, Gorey TF. Operative assessment of intestinal viability. Surg Clin North Am. 1992;72:143–155. doi: 10.1016/s0039-6109(16)45632-x. [DOI] [PubMed] [Google Scholar]
- 10.Hobson RW, Wright CB, Rich NM, Collins GJ. Assessment of colonic ischemia during aortic surgery by Doppler ultrasound. J Surg Res. 1976;20:231–235. doi: 10.1016/0022-4804(76)90145-1. [DOI] [PubMed] [Google Scholar]
- 11.Bergman RT, Gloviczki P, Welch TJ, Naessens JM, Bower TC, Hallett JW, Pairolero PC, Cherry KJ. The role of intravenous fluorescein in the detection of colon ischemia during aortic reconstruction. Ann Vasc Surg. 1992;6:74–79. doi: 10.1007/BF02000672. [DOI] [PubMed] [Google Scholar]
- 12.Moneta GL, Lee RW, Yeager RA, Taylor LM, Porter JM. Mesenteric duplex scanning: a blinded prospective study. J Vasc Surg. 1993;17:79–84; discussion 85-86. doi: 10.1067/mva.1993.41706. [DOI] [PubMed] [Google Scholar]
- 13.Carter MS, Fantini GA, Sammartano RJ, Mitsudo S, Silverman DG, Boley SJ. Qualitative and quantitative fluorescein fluorescence in determining intestinal viability. Am J Surg. 1984;147:117–123. doi: 10.1016/0002-9610(84)90044-8. [DOI] [PubMed] [Google Scholar]
- 14.Boley SJ, Feinstein FR, Sammartano R, Brandt LJ, Sprayregen S. New concepts in the management of emboli of the superior mesenteric artery. Surg Gynecol Obstet. 1981;153:561–569. [PubMed] [Google Scholar]
- 15.Lindblad B, Håkansson HO. The rationale for "second-look operation" in mesenteric vessel occlusion with uncertain intestinal viability at primary surgery. Acta Chir Scand. 1987;153:531–533. [PubMed] [Google Scholar]
- 16.Endean ED, Barnes SL, Kwolek CJ, Minion DJ, Schwarcz TH, Mentzer RM. Surgical management of thrombotic acute intestinal ischemia. Ann Surg. 2001;233:801–808. doi: 10.1097/00000658-200106000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy PJ, Krausz MM, Manny J. Acute mesenteric ischemia: improved results--a retrospective analysis of ninety-two patients. Surgery. 1990;107:372–380. [PubMed] [Google Scholar]
- 18.Denecke H, Stiegler H. Indications and results of second-look operation in acute mesenteric vascular occlusion. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990:311–315. [PubMed] [Google Scholar]
- 19.Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17–27. doi: 10.1002/bjs.4459. [DOI] [PubMed] [Google Scholar]
- 20.Anadol AZ, Ersoy E, Taneri F, Tekin EH. Laparoscopic "second-look" in the management of mesenteric ischemia. Surg Laparosc Endosc Percutan Tech. 2004;14:191–193. doi: 10.1097/01.sle.0000136677.39377.62. [DOI] [PubMed] [Google Scholar]


