Abstract
AIM: To ascertain whether constitutive androstane receptor (CAR) activation by 1,4-bis-[2-(3,5,-dichloropyridyloxy)] benzene (TCPOBOP) modulates steatohepatitis in the methionine choline-deficient (MCD) diet-fed animal.
METHODS: C57/BL6 wild-type mice were fed the MCD or standard diet for 2 wk and were treated with either the CAR agonist, TCPOBOP, or the CAR inverse agonist, androstanol.
RESULTS: Expression of CYP2B10 and CYP3A11, known CAR target genes, increased 30-fold and 45-fold, respectively, in TCPOBOP-treated mice fed the MCD diet. TCPOBOP treatment reduced hepatic steatosis (44.6 ± 5.4% vs 30.4 ± 4.5%, P < 0.05) and serum triglyceride levels (48 ± 8 vs 20 ± 1 mg/dL, P < 0.05) in MCD diet-fed mice as compared with the standard diet-fed mice. This reduction in hepatic steatosis was accompanied by an increase in enzymes involved in fatty acid microsomal ω-oxidation and peroxisomal β-oxidation, namely CYP4A10, LPBE, and 3-ketoacyl-CoA thiolase. The reduction in steatosis was also accompanied by a reduction in liver cell apoptosis and inflammation. In contrast, androstanol was without effect on any of the above parameters.
CONCLUSION: CAR activation stimulates induction of genes involved in fatty acid oxidation, and ameliorates hepatic steatosis, apoptosis and inflammation.
Keywords: Apoptosis, CYP4A, Fatty acid oxidation, Inflammation
INTRODUCTION
Hepatic steatosis or fatty infiltration of the liver has reached epidemic proportions in Western Society. Approximately 30 million adults in the United States have hepatic steatosis, and a subset of these individuals will develop accompanying hepatic inflammation referred to as steatohepatitis[1]. If the steatohepatitis is not associated with significant alcohol intake, it is commonly referred to as nonalcoholic steatohepatitis (NASH). Unfortunately, NASH can progress to cirrhosis and chronic liver failure with considerable morbidity and mortality[2]. Treatment options for NASH are limited and, therefore, there is an unmet need for the pharmacologic treatment of this liver disease.
Although the precise etiopathogenesis of NASH remains to be defined, it is a disease due to perturbations of intermediary fat metabolism. In hepatic steatosis, an excess of non-esterified fatty acids are released from peripheral tissues into the serum[3]. These excess serum-free fatty acids are cleared by the liver where they are esterified and accumulate as neutral fat. The formation of neutral fat is presumably due to a limited capacity to oxidize excess fatty acids. Mechanisms to enhance hepatic fatty acid oxidation are, therefore, a potential strategy to protect the liver from hepatic steatosis. Hepatic fatty acid oxidation occurs by three pathways[4]. β-oxidation is a predominant pathway, which occurs in the mitochondria, and is rate-regulated by carnitine palmitoyltransferase (CPT1) and the mitochondrial trifunctional protein (MPT) complex. Peroxisomal β-oxidation oxidation occurs within peroxisomes and is rate-limited by the peroxisomal L-bifunctional enzyme (L-PBE), acetyl-COA oxidase (ACO), and urate oxidase (UO). The third pathway is ω-oxidation which occurs in the endoplasmic reticulum. This pathway is dependent upon expression of the cytochrome enzymes CYP4A10 and CYP4A14. Stimulation of these pathways either individually or collectively could help remove excess free fatty acids from the liver and attenuate NASH.
Nuclear receptors are a family of transcription factors, which regulate metabolism of endo- and xenobiotic compounds[5]. In particular, the constitutive androstane receptor (CAR), which is highly expressed in the liver, is a biosensor for endo- and xenobiotic compounds, such as toxic bile acids[6-8] and steroids[9]. CAR mediates the induction of detoxifying enzymes by the widely used antiepileptic drug phenobarbital in humans and by the potent synthetic inducer, 1,4-bis-[2-(3,5,-dichloropyridyloxy)] benzene (TCPOBOP) in mice. Once stimulated, CAR increases expression of CYP2B genes in the mouse[7,8,10] and human[11]. From a teleological perspective, this nuclear receptor can be viewed as a general hepatoprotective response system, as it detoxifies potentially injurious endo- and xenobiotics. In addition, it serves as a hepatic mitogen and as an anti-apoptotic agent by increasing transcriptional expression of the anti-apoptotic protein, Mcl-1[12,13]. These composite effects eliminate toxins, enlarge the liver[14], and render it resistant to liver injury-all hepatoprotective responses. Whether CAR also protects the liver from injurious endobiotics, such as free fatty acids, is less clear but a viable concept.
Based on the above concepts, we postulated that CAR would protect the liver from the development of steatohepatitis by enhancing processes involved in fatty acid oxidation. Thus, the overall objective of the current study was to ascertain whether CAR activation by TCPOBOP modulates steatohepatitis induced by the methionine choline-deficient diet, a well known murine model of NASH[15]. Two fundamental questions were formulated. In TCPOBOP-treated MCD diet-fed mice: (1) Does CAR activation modify steatosis, and if so, does it alter expression of genes involved in fatty acid oxidation? and (2) does CAR alter apoptosis and inflammation in this model of NASH? The results indicate that CAR activation can stimulate an induction of genes involved in fatty acid oxidation, ameliorating hepatic steatosis, apoptosis and inflammation. These observations suggest CAR stimulation renders the liver resistant to MCD diet-mediated steatohepatitis. An understanding of these mechanisms may allow the development of CAR agonists as therapeutic strategies for NASH.
