Abstract
Background
Saline-Adenine-Glucose-Mannitol (SAGM) and a variant solution, AS-1 have been used for over 30 years to preserve red blood cells (RBCs). Reputedly these RBC components have similar quality, although no paired study has been reported. To determine whether differences exist, a paired study of SAGM-RBCs and AS-1-RBCs was conducted to identify membrane changes, including microparticle (MP) quantitation and in vitro RBC-endothelial cell (EC) interaction.
Study Design and Methods
Two whole blood packs were pooled-and-split and RBCs prepared (n=6 pairs). One pack was suspended in SAGM and one in AS-1. Samples were collected during 42 days of refrigerated storage. RBC shape/size, glycophorin A (GPA)+ and phosphatidylserine (PS)+ MPs were measured by flow cytometry. RBC adhesion to ECs was determined by an in vitro flow perfusion assay. Routine parameters (pH, hemolysis) were also measured.
Results
Compared to SAGM-RBCs, AS-1-RBCs had lower hemolysis (p<0.04), lower GPA+ MPs (p<0.03) and lower PS+ MPs (p<0.03) from day 14 onwards. AS-1-RBCs had higher (p<0.02) side scatter from day 28 onwards, compared to SAGM-RBCs. SAGM-RBCs were more adherent to ECs at day 28 of storage compared to AS-1 RBCs (p=0.04), but reversed at day 42 (p=0.02). No significant differences in forward scatter or pH were found.
Conclusion
SAGM-RBCs lose more membrane during storage. SAGM-RBCs had increased adherence to ECs at day 28 of storage, while AS-1-RBCs were more adherent at day 42. The effect of these differences on the function and survival of SAGM-RBCs and AS-1-RBCs following transfusion remains to be determined.
INTRODUCTION
Red blood cell (RBC) transfusion is a critical, life-saving treatment for severe anemia caused by disease, trauma or major surgery or chemotherapy. Since the 1980's RBC components have been prepared as concentrates suspended in nutrient additive solution. In conjunction with refrigeration, storage of RBCs in the currently licensed additive solutions allows a shelf-life of up to six weeks.reviewed by 1,2 Nevertheless, during refrigerated storage RBCs undergo a complex and progressive accumulation of physicochemical changes, collectively referred to as the RBC storage lesion.reviewed by 2,3 These changes reduce the efficacy, and potentially the safety, of stored RBC components and dictate their allowed shelf-life.
Some reports from clinical studies have identified RBC transfusion as an independent risk factor for poorer outcomes of certain patient groups and some reports have suggested that older stored RBCs are more strongly correlated with poorer outcomes compared to fresher RBCs.4 Van de Watering5 noted that the concern about the “age of blood” has come from North American studies, rather than European studies, and suggested the existence of a “continental divide”.
A number of differences exist in the manufacturing processes of RBC components produced in North America and Europe. One obvious difference is the choice of RBC additive solutions. Many European blood centers use saline-adenine-glucosemannitol (SAGM) additive solution, whereas in the United States, SAGM is not licensed and other additive solutions are used (i.e. AS-1, AS-3 or AS-5).6 Reported by European researchers in 1981,7 SAGM is a modified version of the earlier SAG formulation supplemented with mannitol to help minimize hemolysis. SAGM was designed to be used for RBC components prepared by the buffy coat method, which was developed and widely implemented in Europe and more recently in Australia, New Zealand and Canada. At the same time that SAGM was being developed in Europe, researchers based in the USA also made modifications to the original SAG formulation by increasing the concentrations of adenine, glucose and mannitol (Table 1) to provide improved energy source and protection against hemolysis.8 The modified US formulation was termed AS-1 (commercial name Adsol, Baxter Healthcare, Deerfield, Illinois).
Table 1.
RBC additive solutions, SAGM and AS-1
| RBC additive solutions |
||
|---|---|---|
| SAGM (Pall) | AS-1 (Adsol; Baxter) | |
| Constituents (mM) | ||
| NaCl | 150 | 154 |
| Adenine | 1.25 | 2 |
| Dextrose (Glucose) | 45 | 111 |
| Mannitol | 30 | 41 |
|
| ||
| Physical properties | ||
| Volume (mL) | 100 | 100 |
| Osmolarity (mOsm)* | 376 | 462 |
| pH | 5.7 | 5.6 |
calculated
Because the formulations for SAGM and AS-1 are very similar, it has been widely assumed that any differences in the efficacy of RBC components stored in these additive solutions would be minimal and outweighed by other variables, such as processing and donor-related factors. Consequently, despite over 30 years of widespread use, few studies have directly compared the quality of RBCs stored in SAGM and AS-1. An unpaired study from Germany compared RBC quality parameters of buffy-coat depleted RBC components that had been stored in SAGM, AS-1 or AS-5 additive solutions for 35 days.9 The RBC components had been manufactured by five independent transfusion services, and the results indicated that this alone significantly contributed to the differences observed and consequently specific conclusions about the effect, if any, of the additive solutions could not be drawn. Two recent studies from India reported on the hemolysis levels in SAGM and AS-1 stored RBC components; however different blood processing procedures had been used for the SAGM packs and AS-1 packs, which made it impossible to distinguish between the effects of processing or the additive solution.10,11 The most solid evidence of differences between RBCs stored in SAGM and AS-1 additive solutions was reported by Hess and colleagues,12 who analysed large data sets of routine hemolysis quality control data and showed significantly higher hemolysis in RBCs stored in SAGM compared to AS-1. These results were from unpaired data collected over several years from a single blood center and although the same general processing practices were used, differences could not be excluded.
To our knowledge there are no published reports of a paired study of the in vitro quality parameters of RBCs stored in SAGM and AS-1 additive solution. Here we report our findings from a pool-and-split paired study specifically designed to compare SAGM and AS-1 stored RBCs. