Summary
Liver fibrosis is a consequence of chronic liver diseases and thus a major cause of mortality and morbidity. Clinical evidence and animal studies suggest that local tissue homeostasis is disturbed due to immunological responses to chronic hepatocellular stress. Poorly defined stress-associated inflammatory networks are thought to mediate gradual accumulation of extracellular-matrix-components, ultimately leading to fibrosis and liver-failure. Here we have reported that hepatic expression of interleukin-33 (IL-33) was both required and sufficient for severe hepatic fibrosis in vivo. We have demonstrated that IL-33’s pro-fibrotic effects related to activation and expansion of liver resident innate lymphoid cells (ILC2). We identified ILC2-derived IL-13, acting through type-II IL-4 receptor-dependent signaling via the transcription factor STAT6 and hepatic stellate-cell activation, as a critical downstream cytokine of IL-33-dependent pathologic tissue remodeling and fibrosis. Our data reveal key immunological networks implicated in hepatic fibrosis and support the concept of modulation of IL-33 bioactivity for therapeutic purposes.
Introduction
Liver fibrosis - a widespread consequence of chronic liver diseases that encompass infection-related hepatic fibrosis (e.g. hepatitis C virus infections, Schistosomiasis) metabolic-type fibrosis (e.g. steatohepatitis), and biliary-type fibrosis (e.g. primary sclerosing cholangitis) - is a major cause of mortality and morbidity. It develops as a result of chronic inflammatory responses to persistent hepatocellular stress which finally culminates in pathologic tissue remodeling and increased deposits of extracellular matrix (ECM) proteins (Bataller and Brenner, 2005). Later stages of progressive liver fibrosis are characterized by fundamental changes in liver architecture, which predispose to organ failure and hepatocellular carcinomas.
Hepatic stellate cells (HSCs) are viewed as a predominant cellular source of ECM components during fibrosis. Injury-associated immunological processes supporting trans-differentiation of quiescent HSC into fibrogenic myofibroblasts in the course of liver injury were shown to be particularly important for fibrosis (Friedman, 2010). The inflammatory response associated with liver injury and fibrosis is a dynamic process that involves intrahepatic accumulation of immune cell subsets. Those are attracted to and activated in the liver by specific patterns of chemokines and cytokines produced by liver-resident cells. Chemokines and cytokines like TGF-β, IL-6, PDGF and IL-1 have been implicated in HSC function in vitro and in vivo (Seki et al., 2009; Wasmuth et al., 2010; Weiler-Normann et al., 2007). However, many of the early events in liver fibrosis, including the exact nature of infiltrating pathogenic immune cell subsets and key molecular mediators which support local inflammation and activation of HSC remain undefined (Friedman, 2008).
Recently, interleukin-33 (IL-33), an IL-1-related cytokine, has been implicated in inflammatory conditions in animal models (Haraldsen et al., 2009; Schmitz et al., 2005; Xu et al., 2008). In addition, IL-33 has been suggested to possess pro-fibrotic properties in skin and lung (Rankin et al., 2010; Yanaba et al., 2011). IL-33 may be actively secreted from cells by yet undefined pathways, but passive release from necrotic cells is reported to be a dominant mechanism for abundance of extracellular IL-33 in vivo (Cayrol and Girard, 2009; Luthi et al., 2009). Therefore, it is proposed that IL-33 maybe considered as a damage-associated molecular pattern (DAMP) which contributes to tissue injury responses as well as inflammatory responses (Palmer and Gabay, 2011). Cytokine-like activity of extracellular IL-33 is mediated through a heteromeric cell surface receptor consisting of the ubiquitous IL-1R associated protein (aka IL-1R3) and the more selectively expressed IL-33 receptor (aka ST2, IL-1R4) (Chackerian et al., 2007; Palmer et al., 2008). Recently, phenotypically similar populations of innate lymphoid cells (ILC) that express ST2 and respond to IL-33 by production of the T helper-2 (Th2) cell-associated cytokines IL-4, IL-5 and IL-13 have been described (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). Furthermore, these cells (ILC2) have been shown to be a major in vivo source of IL-9 (Wilhelm et al., 2011) and intrinsic expression of the transcription factors RORα and GATA3 have been shown to be important for the generation of at least some of these ILC2 populations (Wong et al., 2012, Hoyler et al., 2013). In mice and humans ILC2 are primarily found in lymphoid tissues associated with barrier surfaces and a critical role of ILC2s and ILC2-derived cytokines has been suggested in the context of host protection against helminths and viral infections (Chang et al., 2011; Mjosberg et al., 2011; Monticelli et al., 2011).
Here we have identified IL-33 as key mediator of hepatic fibrosis in vivo. Our data suggest that IL-33 is released in response to chronic hepatocellular stress and that extracellular IL-33, via ST2-dependent signaling, leads to accumulation and activation of ILC2 in the liver. Activated hepatic ILC2 produce IL-13, which in turn triggers activation and trans-differentiation of HSC in an IL-4Rα- and STAT6 transcription factor-dependent fashion. These findings characterize molecular and cellular networks implicated in hepatic fibrosis and highlight the role of IL-33 at the apex of the pro-fibrotic cascade. In addition, a pathogenic capacity of ILC2 in the context of the tissue damage response is unveiled. Finally, our results validate the idea of therapeutic modulation of IL-33-dependent networks in hepatic inflammation and fibrosis.
Results
IL-33 expression is elevated in hepatic fibrosis and IL-33 is sufficient to drive excess ECM deposition in the liver
Previous studies suggest a role for IL-33 in the context of Th2 cell-associated inflammation and fibrosis of skin and lung (Palmer and Gabay, 2011; Rankin et al., 2010). To address its association with development of human fibrotic liver disease, we evaluated IL-33 serum concentrations in a cohort of patients with liver cirrhosis. Significantly higher serum amounts of IL-33 were observed in patients compared to controls (Fig. 1A). In rodent models of hepatic fibrosis, chronic hepatocellular stress is mimicked by repeated application of hepatotoxic chemicals. Hence, we next evaluated the expression of IL-33 in mice in which liver fibrosis was experimentally induced in two independent models by repeated administration of Thioacetamide (TAA) or Carbontetrachloride (CCL4). In mice treated with either chemical, IL-33 serum concentrations were significantly increased (Fig. 1B), and increased hepatic IL-33 protein expression was demonstrated by immunohistochemical staining (Fig. 1C). Furthermore, hepatic IL-33 protein expression was strongly increased in a mouse model of chronic Schistosomiasis, an infection predisposing mice and humans to severe hepatic inflammation and fibrosis (Fig. 1C). In addition, IL-33 mRNA expression in the liver was elevated by TAA and CCl4 treatment (not shown). No differential IL-33 expression was found in other tissues of CCL4 or TAA subjected mice suggesting that increased serum IL-33 is unlikely to originate from non-hepatic cells in these models (not shown).
Figure 1. IL-33 is upregulated in hepatic fibrosis.
(A) Serum IL-33 (n=11/group; ***p<0,001) in healthy controls or cirrhosis patients was determined by ELISA. (B) Serum IL-33 protein concentrations in control mice or mice with TAA or CCL4 induced liver fibrosis. (n=5/group; **p<0.01; ***p<0.001) were determined. (C) Representative images of liver sections from CCL4, TAA, S. mansoni infected and control treated mice stained for IL-33 by immunohistochemistry. Scale bars 50μm. (D) 2,5μg of mcIL-33 expression or control vector (mock) was HD injected into mice. Mice were analyzed 4 weeks later. Liver sections were stained for IL-33 protein, with HE or, for visualization of collagen, with Sirius red. (E) IL-33 serum concentrations in mcIL-33 treated mice (***p<0,001) were determined by ELISA. Scale bars 100μm (IL-33 staining) or 200μm (HE and Sirius Red). (F) Total hepatic collagen of indicated mice was quantified by hydroxyproline assay (n=9-10/group: ***p<0.001)). (G) mRNAs of selected fibrosis associated genes in liver total RNA were quantified by qPCR. (n=9-10/group; ***p<0.001; *p<0,05). Data are representative of at least two different experiments with similar results. (H) CD1 mice infected with ~ 30 S. mansoni cercaria were HD injected with mcIL-33 at week 3 after infection. 3 weeks later mice were sacrificed and organ weights were determined. Hepatic collagen content was determined by hydroxyproline quantification. (n=5/group; **p<0.01; *p<0.05). Data are representative of 2 different experiments with similar results.
In order to establish whether release of IL-33 from liver cells is sufficient for induction of fibrosis we generated a vector encoding a secreted version of IL-33 (mcIL-33) controlled by liver specific regulatory elements and took advantage of the technology of hydrodynamic delivery (HD) for sustained in vivo transduction of hepatocytes with IL-33 (Liu et al., 1999) (Suppl. Fig. 1A). Our data confirmed that IL-33 was overexpressed in hepatocytes of IL-33 vector-injected but not control vector-injected mice (Fig. 1D). Similarly, IL-33 protein was detected in serum (Fig. 1E). Other studies have demonstrated that rIL-33 can induce systemic expression of IL-13 in vivo (Rankin et al., 2010; Schmitz et al., 2005). At the IL-33 doses used for HD in our studies, no serum IL-13 was detectable (not shown) suggesting that liver-specific rather than systemic effects of IL-33 were observed.
Four weeks after HD, IL-33 vector-injected but not control vector-injected animals showed excessive immune cell infiltrates and increased hepatic collagen as evident by hematoxylin and eosin (H&E) and Sirius red staining, respectively (Fig. 1D). A significant increase in total hepatic collagen was confirmed by applying the quantitative hydroxyproline assay to harvested liver tissues (Fig. 1F). Treatment with IL-33 also induced gene expression of fibrogenic markers Acta1, Timp1, and Col1a1 (Fig. 1G). No increase in hepatic collagen was observed upon injection of the same dose of IL-33 vector into IL-33R (ST2)-deficient mice, confirming that ST2 is required for IL-33-mediated hepatic fibrosis (Suppl. Fig. 1B).
Moreover, S. mansoni dependent liver damage strongly increased upon mcIL-33 mediated hepatic IL-33 overexpression (Fig. 1H) and such treated mice display premature death due to severe disease (not shown).
To further analyze the functional role of the IL-33 pathway in hepatic fibrosis in vivo, we expressed biologically active IL-33 in livers of mice. We generated a conditional transgenic line that allowed tissue- or cell type-specific overexpression of IL-33 by Cre recombinase-mediated deletion of an EGFP-Stop cassette (eGFPSTOPfloxIl33 mice; Suppl. Fig. 1C). We induced hepatic IL-33 expression in eGFPSTOPfloxIl33 mice by HD of a Cre-expression plasmid (Suppl. Fig. 1D). Examination of livers of these mice 4 weeks later revealed that hepatic IL-33 expression led to profound collagen depositions and fibrosis (Suppl. Fig. 1D).
IL-33 deficiency ameliorates experimental fibrosis in vivo
We next investigated whether IL-33 deficiency affects development of hepatic fibrosis in vivo; fibrosis was induced in Il33−/− mice and WT littermates by repeated administration of CCL4. After 4 weeks Il33−/− mice displayed a significant reduction in excess collagen deposits as shown by Sirius red staining and hydroxyproline quantification (Fig. 2A), whereas total hepatic collagen was similar in unchallenged Il33−/− mice compared to controls (not shown). Consistent with the ameliorated fibrotic phenotype, a strong reduction in mRNA expression of Timp1, a gene associated with HSC activation and fibrosis in humans and mice (Benyon et al., 1996; Jin et al., 2011), was observed in livers of Il33−/− mice (Fig. 2B). In addition, a biliary fibrosis model with experimental ligation of the common bile duct (BDL) was employed. Again, histologic assessment of liver tissue as well as total collagen quantification showed that fibrosis was markedly reduced in Il33−/− mice compared to controls (Fig. 2C). Reduction of fibrosis was further confirmed by qPCR-based quantification of fibrosis-associated gene expression using liver RNA of mice subjected to BDL for 16 days. Amounts of Col1a1, Col3a1 and Timp1 were dramatically reduced in livers of Il33−/− mice (Fig. 2D). To evaluate whether modulation of IL-33 bioactivity affects liver pathology in Schistosomiasis, we next treated mice 3 weeks post infection with a construct for soluble ST2 (sST2), a naturally occurring IL-33 decoy receptor. Compared to controls, mice subjected to sST2 delivery developed significantly less liver disease (Fig. 2E) indicating that IL-33 has proinflammatory and/or profibrotic functions in the context of parasite induced liver disease.
Figure 2. IL-33 deficiency ameliorates experimental fibrosis in vivo.
Il33−/− or C57BL/6 control mice (n=5/group) were treated with CCL4 for 4 weeks. (A) Livers were stained for determination of fibrillar collagen depositions with Sirius red and hydroxyproline amounts in livers were determined (*p<0.01). (B) Timp1 mRNA in liver total RNA of CCL4 treated mice or controls was quantified by qPCR (**p<0.01). (C, D) Il33−/− or C57BL/6 controls underwent BDL surgery. 16d later mice were sacrificed and livers were analyzed: (C) Sections were stained with Sirius red and hydroxyproline amounts in livers were quantified (*p<0.05), (D) mRNAs of selected fibrosis-associated genes in liver total RNA of BDL mice or controls were quantified by qrtPCR. (E) CD1 mice were infected with ~ 30 S. mansoni cercaria. 3 weeks later mice were HD injected with a liver specific expression construct for the soluble ST2 receptor. 4 weeks later mice were sacrificed. Collagen deposits in livers of mice were determined by Sirius red staining and hydroxyproline quantification. Granulomas were counted in 10 low power fields per slide. (n=5/group; ***p<0.001; *p<0.05). Scale bars 200μm.
(F, G, H) Il33−/− mice were injected with 5μg of a liver specific expression construct for full-length IL-33 (aa 1-260). (F) IL-33 specific immunostaining demonstrating nuclear IL-33 expression in hepatocytes. (G) Analysis of livers of mice 7d after treatment (H) 7d after HD mice were treated with 1,8 ml/kg CCL4 or 350 mg/kg TAA. 3d later serum IL-33 concentrations were determined by ELISA. (***p<0.001) Data are representative of at least two different experiments with similar results. Scale bars 200μm or 50μm (IL-33 staining).
Some reports describe IL-33 as intracellular protein which unfolds its ST2-dependent cytokine activity only after passive release from damaged cells (Palmer and Gabay, 2011). However, whether prominent functional roles exist for intracellular IL-33 appears poorly understood at this time. Therefore, one possible interpretation of our result of reduced hepatic fibrosis in Il33−/− mice is that intracellular IL-33 possesses pro-fibrotic properties. To address this, a vector encoding an intracellular expressed version of IL-33 including the nuclear localization signal was delivered to Il33−/− mice via HD. While hepatic IL-33 protein expression was evident from IHC staining (Fig. 2F), no IL-33 was detectable in the serum of those animals (not shown) and no liver pathology was observed by histology (Fig. 2G). However, when those animals where treated with CCL4, IL-33 became detectable in the serum (Fig. 2H) and hepatic fibrosis was observed (not shown).
To obtain further information about molecular networks involved in the progression of hepatic fibrosis in this model, we next generated mRNA expression profiles of cytokines, chemokines, profibrogenic, and fibrolytic genes in livers of mice with IL-33 dependent fibrosis by qPCR. Thereby, numerous differentially regulated genes were identified (Suppl. Table 1) that when analyzed for relationships and biological significance by Ingenuity Pathway Analysis are clearly implicated in gene expression networks associated with development of liver fibrosis and HSC activation (Suppl. Fig. 2).
IL-13 dependent type II IL-4 receptor signaling mediates IL-33 dependent fibrosis
Our next objective was to define pathways required for IL-33-dependent fibrotic liver disease in more detail. Because IL-13 mRNA was most prominently increased in livers of mice with IL-33 induced fibrosis and IL-33 treated S. mansoni infected mice (Suppl. Fig. 3A, B), we treated Il13−/− mice and WT littermates with mcIL-33 and examined livers 4 weeks later. Sirius red staining and quantification of hepatic collagen revealed that fibrosis in IL-33 treated Il13−/− mice was reduced compared with controls suggesting critical profibrotic roles of IL-13 in this model (Fig. 3A). IL-13 mediates biological functions via the type II IL-4 receptor, which is composed of IL-4Rα and IL-13Rα1 (Wills-Karp and Finkelman, 2008). To test whether type II IL-4R signaling is involved in IL-33 dependent fibrosis, we injected mcIL-33 into Il4Rα−/− mice. As demonstrated by Sirius red staining and hydroxyproline assay, Il4Rα−/− mice had a significant reduction in fibrosis compared to Il4Rα+/+ mice (Fig 3B). Furthermore, HD of an IL-13 construct, but not constructs for the Th2-related cytokines IL-5 and IL-9 (Suppl. Fig. 4A), clearly induced ECM depositions in livers of WT mice. In contrast, Il4Rα−/− mice were not susceptible for IL-13 dependent fibrosis (Suppl. Fig. 4A). Staining of liver sections from cirrhosis patients demonstrated that both IL-4Rα and IL-13Rα1 are strongly upregulated in diseased areas suggesting that these mechanistic findings in mice may translate to pathogenesis of human liver disease (Fig. 3C).
Figure 3. IL-13 dependent type II IL-4 receptor signaling is required for IL-33-mediated fibrosis and HSC activation.
IL-33 expression constructs were HD injected into (A) Il13−/− mice, (B) Il4Rα−/− mice or Balb/c controls. Mice were sacrificed 4 weeks later and collagen depositions in livers were analyzed by Sirius red staining or hydroxyproline assay (n=10 mice/group; ***p<0,001;**p<0,01). Scale bars 200μm. (C) Representative pictures of liver tissue sections from healthy controls or patients with liver cirrhosis that were stained for IL-4Rα or IL-13Rα by immunohistochemistry (D) Detection of activated STAT6 by Western-Blot in whole liver lysates (top panel) or IL-13 stimulated HSC (lower panel). (E) HSCs were stimulated in vitro with rIL-13 for 4d. Cell growth was assessed using WST-1 assay. Control: medium alone (***p<0,001; *p<0,05). (F) HSCs were cultivated for 48h with or without the presence of 30ng/ml IL-13. Chemokines in supernatants were quantified by ELISA. Data are representative of at least 2 different experiments.
IL-13 dependent type II IL-4R signaling mediates HSC activation
Our observation that IL-13 is a dominant effector of the profibrogenic function of IL-33 prompted us to further investigate the biological role of IL-13 in hepatic fibrogenesis. Effects of IL-13 on both immune and non-hematopoietic cells have been described (Koyasu and Moro, 2011). We therefore assessed whether IL-4Rα signaling in hematopoietic or nonhematopoietic cells is required for fibrogenesis by generating chimeras in which IL-4Rα–deficient or wildtype bone marrow was transferred into recipient mice that were pretreated with clodronate-liposomes to deplete Kupffer cells. However, IL-33 induced a similar degree of hepatic fibrosis in both chimeric strains (Suppl. Fig. 4B). Since HSCs have been suggested to be predominant ECM-producers in the course of fibrosis, we analyzed whether IL-33 dependent IL-13 production directly regulates these non-immune cells. We isolated HSC and found that they produced mRNAs for both components of the functional type II IL-4R, IL-4Rα and IL-13Rα1. In addition, surface expression of IL-13Rα1 was demonstrated by flow cytometry (Suppl. Fig. 4C). Western-Blot analysis with liver lysates demonstrated increased STAT6 phosphorylation after injection of mcIL-33 in vivo (Fig. 3D top panel). Moreover, purified primary HSC, stimulated with IL-13 ex vivo, responded by phosphorylation of STAT6, a critical transcription factor for IL-4- and IL-13-dependent cellular responses (Hebenstreit et al., 2006) (Fig. 3D lower panel). Additionally, stimulation of HSC with IL-13 increased their proliferation in vitro (Fig. 3E), and elicited an IL-13 dependent increase in mRNA of profibrotic genes (Suppl. Fig. 4D) and chemokines (Fig. 3F). Collectively, these data suggest that IL-33 through IL-13 modulates HSC functions.
ILC2 are increased in livers of mice with hepatic fibrosis
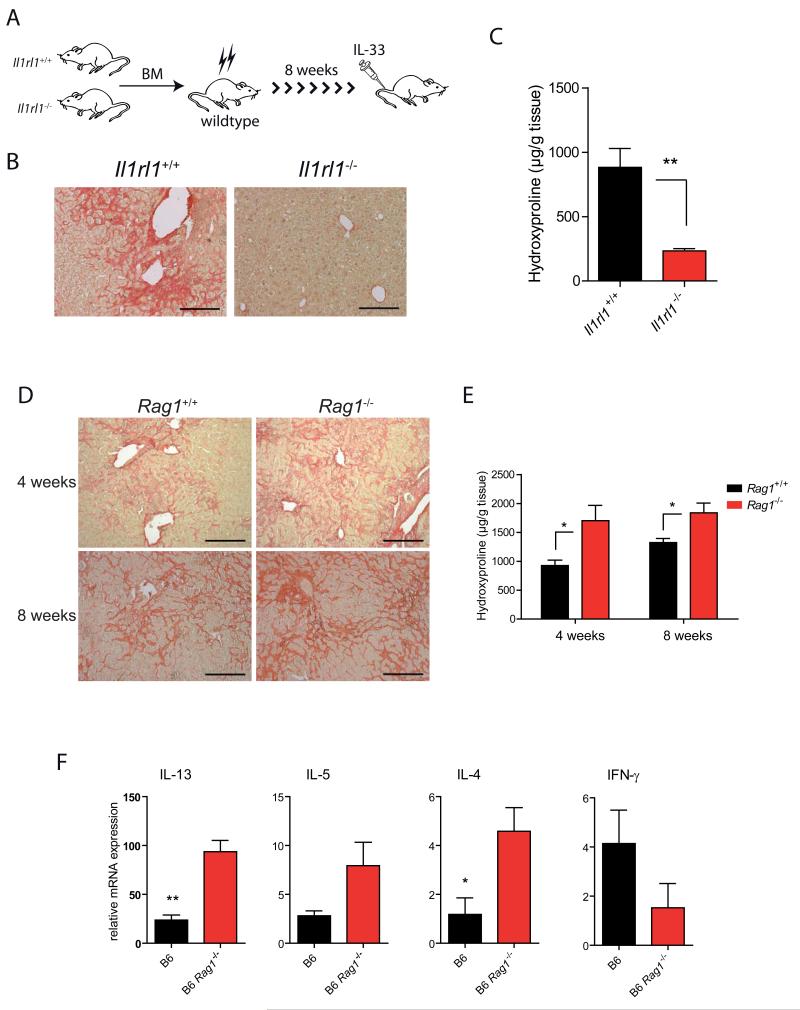
Different cell types have been described as possible sources of IL-13 in response to IL-33 stimulation (Besnard et al., 2011; Kroeger et al., 2009; Stolarski et al., 2010). To identify which cells are the dominant producers of IL-33-dependent IL-13 in this system we first assessed whether immune cells or liver-resident cells are critical targets of IL-33 by generating chimeras in which ST2-deficient or wildtype BM cells were transferred into wildtype recipients (Fig. 4A). Interestingly, mcIL-33 treatment induced different responses in the two strains: whereas mice reconstituted with wildtype BM developed severe fibrotic disease, chimeras with defective IL-33 signaling in BM-derived cells did not show liver pathology or excess collagen (Fig. 4B, C).
Figure 4. Innate immune cells promote IL-33 dependent fibrosis.
(A) Il1rl1−/− or Il1rl1+/+ BM cells were i.v. injected into irradiated C57BL/6 recipient mice. 8 weeks later mice received mcIL-33 via HD injection. (B) Representative Sirius red stainings of livers of mice four weeks after treatment. (C) Hepatic collagen content was determined by hydroxyproline quantification (n=5/group; **p<0.01). (D, E, F) IL-33 expression constructs were HD injected into C57BL/6 or Rag1−/− mice. (D) Sirius red stainings of livers of mice 4 or 8 weeks after treatment. (E) Total collagen in livers was determined by hydroxyproline quantification (N=5-6 mice/group; *p<0.05). (F) Hepatic mRNAs of fibrosis-associated genes 4 weeks after mcIL-33 treatment in mice were quantified by qPCR (N=5/group; **p<0.01; *p<0.05). Data are representative of at least 2 different experiments with similar results. Scale bars 200μm.
To characterize the role of these IL-33-responsive hematopoietic cells further, we determined the contribution of lymphocytes for progression of liver fibrosis. Rag1−/− mice treated with mcIL-33 developed marked liver pathology at 4 weeks post HD (Fig. 4D). In fact, Sirius Red staining and hydroxyproline determination indicated that the extent of fibrosis was more pronounced in these mice compared to controls (Fig. 4D, 4E). In contrast, Rag1−/−gc−/− mice, which – in addition to B- and T cells - lack γc -dependent lymphocytes, e.g. NK cells and ILCs, were resistant to IL-33 mediated hepatic fibrosis (data not shown). We next did qPCR analysis to compare the expression of several fibrosis-associated genes in livers from mcIL-33 treated Rag1−/− and WT mice. Indeed, and consistent with our data described above, hepatic mRNAs for Th2 cell-associated cytokines, particularly IL-13, were strongly elevated in Rag1−/− mice. In contrast, amounts of the antifibrotic cytokine interferon-γ were lower in Rag1−/− mice (Fig. 4F).
Recently, several reports have described innate lymphoid cell populations (nuocytes, natural helper cells, innate helper cells, ILC2) that respond to stimulation with IL-25 and/or IL-33, are capable of producing substantial amounts of IL-13 and are important for host responses to infections (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). Our observation that IL-33 induced hepatic fibrosis developed independently of adaptive immune cells, and largely depended on IL-13, but not on the presence of basophils and mast cells (Suppl. Fig. 5A, 5B), prompted us to analyze the functional role of ILC2 in fibrotic liver disease. First, we treated Il4-eGFP (4get) mice, a reporter strain marking type-2 competent cells by expression of eGFP (Mohrs et al., 2001), with mcIL-33 and analyzed eGFP expression in isolated liver cells by flow cytometry (Fig. 5A). As a result, the number of eGFP+Sca-1+ cells increased dramatically with IL-33 treatment. Sca-1 is expressed on hematopoietic progenitors and was previously shown to be expressed by ILC2 (Saenz et al., 2010a). In further experiments we were able to recover small numbers of lin−, CD45+ cells, characterized by expression of IL-33R, IL-7Rα, CD44, CD90.2, ICOS and Sca-1, resembling ILC2, from the livers of naïve mice. In livers of mcIL-33 treated mice, we found these cells strongly enriched (Fig. 5B) and moreover, these ILC2 produced IL-13 as shown by intracellular flow cytometry analysis (Fig. 5C). Recently, the transcription factor RORα has been shown to be critical for nuocyte development (Wong et al., 2012) and mice treated with mcIL-33 had increased liver RORα mRNA expression compared to controls (Fig. 5D). To determine a potential impact of RORα on the development of liver ILC2, we analyzed RORα expression in purified liver ILC2. Quantitative analysis of RORα mRNA showed high RORα abundance in ILC2 compared to ROR-γt, a factor involved in the developmental program of other ILC lineages (ILC3) (Fig. 5E). In addition, hepatic numbers of lin−Sca-1+ICOS+ cells were reduced in chimeric mice reconstituted with BM from mice lacking RORα suggesting that liver ILC2 are developmentally related to ILC2 described in other tissues (Wong et al., 2012) (Fig. 5F). Next we investigated the frequency of ILC2 in mice with hepatic fibrosis. We found that lin−ST2+ICOS+ cells were increased in livers of mice with CCL4 induced fibrosis (Fig. 5G). However, in livers of CCL4 treated Il1rl1−/− mice, ILC2 amounts were lower than in WT mice (Fig. 5H). Moreover, hepatic ILC2 numbers were also increased in S. mansoni infected mice (Fig. 5I), whereas blockade of IL-33 by sST2 treatment reduced liver ILC2 and RORα mRNA (Fig. 5J). These findings indicate that IL-33 signaling is important for hepatic accumulation of ILC2 during chronic liver disease. Similar numbers of hepatic ILC2 were present in naïve Il33−/−/4get versus Il33+/+/4get mice suggesting that IL-33 signaling may not be required for ILC2 survival in conditions of liver homeostasis (Suppl. Fig. 5C). Accordingly, hepatic overexpression of IL-25 expanded liver ILC2 in the absence of both IL-33 and ST2 (Suppl. Fig. 5D, 5E). However, although hepatic IL-25 mRNA was somewhat increased in mice with CCL4 or infection dependent liver fibrosis (Suppl. Fig. 5F), no IL-25 protein could be detected in the sera of these mice suggesting that rather IL-33 than IL-25 is responsible for ILC2 expansion in chronic liver diseases.
Figure 5. IL-33 expands liver resident type-2 related ILC that produce IL-13.
(A) Flow cytometric analysis of isolated hepatic immune cell populations from naïve IL-4/eGFP reporter mice or 5d after treatment with mcIL-33. (N=3-4/group; **p<0.01) (B) Hepatic immune cell populations of mock or mcIL-33 injected Rag1−/− mice were isolated and depleted from lin+ (CD5+, B220+, CD11b+, Gr1+, 7/4+, Ter119+) cells. The expression of IL-7Rα and ST2 was analyzed by flow cytometry. Gated IL-7Rα+/ST2+ cells were further analyzed for expression of CD45, Thy1.2, CD44, ICOS and Sca-1. Blue histograms represent isotype controls. Numbers of lin− IL-7Rα+/ST2+ cells in livers of controls or mcIL-33 injected Rag1−/− mice. (N=4-8/group; ***p<0.001) (C) Hepatic immune cells from 4get/Rag1−/− mice were stimulated for 4h with PMA and Ionomycin. Surface expression of Sca-1 and ICOS and intracellular IL-13 expression were analyzed by flow cytometry (D) Total liver RNA from 5d control or mcIL-33 treated mice was isolated and used for RORα specific qPCR analysis. (E) Total RNA from sort purified liver ILC2 isolated from Rag1−/− mice treated for 5d with mcIL-33 was used for RORα and RORγ specific qPCR (*p<0.05). (F) Flow cytometry -based quantification of lin−Sca-1+ICOS+ cells in irradiated mice reconstituted with BM from WT or sg/sg mice. (N=2-4/group) (G) Flow cytometry analysis of lin−, eGFP+, ICOS+ cells in livers of 4get mice treated for 4 weeks with CCL4. (H) Il1rl1+/+ WT or Il1rl1−/− mice were treated for 4 weeks with CCL4 before hepatic immune cells were isolated and analyzed by flow cytometry for the presence of lin−Sca-1+ICOS+ cells. Data are representative of at least two different experiments with 5 mice/group with similar results. (I) CD1 mice were infected with ~ 200 S. mansoni cercaria or left uninfected. 3 weeks later mice liver ILC2 numbers were determined by flow cytometry. (J) CD1 mice were infected with ~ 30 S. mansoni cercaria. 4 weeks later they were HD injected with a expression construct for sST2. 4 weeks later mice were sacrificed and liver ILC2 numbers were determined. Hepatic Rora mRNA was quantified by qPCR. n=5/group; *p<0.05).
ILC2 mediate hepatic fibrosis
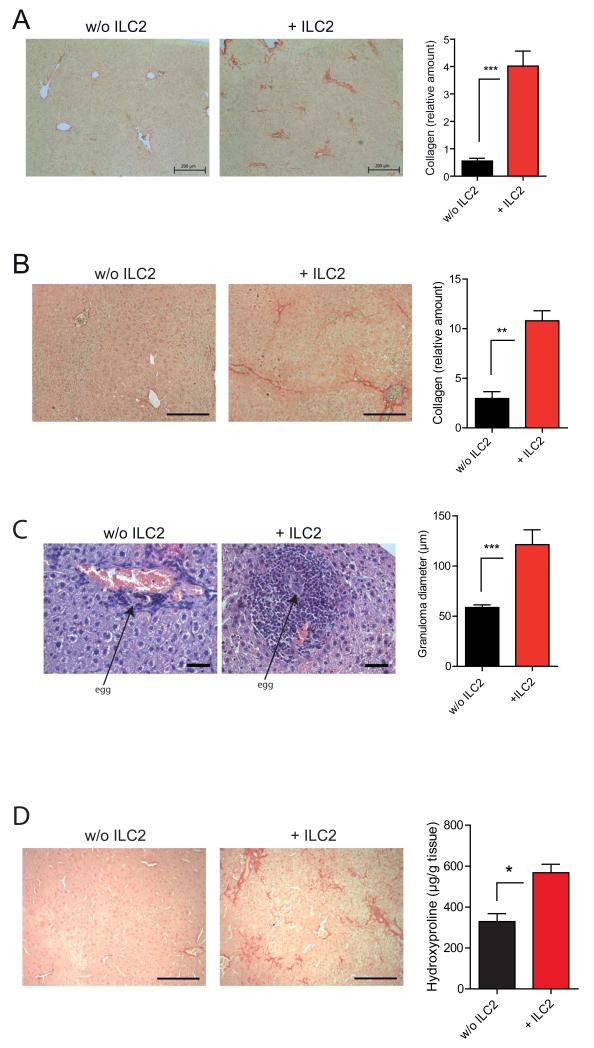
IL-33 treated Rag1−/− mice showed higher numbers of ILC2 in their livers than WT mice (not shown), consistent with a more severe IL-33 dependent fibrotic phenotype (Fig. 4D). To directly investigate a possible link between ILC2 and liver tissue remodeling, we performed depletion and ILC2 transfer experiments. ILC2 counts can be minimized in livers of Rag1−/− mice by treatment with αThy1.2 (CD90.2) antibodies (Fig. 6A). ILC2 depletion prior to IL-33 administration strongly reduced IL-33 dependent hepatic fibrosis (Fig. 6B), although αThy1.2 treatment 2 weeks after mcIL-33 administration was less protective (Suppl. Fig. 6A). Moreover, αThy1.2 treatment of Rag1−/− mice (Suppl. Fig. 6B, Fig. 6C) and C57BL/6 wildtype mice (Fig. 6D) resulted in significantly decreased CCL4 dependent fibrosis as evident by reduced Sirius red staining and hydroxyproline assay at week 4. Similarly, ILC2 depletion in chronic CCL4 dependent fibrosis was also efficient in Rag1−/− mice reconstituted with T and B cells from Thy1.1+ mice (Fig. 6E) and resulted in decrease expression of the fibrosis associated genes Acta1 and Timp1 (Fig. 6F). Next, we adoptively transferred purified ILC2 from Il1rl1+/+ mice into Il1rl1−/− mice and treated these mice with mcIL-33. Analysis of liver tissue 4 weeks after treatment showed that ILC2 treated mice but not controls had marked inflammatory cell infiltrations and collagen depositions. In contrast, no such liver alterations were observed in mcIL-33 treated Il1rl1−/− control mice that received no ILC2s (Fig. 7A). Similarly, ILC2 transfer into Il1rl1−/− mice subjected to the TAA model to mimic chronic hepatocellular stress clearly increased the degree of hepatic fibrosis (Fig. 7B). Moreover, in a model of hepatic granuloma formation by intra portal vein transfer of Schistosome eggs (Suppl. Fig. 7C) the size of egg induced granulomas in Il1rl1−/− mice was significantly increased when ILC2 were adoptively transferred (Fig. 7C).
Figure 6. ILC2 are important mediators of hepatic fibrosis.
(A) Naïve 4get/Rag1−/− mice were treated every other day with 200 μg αThy.1.2 mAb for 6d. lin−eGFP+ICOS+ cells in livers were analyzed by flow cytometry. (B) 4get/Rag1−/− mice were treated every 3 days with 200 μg αThy.1.2 mAb for 4 weeks. At day 6 mice were HD injected with mcIL-33. Collagen depositions in livers of mice were determined by Sirius red staining (n=5/group; **p<0.01). Rag1−/− (C) or C57BL/6 (D) mice were treated every 3d with 200μg αThy.1.2 or control mAbs for 4 weeks. Starting at day 6 mice were treated twice weekly with 0,8 ml/kg CCL4 for 4 weeks. Collagen deposits in livers of mice were determined by Sirius red staining (n=5/group; **p<0.01). Scale bars = 200 μm. (E, F) Rag1−/− mice were intravenously reconstituted with 4×106 purified splenic T and B cells from Thy.1.1+ mice. 7d later, mice were treated every 3 days with 200 μg αThy.1.2 mAb for 4 weeks. Starting at day 6 mice were treated twice weekly with 0,8 ml/kg CCL4. Collagen deposits in livers of mice were determined by Sirius red staining. Scale bars = 200 μm. Hepatic mRNAs of Acta1 and Timp1 were determined by qPCR (N=6-7/group; **p<0.01;*p<0.05). Data represent 2 independent experiments with similar results.
Figure 7. Adoptive transfer of ILC2 restores hepatic fibrosis.
(A) Il1rl1−/− mice were injected i.v. with 2×106 sorted, WT ILC2 or saline only. 24 h later mice were treated with mcIL-33. Hepatic collagen content was assessed by Sirius red staining (n=4/group; ***p<0,001). (B) Il1rl1−/− mice were injected i.v. with 2×106 ILC2 or saline. Subsequently mice were treated i.p. for 4 weeks with TAA as described in methods. Hepatic collagen content was assessed by Sirius red staining (n=5/group; p<0.01). (C) Il1rl1−/− mice were injected i.v. with 2×106 ILC2. 24 h later mice were injected with 5000 freshly isolated S. mansoni eggs via the portal vein system. 4 weeks later mice were sacrificed and granuloma formation was analyzed in HE stained liver sections. (n=3-4/group; p<0,001). (D) Il13−/− mice were injected i.v. with 2×106 ILC2 from WT Balb/c mice. 24h later mice were treated with mcIL-33 via HD. Collagen depositions in livers were determined by Sirius red staining and hydroxyproline assay. (n=4/group; **p<0.01). Scale bars 200μm (A, B, D) or 50μm (C). Data are representative of at least 2 independent experiments with similar results.
Given that Il13−/− mice are largely protected from IL-33 dependent fibrosis (Fig. 3A) and that ILC2 transferred Il1rl1−/− mice treated with a vector encoding a neutralizing IL-13Rα2-Fc fusion protein developed less IL-33 dependent fibrosis (Suppl. Fig. 7A), we directly tested whether ILC2 promote hepatic tissue remodeling via IL-13 production. By transferring wildtype ILC2 into Il13−/− mice, we demonstrated that ILC2 even when they are the only IL-13 secreting cell-type were sufficient to mediate fibrosis (Fig. 7D). Accordingly other ST2+ IL-13 producing lineages such as basophils and mast cells were less important for IL-33 mediated disease (Suppl. Fig. 5 A, B) and moreover other cytokines that were reported to be produced by ILC2 such as IL-9 (Wilhelm et al., 2011) are not as critical for IL-33 dependent fibrosis as IL-13 (Suppl. Fig. 7B). Collectively, these data suggest a critical role of IL-33 dependent ILC2 in promoting hepatic fibrosis. Results from these experiments indicate that IL-33 is critical for hepatic accumulation of ILC2 and that ILC2, through an IL-13-dependent mechanism, are both required and sufficient for hepatic fibrosis.
Discussion
Hepatic fibrosis develops as a consequence of chronic liver infections, such as HBV, HCV or Schistosomiasis, or can occur due to sustained metabolic or biliary imbalances. Irrespective of the underlying cause, hepatic fibrosis in humans and experimental animal models is closely associated with persistent activation of inflammatory pathways. However, early molecular and cellular networks that link hepatocellular stress to inflammation and subsequently to initiation and progression of liver fibrosis remain poorly defined. Here we present compelling evidence that IL-33 - released from liver cells in the context of hepatocellular stress - is a key mediator of hepatic fibrosis in vivo. We show that IL-33 release from liver cells leads to activation and expansion of IL-13 producing liver resident ILC2 and identify a previously unrecognized role for these cells during hepatic tissue remodeling.
We demonstrate that elevated concentrations of IL-33 protein in the serum and liver tissue relate to the condition of hepatic fibrosis in humans and also are observed across different mouse models of hepatic fibrosis which is consistent with a recent study reporting an association of IL-33 overexpression with hepatic fibrosis (Marvie et al., 2010). Importantly, our data provide multiple lines of evidence that intrahepatic release of IL-33 in the context of stress-dependent cellular damage is associated with fibrogenic changes in the liver in vivo. Taken together, these results provide a rationale for investigation of serum IL-33 as possible non-invasive diagnostic biomarker of chronic hepatocellular injury and fibrosis; such biomarker maybe valuable in uncovering early inflammatory and fibrogenic events and may complement existing clinical markers of hepatic fibrosis.
To address the question if IL-33 was not only sufficient but also required for hepatic fibrosis, IL-33-deficient mice were challenged in two different models of hepatic fibrosis: CCL4 and BDL. In both models, IL-33-deficient animals developed significantly reduced collagen deposits compared to WT littermates. Similar to the reduction in collagen, expression of ECM-associated genes was diminished in IL-33-deficient mice. Collectively, these data support the concept that IL-33 contributes to severe hepatic fibrosis in vivo.
Transcriptional profiling identified IL-13, a cytokine previously described as inflammatory and fibrogenic mediator in other organs, as one of the most prominently upregulated genes during IL-33-mediated hepatic fibrosis. Indeed, Il13−/− and Il4Rα−/− mice were markedly protected from IL-33-mediated disease suggesting that IL-33 and IL-13 constitute a profibrotic axis in the course of the disease. This concept is further supported by our studies demonstrating IL-33- or IL-13-dependent hepatic activation of STAT6 in vivo and direct activation of signaling, proliferation and gene expression of HSC in vitro. A recent report suggested that IL-13 activates STAT6 but also SMAD-family transcription factors in rat HSC. Through this mechanism IL-13 bypasses requirements for pro-fibrotic transforming growth factor-β (TGF-β) signaling for full HSC activation (Liu et al., 2011). Consistent with the murine data, we have demonstrated elevated hepatic protein expression of both components of the functional IL-13 receptor, IL-4Rα and IL-13Rα, in human liver cirrhosis sections compared to controls. This indicates increased sensitization to IL-13 dependent signals in human fibrotic liver disease and suggests at least partial functional congruence of pro-fibrotic hepatic networks in mouse and human. Taken together, these findings suggest a hepatic IL-33/IL-13 axis, which promotes HSC activation and triggers a potent fibrogenic response.
A number of different cell types have been identified as candidates for IL-13 production after IL-33 stimulation (Liew et al., 2010). While other IL-13 producing cell types such as lymphocytes, basophils and mast cells did not contribute profoundly, we identified in a series of in vivo experiments a γc-dependent non-T and non-B cell lymphoid cell population that was a strong IL-13 producer and important pathogenic cell type in our system. These cells depend on the expression on the transcription factor RORα and express a panel of cell surface receptors characteristic of ILC2 a recently described innate lymphocyte subset with a key role in host defense to viral or parasitic infection (Chang et al., 2011; Kang et al., 2012; Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). Reduction of ILC2 numbers by administration of a depleting mAb to Thy1.2 correlated with reduced sensitivity to hepatic fibrosis after HD administration of IL-33, as well as after chronic CCL4 challenge. Moreover, adoptive transfer of ILC2 purified from livers of WT mice into IL-33 unresponsive Il1rl1−/− mice restored this strains’ susceptibility to fibrosis in the different models that we employed indicating that intra-hepatic ILC2 have a critical role in promoting IL-33-mediated liver fibrosis in vivo. For the first time to our knowledge this report describes a role for ILC2 outside the realm of host defense at mucosal surfaces and establishes a role of ILC2 in hepatic tissue remodeling networks via IL-33-dependent IL-13 production. Further studies are required to clarify the exact role of both IL-33 and ILC2 in normal liver physiology, although our results indicate that IL-33 may not be essential for survival of hepatic ILC2 in conditions of normal liver function. Recently, IL-33 upregulation in hepatocytes and a protective role of IL-33/ST2 signaling in ConA hepatitis, a model of acute, fulminant hepatitis in humans, has been described (Arshad et al., 2011; Volarevic et al., 2012). Although in another study Il33−/− mice do not show differential susceptibility to ConA hepatitis (Oboki et al., 2010), it is tempting to speculate that IL-33 may exert a protective role in tissue repair responses following transient injury, while sustained IL-33 release due to chronic hepatocellular stress drives pathological tissue remodeling in the liver, as demonstrated here. Consistent with this idea, IL-33-dependent ILC2 promote early lung tissue homeostasis during acute influenza infection (Monticelli et al., 2011), but also mediated severe airway hypereactivity and asthma (Barlow et al., 2012; Bartemes et al., 2011; Chang et al., 2011). Thus, ILC2 may have evolved to establish a direct link between tissue damage responses and host defense, and some of the stereotypical Th2-associated genes may more accurately be viewed as dual role factors coordinating host defenses as well as tissue remodeling.
Lastly, our findings validate the concept of therapeutic modulation of IL-33 or IL-33-dependent ILC2 responses for treatment of conditions that involve chronic hepatic inflammation and fibrosis.
Experimental procedures
Animals and mouse models. C57BL/6, Balb/cJ, Rag1−/−, sg/sg and Il4Rα−/− (Balb/c background) mice were obtained from Jackson Laboratory. Il13−/− mice were kindly provided by A. McKenzie. 4get/eGFP reporter (Mohrs et al., 2001) and Mcpt8Cre (Ohnmacht et al., 2010) mice were obtained from D. Voehringer. Il33−/− mice were recently described (Louten et al., 2011). Il1rl1−/− mice were originally obtained from Shizuo Akira’s lab and backcrossed to the C57BL/6 background for 10 generations. 8-12 week old age and sex matched mice were used for experimental procedures. Chronic CCL4 dependent hepatic fibrosis was induced by 8 intraperitoneal injections of CCL4 (Merck) at 0,8 ml/kg in mineral oil (Sigma). For eliciting thioacetamide (TAA) induced toxic fibrosis TAA (Sigma) was i.p. injected at 200 mg/kg thrice a week for 4 weeks. In some experiments cholestasis dependent fibrosis was induced by surgical ligation of the common bile duct under ketamine and xylazine anaesthesia. Sham-operated mice underwent laparotomy without ligation. For analysis of hepatic IL-33 expression after Schistosoma infection Swiss Webster mice were infected with ~ 176 - 200 (high dose) or ~ 30 (low dose) S. mansoni cercaria larvae (Blank et al., 2010).
For generation of bone marrow chimeras 2×107 cells from femurs and tibias of donor mice were i.v. injected into lethally irradiated (10Gy : C57BL/6; 7,8 Gy : Balb/c) recipient mice. In some experiments recipients were i.p. pre-treated for 24h with 200μl clodronate liposomes to deplete Kupffer cells (van Rooijen and van Kesteren-Hendrikx, 2003). Mice were let to recover for 8 weeks followed by mcIL-33 injection into the tail veins. Animal experiments were approved by the governments of Rheinland-Pfalz and Mittelfranken.
Liver cell isolation procedures
Livers were aseptically removed from experimental mice and non-parenchymal liver cells were isolated using the gentleMACS device according to the manufactures instructions (Miltenyi Biotec). Briefly, livers were digested 2 times for 30 min in Krebs-Ringer solution containing 5000 U Collagenase IV (Sigma) and 30000 U/ml DNase I (Roche) in gentleMACS C tubes at 37° C. Subsequently, cells were passed through a 100-μm filter mesh and remaining hepatocytes were removed by centrifugation at 20g for 4 minutes.
For further purification of HSCs, pellets of nonparenchymal liver cells suspended in 15% (w/v) iodixanol were overlaid with a solution containing 10% (w/v) iodixanol and HBSS and centrifuged at 1400g for 20 minutes as described previously (Elsharkawy et al., 2010). HSCs were collected at the interface of the low-density barrier. Immediately after cell isolation purity of isolated HSC was examined by analysis of autofluorescence excitation under UV light or by FACS for autofluorescence and negativity for CD45.
For further purification of immune cell populations, cells were suspended in 30% Percoll , underlaid with 100% Percoll and centrifuged at 1400g for 20 minutes. Cells from the interface were collected, counted and processed for FACS analysis or cell culture. In some experiments ILC2 populations were enriched using a lineage cell depletion kit according to the manufacturer’s instructions (Miltenyi Biotec).
Flow cytometry
Single cell suspensions from livers were stained with combinations of the following fluorochrome-tagged antibodies (all from eBioscience unless specified otherwise) : Allophycocyanin-conjugated anti-CD45 (30-F11), anti-Sca-1 (D7), anti-Thy1.2 (53-2.1), anti-CD44 (IM7), Fluoresceinisothiocyanat-conjugated anti-T1-ST2 (DJ8, MD Biosciences, Zuerich, Switzerland), Phycoerythrin-conjugated anti-IL-13Rα1 (13MOKA), anti-IL-13 (eBio13A), anti-ICOS (7E.17G9) and Brilliant Violet 421-conjugated anti IL-7Rα (A7R34), anti-mouse lineage-cocktail (Biolegend). Samples were measured on a LSRFortessa cell analyser (BD Biosciences) and were analysed with Flowjo (Treestar).
In vivo depletion of ILC2
For depletion of ILC2 in mice anti-Thy1.2 monoclonal Ab (clone 30H12) from BioXCell was used. 200 μg/mouse were administered i.p. every 3 days as described in the figure legends.
Adoptive transfers of liver ILC2s
Single cells suspensions from livers of 4get/Rag1−/− mice treated with mcIL-33 for 5 days were enriched for lineage- cells a lineage cell depletion kit according to the manufacturer’s instructions (Miltenyi Biotec). Cells were stained with PE conjugated antibodies to ICOS and APC conjugated antibodies to Sca-1 and 4get/eGFP, PE and Sca-1 positive cells were collected using a FACSAria II cell sorter (Becton Dickinson). Sorted cells were adoptively transferred to recipient mice by i.v. tail vein injection.
Cytokine and chemokine measurements
For determination of mouse and human IL-33, soluble ST2 ELISA and IL-13 specific Duoset ELISA Kits from R&D Systems were used. Chemokine concentrations in cell culture supernatants and organ lysates were measured by a mouse Flowcytomix kit (Ebioscience) according to manufacturer’s instructions.
Cell proliferation assay
Isolated HSCs were stimulated with 20 ng/ml rIL-13 (R&D Systems, Wiesbaden, Germany) for 72 h, WST-1 proliferation reagent (Roche) was added (1:10 dilution), followed by incubation for 4 hours at 37° C. The absorbance was measured at 450 nm.
Statistical analysis
Student’s t tests or Mann-Whitney tests were performed using Graphpad 5 software (Prism). P values < 0,05 using a 95 % confidence interval were considered significant.
Supplementary Material
Acknowledgments
The authors thank D. Freytag, C. Lindner, and A. Taut for excellent technical assistance. We thank the Institute of Pathology, University Medical Center Mainz (PD Dr. Hansen) for assistance and the NIAID Schistosoma Resource Center for materials and technical support. This work was supported by the Collaborative Research Center (SFB796 of the DFG (to T.M., S.W. and M.F.N.), by an ERC starting grant (PAS_241506 to D.V.) and by the Interdisciplinary Center for Clinical Research (IZKF) and the ELAN program of the University Medical Center of Erlangen (to S.W).
References
- Arshad MI, Rauch M, L’Helgoualc’h A, Julia V, Leite-de-Moraes MC, Lucas-Clerc C, Piquet-Pellorce C, Samson M. NKT cells are required to induce high IL-33 expression in hepatocytes during ConA-induced acute hepatitis. European Journal of Immunology. 2011;41:2341–2348. doi: 10.1002/eji.201041332. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-Responsive Lineage-CD25+CD44hi Lymphoid Cells Mediate Innate Type 2 Immunity and Allergic Inflammation in the Lungs. J Immunol. 2011 doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJP. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–831. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. European Journal of Immunology. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- Blank WA, Test MR, Liu SF, Lewis FA, Blanton RE. Long-Term Genetic Stability and Population Dynamics of Laboratory Strains of Schistosoma Mansoni. Journal of Parasitology. 2010;96:900–907. doi: 10.1645/GE-2463.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. P Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. Journal of Immunology. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsharkawy AM, Oakley F, Lin F, Packham G, Mann DA, Mann J. The NF-kappa B p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. Journal of Hepatology. 2010;53:519–527. doi: 10.1016/j.jhep.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine & Growth Factor Reviews. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2013;19:634–48. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology. 2011;53:219–229. doi: 10.1002/hep.23983. [DOI] [PubMed] [Google Scholar]
- Kang Z, Swaidani S, Yin W, Wang C, Barlow JL, Gulen MF, Bulek K, Do JS, Aronica M, McKenzie AN, et al. Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(−)c-Kit(+) innate cell population. Immunity. 2012;36:821–833. doi: 10.1016/j.immuni.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S, Moro K. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Current Opinion in Allergy and Clinical Immunology. 2011;11:109–114. doi: 10.1097/ACI.0b013e3283448808. [DOI] [PubMed] [Google Scholar]
- Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88-and p38 alpha-dependent pathway. Journal of Leukocyte Biology. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- Liu F, Song YK, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- Liu Y, Meyer C, Muller A, Herweck F, Li Q, Mullenbach R, Mertens PR, Dooley S, Weng HL. IL-13 Induces Connective Tissue Growth Factor in Rat Hepatic Stellate Cells via TGF-beta-Independent Smad Signaling. Journal of Immunology. 2011;187:2814–2823. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, Bourne P, McClanahan TK, Pflanz S, de Waal Malefyt R. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, et al. Suppression of Interleukin-33 Bioactivity through Proteolysis by Apoptotic Caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Marvie P, Lisbonne M, L’Helgoualc’h A, Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Theret N, Gascan H, Piquet-Pellorce C, Samson M. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. Journal of Cellular and Molecular Medicine. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011 doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. P Natl Acad Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nature Reviews Rheumatology. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- Palmer G, Lipsky BP, Smithgall MD, Meininger D, Siu S, Talabot-Ayer D, Gabay C, Smith DE. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine. 2008;42:358–364. doi: 10.1016/j.cyto.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin AL, Mumm JB, Murphy E, Turner S, Yu N, McClanahan TK, Bourne PA, Pierce RH, Kastelein R, Pflanz S. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2010;184:1526–1535. doi: 10.4049/jimmunol.0903306. [DOI] [PubMed] [Google Scholar]
- Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends in Immunology. 2010a;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr., Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010b;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. Journal of Clinical Investigation. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 Exacerbates Eosinophil-Mediated Airway Inflammation. Journal of Immunology. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- Volarevic V, Mitrovic L, Milovanovic M, Zelen I, Nicolic I, Mitrovic S, Pejnovic N, Arsenijevic N, Lukic ML. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol. 2012;56:26–33. doi: 10.1016/j.jhep.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Wasmuth HE, Tacke F, Trautwein C. Chemokines in Liver Inflammation and Fibrosis. Seminars in Liver Disease. 2010;30:215–225. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- Weiler-Normann C, Herkel J, Lohse AW. Mouse models of liver fibrosis. Zeitschrift Fur Gastroenterologie. 2007;45:43–50. doi: 10.1055/s-2006-927387. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M, Finkelman FD. Untangling the Complex Web of IL-4-and IL-13-Mediated Signaling Pathways. Science Signaling. 2008;1 doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum IL-33 levels are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Clinical Rheumatology. 2011;30:825–830. doi: 10.1007/s10067-011-1686-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.