MATERIALS AND METHODS
Animal models
The care and use of the animals for this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). C57/BL wild-type mice (Jackson laboratories, Bar Harbor, ME), weighing 20-25 g, were fed a methionine choline-deficient (MCD) diet (Harland Tech Lad, Madison, WI) for 2, 3 or 4 wk. This diet rapidly induces steatosis and steatohepatitis in rodents[16]. The mice were maintained in a temperature-controlled, pathogen-free environment and fed a standard rodent chow diet and water ad libitum. To assess the effect of CAR modulation, mice were intraperitoneally (ip) injected with either vehicle (corn oil), 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP) (Sigma-Aldrich, St Louis, MO) (3 g/kg) daily for the first 3 d[7] at the starting of the MCD diet, or 5β-androstan-3β-ol (androstanol) (a CAR inverse agonist) (Steraloids, Newport, RI) (100 mg/kg) daily for 3 d at the starting of weeks one and two of the MCD diet (total of 6 injections). Androstanol was used as negative control for CAR target genes. At selected times, the animals were anesthetized with ether, and a hepatectomy was performed prior to euthanasia via exsanguination. Blood was drawn via the portal vein to examine triglyceride levels. Liver tissue sections were placed in fixative for subsequent microscopic analyses. Liver sections were also subjected to RNA extraction using the Trizol Reagent (Invitrogen, Carlsbad, CA).
Oil red O staining and fat quantification
Liver sections were cut into a thickness of 20 μm in a cryostat, air dried, and then stained with oil red O as per standard techniques[17]. Slides were then viewed under microscopy (Axioplan 2, Carl Zeiss, Inc. Oberkochen, Germany). Digital pictures were captured to quantitate percent fat (red color in area/field area × 100) of digital photomicrographs as described previously[18].
Histology, TUNEL assay and immunohistochemical identification of activated caspases 3/7
Histology, TUNEL assay, and immunohistochemical analysis for activated caspases 3/7 was performed as previously described by us[18]. To accurately quantitate TUNEL-positive and caspase 3/7-positive cells, slides were examined by digital image analysis to quantitate the percent fluorescence/field area of digital photomicrographs as described previously[13].
Measurement of Leukotriene B4
LTB4 was quantitated using a specific ELISA kit (R&D systems) according to the manufacturer's instructions[19].
Immunohistochemistry for CD68
Unstained slides of liver tissue specimens were deparaffinized and hydrated. Antigen retrieval was performed using EDTA (1 mmol/L, pH 8.0). Slides were placed in a vegetable steamer for 40 min at 97°C, followed by a cooling for 2 min. Thereafter, the catalyzed signal amplification system (DAKO, Carpinteria, CA) was used according to manufacturer's instructions. CD68 immunostaining was performed as previously described by us[20].
Real time-polymerase chain reaction (RT-PCR)
Total RNA was obtained from whole liver as previously described by us[21]. After the reverse transcription reaction, the cDNA template was amplified by PCR with Taq polymerase (Invitrogen) and mRNA was quantitated for acetyl-CoA oxidase (ACO), carnitine palmitoyltransferase (CPT1); peroxisomal 3-ketoacyl-CoA thiolase (ketoacyl thiolase), L-peroxisomal bifunctional enzyme (L-PBE), mitochondrial trifunctional protein (MTP) subunits alpha and bet, peroxisome proliferators-activated receptor-alpha (PPAR-α) as previously described[22]. Primers used are listed in Table 1. 18S primers (Ambion, Austin TX) were used as a control for RNA isolation and integrity. All PCR products were confirmed by gel electrophoresis. Real-time PCR was performed using the LightCycler (Roche Diagnostics, Mannheim, Germany) and SYBR green as the fluorophore (Molecular probes). The results were expressed as a ratio of product copies per milliliter to copies per milliliter of housekeeping gene 18S from the same RNA (respective cDNA) sample and PCR run.
Table 1.
Primer sequences used for RT-PCR
| Target | Primer sequence | Product size (bp) |
| ACO | F 5'-GAACTCCAGATAATTGGCACCTA-3' | 75 |
| R 5'-AGTGGTTTCCAAGCCTCGAA-3' | ||
| CPT-1b | F 5'-ATCATGTATCGCCGCAAACT-3' | 85 |
| R 5'-CCATCTGGTAGGAGCACATGG-3' | ||
| CYP2B10 | F 5'-CAA TGGGGA ACG TTG GAA GA-3' | 176 |
| R 5'-TGATGCACTGGAAGAGGA AC-3' | ||
| CYP3A11 | F 5'-CTCAATGGTGTGTATATCCCC-3' | 423 |
| R 5'-CCGATGTTCTTAGACACTGCC-3' | ||
| CYP4A10 | F 5'-AGTGTCTCTGCTCTAAGCC-3' | 180 |
| R 5'-CCCAAAGAACCAGTGAAA-3' | ||
| Ketoacyl thiolase | F 5'-GCATCCCAGAGACTGTACCTTT-3' | 202 |
| R 5'-GTCTCTGGCCTTCTCGTTCT-3' | ||
| L-PBE | F 5'-TGGCTCTTGGAGGAGGACTAG A-3' | 125 |
| R 5'-AAGCTGCGTTCCTCTTGCA-3' | ||
| MPT-α | F 5'-GGCAGTCTCAGTCGCTTCTC-3' | 240 |
| R 5'-GCACTCCTGATTTGGTCGTT-3' | ||
| MPT-β | F 5'-AGAGCTGCACTTTCGGGTTT-3' | 202 |
| R 5'-CTGTGGTCATGGCTTGGTTT-3' | ||
| PPAR-α | F 5'-GTACGGTGT GTATGAAGCCATCTT-3' | 76 |
| R 5'-GCCGTACGCGATCAGCAT-3' | ||
| Urate oxidase | F 5'-ACCTCCCGTCATTCACTCT-3' | 438 |
| R 5'-ACTGTCCCTGTTATTTTGCC-3' |
ACO: Acetyl-CoA oxidase; CPT1: Carnitine palmitoyltransferase; Ketoacyl thiolase: Peroxisomal 3-ketoacyl-CoA thiolase; L-PBE: L-peroxisomal bifunctional enzyme; MTP: Mitochondrial trifunctional protein subunits alpha and beta; PPAR-α: Peroxisome proliferators-activated receptor-alpha.
Statistical analysis
All data represent at least three independent experiments and are expressed as mean ± SE of the mean. Differences between groups were compared using Student t-tests and one-way analysis of variance with post hoc Dunnett test was used for multiple comparisons. P < 0.05 was considered statistically significant.
RESULTS
Stimulating CAR target genes in the MCD diet-fed mouse by TCPOBOP
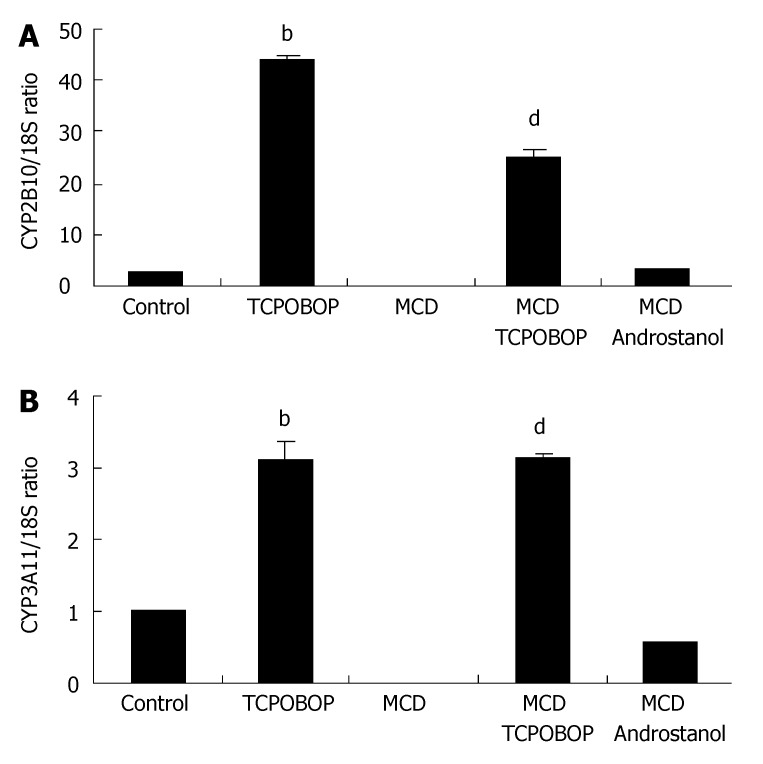
CYP2B10 and CYP3A11 have been identified as CAR target genes[10,23,24]. Therefore, to determine if TCPOBOP activates CAR in the MCD diet-fed mouse, expression of these genes was examined. When TCPOBOP treatment was administered to the MCD diet-fed mice, expressions of both CYP2B10 and CYP3A11 increased (Figure 1A and B). We also observed 45-fold and 30-fold elevations in CYP2B10 mRNA level in TCPOBOP-treated standard diet-fed mice and MCD diet-fed mice, respectively. CYP3A11 mRNA levels were also similarly increased in TCPOBOP-treated animals. Administration of the CAR inverse agonist, androstanol[10,25], did not alter expression of these gene products (Figure 1A and B). Androstanol was, therefore, used as a negative control for CAR target genes in this model, as it blocks basal activity of CAR[26]. Unexpectedly, CYP2B10 and CYP3A11 mRNA levels remained elevated for 2 wk after initial TCPOBOP administration, mRNA expression of these target enzymes began to decrease by week three (down to 7-fold elevation) and further decreased by week four (down to 6-fold elevation) (data not shown). This data illustrates that TCPOBOP is a potent CAR agonist in the fatty liver and its effects are long-lasting after initial treatment.
Figure 1.
Over-expression of CAR target genes in MCD diet-fed mice treated with TCPOBOP. CAR activation was assessed by measuring CYP2B10 and CYP3A11 (CAR target genes) expression in whole liver from vehicle-treated and TCPOBOP-treated chow-fed and MCD diet-fed mice. Expression was measured by real-time PCR and normalized as a ratio using 18S mRNA as housekeeping genes. A value of 1 for this ratio was arbitrarily assigned to the data obtained from vehicle-treated CAR+/+ mice. (A) CYP2B10 and (B) CYP3A11 mRNA expressions were increased in TCPOBOP-treated (3 mg/kg ip for 3 d) chow-fed mice (bP < 0.01 for CYP2B10 and CYP3A11 compared to control) and MCD diet-fed mice (dP < 0.01 for CYP2B10 and CYP3A11 compared to untreated). This phenomenon was abated by treatment with a CAR inhibitor androstanol (100 mg/kg ip) daily for 3 d at the starting of weeks one and two of the MCD diet (total of 6 injections), (n = 5 in each group).
Effects of TCPOBOP on hepatic and serum lipid content in MCD diet-fed mice
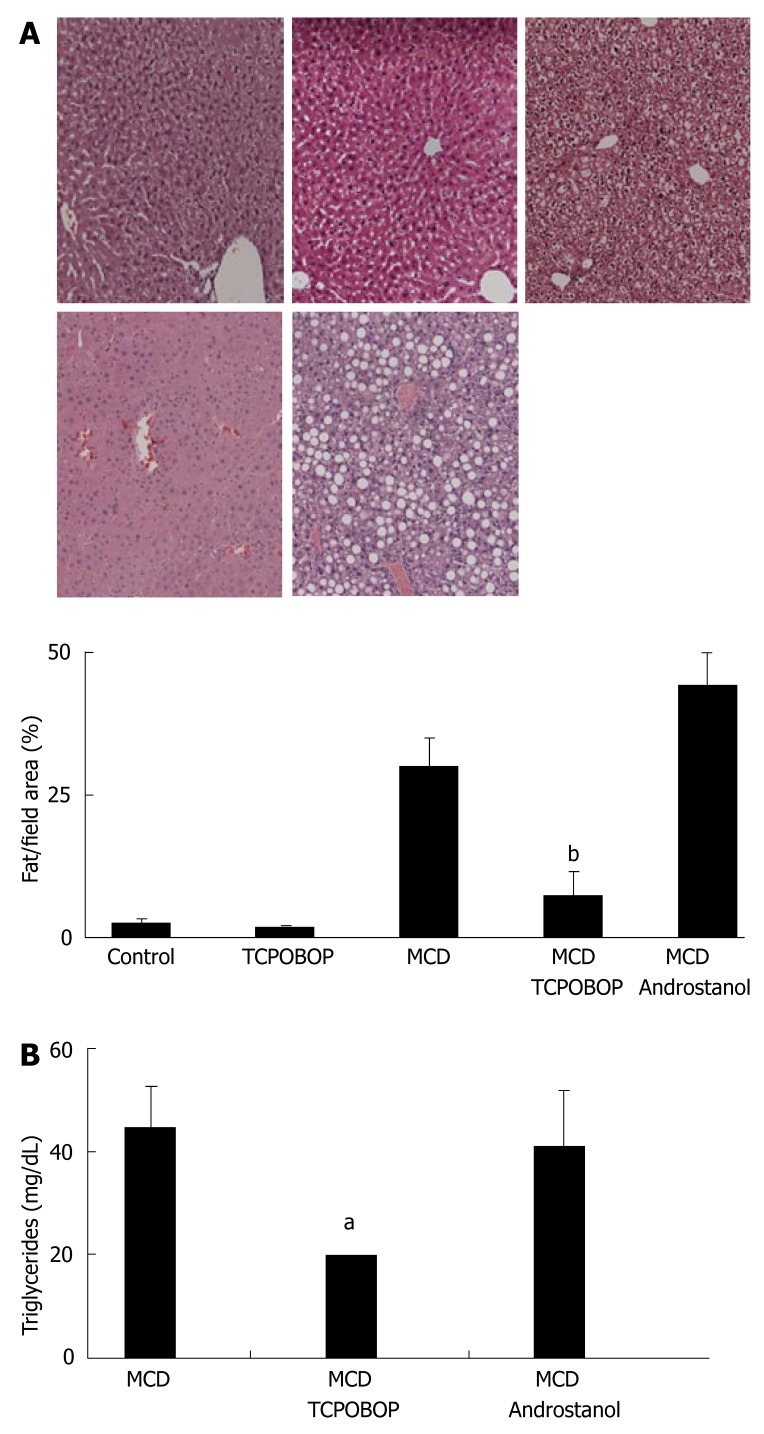
Animals treated with TCPOBOP and fed the MCD diet for 2 wk had 25% less hepatic steatosis than animals fed the MCD diet alone (Figure 2A). Interestingly, MCD diet-fed animals treated with androstanol had 15% more hepatic steatosis per surface area than untreated MCD diet-fed littermates. Of note, the fat pattern observed in the androstanol-MCD group was homogenously macrovesicular, where in untreated MCD diet-fed animals, the steatosis pattern was heterogeneous with both microvesicular and macrovesicular steatosis. Administration of TCPOBOP to the MCD diet-fed animals also markedly lowered serum triglyceride levels by 2-fold (Figure 2B). As expected, treatment with androstanol did not alter serum triglyceride levels. Collectively, these data demonstrate that TCPOBOP treatment decreased hepatic fat accumulation and improved serum triglyceride levels.
Figure 2.
Hepatic and serum fat content reduced by TCPOBOP treatment in the MCD diet-fed mice. (A) Top panel, from left to right: Fixed liver specimens from vehicle-treated chow-fed mice, TCPOBOP-treated (3 mg/kg ip for 3 d) chow-fed mice, vehicle-treated (corn oil) MCD diet-fed diet mice for 2 wk, TCPOBOP-treated MCD diet-fed mice for 2 wk; CAR inhibitor, androstanol-treated MCD diet-fed mice for 2 wk were stained by conventional H&E. Lower panel: Quantitation of hepatic fat content by Oil red O staining. Note the marked reduction of hepatic fat in the TCPOBOP-treated MCD diet-fed mice (bP = 0.001); (B) Serum triglyceride levels from mice fed the MCD diet for 2 wk with either TCPOBOP or androstanol treatment. Treatment with TCPOBOP reduced serum triglyceride levels by half (aP = 0.03).
Modulation of hepatic expression of genes involved in fatty acid oxidation by TCPOBOP
The reduction in hepatic steatosis was consistent with stimulation of fatty acid oxidation by TCPOBOP. Therefore, we evaluated the effect of TCPOBOP on expression of several target genes involved in fatty acid oxidation (Table 2). In the liver, microsomal, mitochondrial and peroxisomal fatty acid oxidation systems are regulated by PPAR-α. PPAR-α mRNA expression levels were not altered by treatment with TCPOBOP in the MCD diet-fed animal (data not shown). However, CYP4A10 and CYP4A14, target enzymes for PPAR-α involved in the microsomal ω-oxidation system, were altered by TCPOBOP administration (Table 2). CYP4a10 mRNA expression was increased 4-fold (P < 0.001), while, CYP4A14 mRNA expression was actually decreased by TCPOBOP treatment (P < 0.01). L-PBE and peroxisomal 3-ketoacyl-CoA thiolase (ketoacyl thiolase), genes involved in peroxisomal β-oxidation were also significantly modified by TCPOBOP. mRNA expression was increased 6-fold for L-PBE, (P < 0.001) and 2-fold for ketoacyl thiolase (P < 0.05), when compared to MCD diet-fed untreated animals (Table 2). Two key genes involved in mitochondrial β-oxidation, carnitine palmitoyltransferase (CPT1) and mitochondrial trifunctional protein (MTP), showed no significant difference between TCPOBOP-treated and -untreated MCD diet-fed mice. These data demonstrated a PPAR-α-like effect on microsomal ω-oxidation and peroxisomal β-oxidation by TCPOBOP, which may confer protection against steatosis in the MCD diet-fed animal.
Table 2.
Effect of TCPOBOP on genes involved in fatty acid oxidation
| Type of fatty acid oxidation | Gene | MCD | MCD-TCPOBOPa | Fold-change | P value |
| ω-oxidation | CYP4A10 | 1.40 ± 1.69 | 6.07 ± 1.73 | 4.3 | 0.001 |
| (ER) | CYP4A14 | 0.81 ± 0.04 | 0.07 ± 0.02 | -11.6 | 0.008 |
| β-oxidation | L-PBE | 0.07 ± 0.01 | 0.39 ± 0.01 | 5.6 | 0.0004 |
| (peroxisome) | Ketoacyl thiolase | 0.12 ± 0.03 | 0.28 ± 0.06 | 2.3 | 0.04 |
| ACO | 0.25 ± 0.14 | 0.32 ± 0.08 | 1.3 | NS | |
| Urate oxidase | 0.29 ± 0.02 | 0.16 ± 0.02 | -1.8 | NS | |
| β-oxidation | CPT1 | 0.79 ± 0.21 | 0.77 ± 0.19 | -1.0 | NS |
| (mitochondria) | MTP-α | 0.60 ± 0.30 | 0.88 ± 0.71 | 1.5 | NS |
| MTP-β | 0.50 ± 0.40 | 0.59 ± 0.07 | 1.2 | NS |
Values are expressed as mRNA expression/18S ratio ± SD.
Animals fed methionine choline-deficient (MCD) diet for 2 wk and pre-treated with the CAR agonist, TCPOBOP (3 mg/kg ip for 3 d at starting of diet). NS: Not significant; ER: Endoplasmic reticulum; L-PBE: L-peroxisomal bifunctional enzyme; ACO; Acetyl-CoA oxidase; Ketoacyl thiolase: Peroxisomal 3-ketoacyl-CoA thiolase; CPT1: Carnitine palmitoyltransferase; MTP: Mitochondrial trifunctional protein subunits alpha and beta.
Alteration of apoptosis and inflammation in the MCD diet-fed animals by TCPOBOP treatment
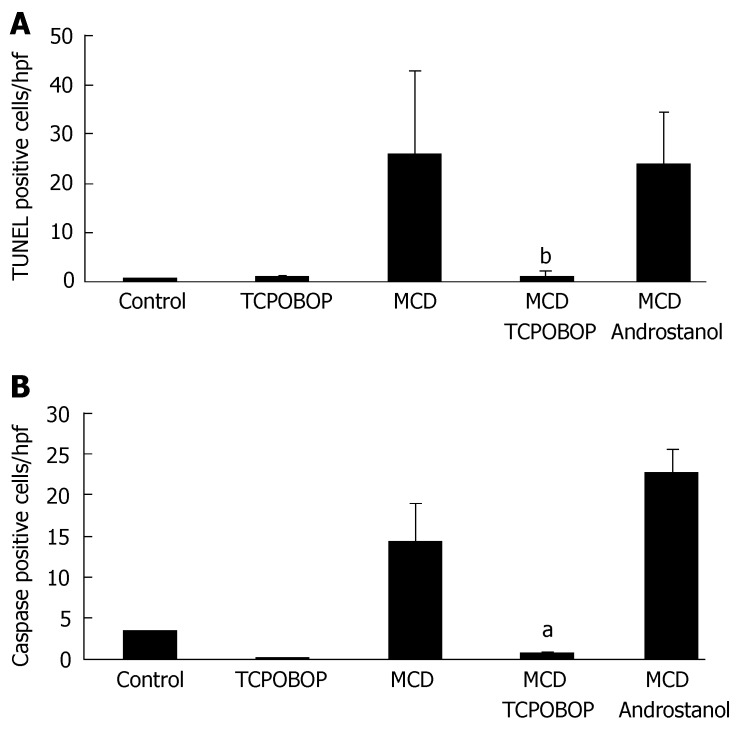
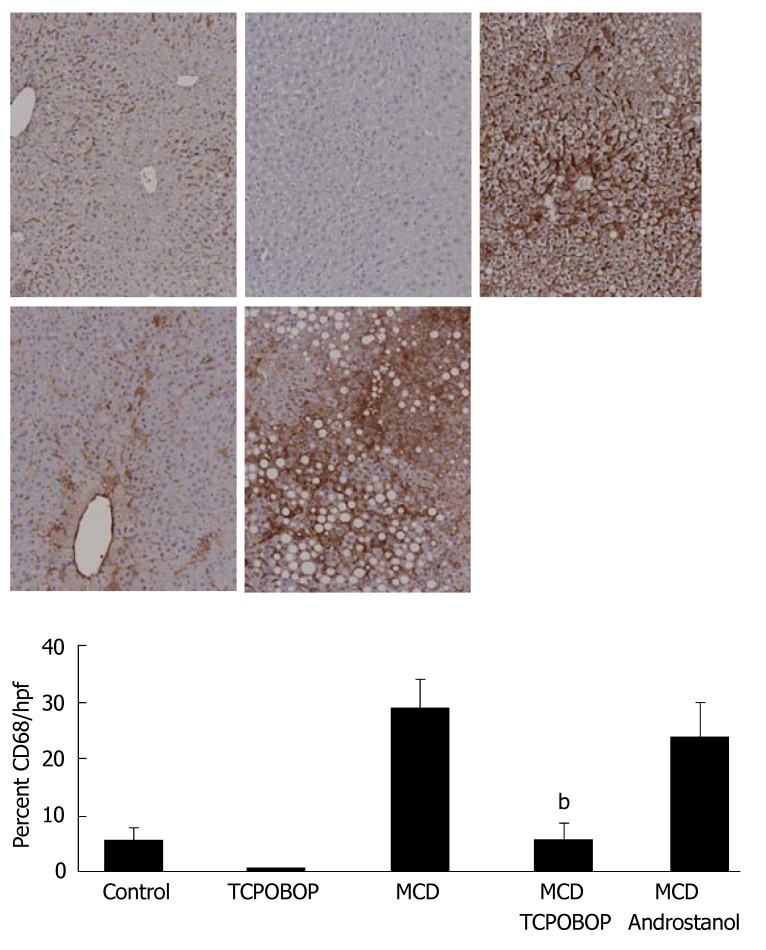
Although TCPOBOP reduced hepatic steatosis, this reduction may or may not be sufficient to attenuate liver inflammation. Therefore, we assessed the effect of TCPOBOP on hepatocyte apoptosis and its effect on inflammation by quantitating macrophage/Kupffer cell numbers. Apoptosis was assessed by quantitating TUNEL- and caspase 3/7-positive cells. TUNEL- and caspase 3/7-positive cells were reduced in TCPOBOP-treated MCD diet-fed mice; this reduction of apoptosis was not observed with androstanol treatment (Figure 3A and B). Hepatic inflammation was assessed by immunohistochemical staining for CD68, a marker for macrophage/Kupffer cells[27] (Figure 4). CD68-positive cells were less abundant in the MCD diet-fed mice treated with TCPOBOP as compared with the untreated mice on MCD diet; androstanol treatment did not reduce the number of CD68 immunoreactive cells. Consistent with these findings, leukotriene B4 (LTB4), a potent chemotactic agent that initiates, sustains and amplifies the inflammatory response, was also reduced in TCPOBOP-treated MCD diet-fed animals (55.6 ± 2.8 pg/mL) as compared to untreated MCD diet-fed mice (266.9 ± 12.1 pg/mL) (P = 0.001). These results suggest that TCPOBOP pre-treatment abrogates apoptosis and inflammation induced by the MCD diet.
Figure 3.
Hepatocyte apoptosis is attenuated by TCPOBOP in the MCD-diet fed animal. (A and B) Fixed liver specimens were analyzed by TUNEL and immunofluorescence for active caspase 3/7 to identify apoptotic liver cells. The percent/field area of TUNEL- (bP = 0.01) and active caspase 3/7-positive cells (aP = 0.03) were significantly higher in vehicle-treated MCD diet-fed mice than TCPOBOP-treated (3 mg/kg ip for 3d) MCD diet-fed mice (n = 5 in each group).
Figure 4.
Hepatic inflammation attenuated by TCPOBOP treatment in the MCD diet-fed mice. Top panel: Representative photomicrographs of immunohistochemistry for CD68, a marker of Kupffer cells; Lower panel: The percentage of CD68-positive areas in the liver sections was quantitated using digital image analysis. Immunoreactivity for CD68 was significantly reduced in TCPOBOP-treated MCD diet-fed mice as compared with vehicle- and androstanol-treated mice (bP = 0.002) (n = 5 in each group).
DISCUSSION
The principle findings of this study relate to the effect of CAR modulation of steatohepatitis. The observations suggest TCPOBOP stimulation of CAR in the MCD diet-fed mice model of NASH: (1) reduces hepatic steatosis; (2) increases expression of genes involved in microsomal ω-oxidation and peroxisomal β-oxidation pathways; (3) and reduces hepatic inflammation.
We utilized the MCD animal model of steatosis for this study. The MCD diet stimulates steatosis by inhibition of fatty acid oxidation[15]. This animal model of hepatic steatosis is unique in that mice fed the MCD diet develop inflammation, thereby mimicking human NASH. Recently, peroxisome proliferator-activated receptor α (PPAR-α) agonists have been shown to be useful in ameliorating steatohepatitis induced by the MCD diet in mice by increasing genes involved in fatty acid oxidation[28,29]. PPAR-α is a transcription factor belonging to the nuclear receptor superfamily (NRSF) that increases hepatic uptake and breakdown of fatty acids by up-regulating genes involved in peroxisomal and mitochondrial β-oxidation and microsomal ω-oxidation[5]. In our current study, TCPOBOP administration demonstrated a PPAR-α-like effect on fatty acid microsomal ω-oxidation and peroxisomal β-oxidation enzymes, by increasing expression of CYP4A10, L-PBE and 3-ketoacyl-CoA thiolase. Our observations are consistent with findings from other studies in which protection from hepatic steatosis was afforded by changes in fatty acid microsomal ω-oxidation and peroxisomal β-oxidation without alteration of genes involved in the mitochondrial β-oxidation pathway[30]. These results indicate that upregulation of peroxisomal and microsomal enzymes was primarily responsible for the CAR-dependent reduction in hepatic steatosis.
Our data indicated that CAR activation by TCPOBOP had a PPAR-α-like effect on enhancing expression of genes involved in fatty acid oxidation. How CAR activation mimics PPAR-α activation is unclear, but suggests a potential interaction between the two nuclear receptors. CAR binds to its cognate DNA motif as a heterodimer with the retinoid X receptor (RXR), which serves as a common heterodimerization partner for other nuclear receptors in the superfamily, such as PPAR-α, pregnane X receptor (PXR), liver X receptor (LXR), and farnesol X receptor (FXR)[31]. Although evidence exists for cross-talk between nuclear receptors of this superfamily[32,33], it is unknown if there is a direct link between CAR and PPAR-α activation. CAR could potentially bind to PPAR-α/RXR heterodimer, as LXR has been shown to bind directly to the PPAR-α/RXR complex[34]. This may afford CAR's ability to enhance expression of PPAR-α target genes involved in fatty acid oxidation. CAR has been shown to increase expression of gene targets otherwise thought to be regulated by other nuclear receptors. Recent experiments have illustrated CAR's ability to activate CYP3A, normally thought as the target of PXR[35]. Further studies are necessary to define the nature of the potential cross-talk between CAR and PPAR-α.
Apoptosis of markedly steatotic hepatocytes may incite the inflammatory response in NASH[36]. Apoptotic markers were reduced in TCPOBOP-treated MCD diet-fed animals in the present study. Indeed, previous work by us[13] has demonstrated CAR to have anti-apoptotic properties. In these studies, CAR activation by TCPOBOP depleted hepatocytes of the pro-apoptotic proteins Bak and Bax and increased expression of the potent anti-apoptotic protein Mcl-1 by directly promoting Mcl-1 transcription. These CAR-dependent processes rendered the liver resistant to death receptor-induced liver injury by Fas. The steatotic liver is highly susceptible to Fas-mediated apoptosis[37]. Perhaps, the CAR-induced resistance to Fas or other death ligands, such as tumor necrosis factor-alpha (TNF-α), accounts for its cytoprotective effects on the MCD diet-fed mouse. Finally, we noted that CAR can modify bile acid metabolism, and the role of bile acids in lipotoxicity remains unexplored[38].
The accumulation of fat in hepatocytes (steatosis) and the onset of steatohepatitis may reflect successive stages in fatty liver disease. The "two-hit" hypothesis postulates that the steatotic liver is susceptible to secondary insults including vulnerability to reactive oxygen species, tumor necrosis factor-α and other cytokines, which ultimately culminates in a sustained inflammatory response[39]. TCPOBOP treatment of MCD diet-fed mice resulted in a marked reduction in steatosis as well as inflammation. CAR-stimulated MCD diet-fed animals had decreased hepatic macrophage/Kupffer cell numbers and less circulating serum leukotriene B4 (LTB4). LTB4 is a potent chemotactic agent that initiates, sustains and amplifies the inflammatory response. Catabolism of LTB4 occurs in hepatocytes, as the liver is the principle organ for clearance of LTB4 from circulating blood[40]. In the liver, degradation of the fatty acid-like derivative LTB4 is modulated by PPAR-α regulation of microsomal ω-oxidation and peroxisomal β-oxidation pathways. Upregulation of PPAR-α-regulated genes involved in fatty acid oxidation by CAR activation may have attributed to a reduction in the inflammatory response, in part by enhancing LTB4 hepatic clearance.
In summary, our findings suggest that CAR activation is sufficient to attenuate hepatic steatosis, apoptosis and inflammation in the MCD diet-fed mice. The CAR-dependent PPAR-α-like stimulation of genes involved in microsomal ω-oxidation and peroxisomal β-oxidation pathways may, in part, be responsible for the hepatic cytoprotective effects observed in this model. These data also implicate a mechanistic link between fatty acid oxidation, hepatocyte apoptosis and Kupffer cell infiltration or inflammation. These preclinical studies suggest the employment of CAR agonists in the treatment of fatty liver diseases merits further attention.
ACKNOWLEDGMENTS
We appreciate the excellent secretarial service provided by Erin Bungum.
COMMENTS
Background
Nonalcoholic steatohepatitis (NASH) is a progressive spectrum of fatty liver disease for which there are limited therapeutic options. Apoptosis or programmed cellular death is a prominent histopathologic feature of NASH and correlates with disease severity. Recently, the constitutive androstane receptor (CAR), a nuclear receptor, has been reported to promote hepatic cytoprotection against apoptosis. Therefore, we hypothesized that CAR may ameliorate apoptosis induced by NASH.
Research frontiers
The incidence of nonalcoholic steatohepatitis is increasing with the rise in obesity over the last decade. New therapeutic regimens to slow down the process of fibrosis and cirrhosis in these patients will be important.
Innovations and breakthroughs
There is a prominent role for CAR cytoprotection against Fas-mediated hepatocyte injury via a mechanism involving upregulation of Mcl-1 (anti-apoptotic proteins) and, likely, downregulation of Bax and Bak (pro-apoptotic proteins).
Applications
At this time the use of CAR agonists to reduce the liver injury caused by NASH-induced hepatocyte apoptosis is still in its early phase of development. We need more data to fully evaluate its role in NASH.
Terminology
CAR is highly expressed in the liver and the small intestine, two key tissues expressing xenobiotic metabolizing enzymes, and mediates the induction of their expression by the widely used antiepileptic drug, phenobarbital (PB) and the potent synthetic inducer 1,4-bis-[2-(3,5,-dichloropyridyloxy)] benzene (TCPOBOP). TCPOBOP is an agonist ligand for CAR. PB induces its nuclear translocation, which results in increased expression of CAR target genes. The nuclear receptor, peroxisome proliferator-activated receptor alpha (PPAR-α), mediates many, if not all, of the adaptive consequences of peroxisome proliferator exposure in the liver, including alteration in lipid metabolism genes, hepatomegaly, and increases in liver tumors. Apoptosis is executed by caspases, a family of proteases that sequentially disassemble a cell. The pathways leading to caspase activation are dependent on the cytotoxic stimulus. Cytotoxic stress activates caspases by initiating signaling pathways that converge on the Bcl-2 family of proteins. After apoptotic stimulation, changes in the balance between pro- and anti-apoptotic members of this family lead to alteration of mitochondrial pore structure or integrity and permeabilization and release of proteins that promote cell death.
Peer review
This paper describes a series of experiments investigating the modulation of the CAR of steatotohepatitis in experimental rat model of NASH. The paper is well written and the methodology seems adequate. There are some issues that have to be addressed.
Footnotes
Supported by NIH grants T32 DK07198-26 (to ESB) and DK41876 (to GJG), and the Palumbo and Mayo Foundation
S- Editor Liu Y L- Editor Kumar M E- Editor Wang HF
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 5.Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 6.Uppal H, Toma D, Saini SP, Ren S, Jones TJ, Xie W. Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology. 2005;41:168–176. doi: 10.1002/hep.20512. [DOI] [PubMed] [Google Scholar]
- 7.Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, Moore DD. Induction of bilirubin clearance by the constitutive androstane receptor (CAR) Proc Natl Acad Sci USA. 2003;100:4156–4161. doi: 10.1073/pnas.0630614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113:137–143. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki Y, Kakizaki S, Horiguchi N, Takagi H, Mori M, Negishi M. Role of nuclear receptor CAR in carbon tetrachloride-induced hepatotoxicity. World J Gastroenterol. 2005;11:5966–5972. doi: 10.3748/wjg.v11.i38.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue K, Borchers CH, Negishi M. Cohesin protein SMC1 represses the nuclear receptor CAR-mediated synergistic activation of a human P450 gene by xenobiotics. Biochem J. 2006;398:125–133. doi: 10.1042/BJ20060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledda-Columbano GM, Pibiri M, Concas D, Molotzu F, Simbula G, Cossu C, Columbano A. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis. 2003;24:1059–1065. doi: 10.1093/carcin/bgg063. [DOI] [PubMed] [Google Scholar]
- 13.Baskin-Bey ES, Huang W, Ishimura N, Isomoto H, Bronk SF, Braley K, Craig RW, Moore DD, Gores GJ. Constitutive androstane receptor (CAR) ligand, TCPOBOP, attenuates Fas-induced murine liver injury by altering Bcl-2 proteins. Hepatology. 2006;44:252–262. doi: 10.1002/hep.21236. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- 15.Koteish A, Mae Diehl A. Animal models of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:679–690. doi: 10.1053/bega.2002.0332. [DOI] [PubMed] [Google Scholar]
- 16.Ghoshal AK, Ahluwalia M, Farber E. The rapid induction of liver cell death in rats fed a choline-deficient methionine-low diet. Am J Pathol. 1983;113:309–314. [PMC free article] [PubMed] [Google Scholar]
- 17.Hausman GJ. Techniques for studying adipocytes. Stain Technol. 1981;56:149–154. doi: 10.3109/10520298109067302. [DOI] [PubMed] [Google Scholar]
- 18.Baskin-Bey ES, Canbay A, Bronk SF, Werneburg N, Guicciardi ME, Nyberg SL, Gores GJ. Cathepsin B inactivation attenuates hepatocyte apoptosis and liver damage in steatotic livers after cold ischemia-warm reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2005;288:G396–G402. doi: 10.1152/ajpgi.00316.2004. [DOI] [PubMed] [Google Scholar]
- 19.Weller CL, Collington SJ, Brown JK, Miller HR, Al-Kashi A, Clark P, Jose PJ, Hartnell A, Williams TJ. Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors. J Exp Med. 2005;201:1961–1971. doi: 10.1084/jem.20042407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wuestefeld T, Klein C, Streetz KL, Beraza N, Schölmerich J, Burgart LJ, Zender L, Kubicka S, Baskin-Bey E, Gores GJ, et al. Lack of gp130 expression results in more bacterial infection and higher mortality during chronic cholestasis in mice. Hepatology. 2005;42:1082–1090. doi: 10.1002/hep.20912. [DOI] [PubMed] [Google Scholar]
- 21.Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- 22.Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- 23.Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 25.Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 26.Moore DD. CAR: three new models for a problem child. Cell Metab. 2005;1:6–8. doi: 10.1016/j.cmet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Kwekkeboom J, Kuijpers MA, Bruyneel B, Mancham S, De Baar-Heesakkers E, Ijzermans JN, Bouma GJ, Zondervan PE, Tilanus HW, Metselaar HJ. Expression of CD80 on Kupffer cells is enhanced in cadaveric liver transplants. Clin Exp Immunol. 2003;132:345–351. doi: 10.1046/j.1365-2249.2003.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–132. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- 29.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 30.Huong DT, Takahashi Y, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition. 2006;22:546–552. doi: 10.1016/j.nut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 32.Columbano A, Ledda-Columbano GM, Pibiri M, Concas D, Reddy JK, Rao MS. Peroxisome proliferator-activated receptor-alpha mice show enhanced hepatocyte proliferation in response to the hepatomitogen 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene, a ligand of constitutive androstane receptor. Hepatology. 2001;34:262–266. doi: 10.1053/jhep.2001.26172. [DOI] [PubMed] [Google Scholar]
- 33.Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- 34.Miyata KS, McCaw SE, Patel HV, Rachubinski RA, Capone JP. The orphan nuclear hormone receptor LXR alpha interacts with the peroxisome proliferator-activated receptor and inhibits peroxisome proliferator signaling. J Biol Chem. 1996;271:9189–9192. doi: 10.1074/jbc.271.16.9189. [DOI] [PubMed] [Google Scholar]
- 35.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 36.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 37.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 38.Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 40.Harper TW, Garrity MJ, Murphy RC. Metabolism of leukotriene B4 in isolated rat hepatocytes. Identification of a novel 18-carboxy-19,20-dinor leukotriene B4 metabolite. J Biol Chem. 1986;261:5414–5418. [PubMed] [Google Scholar]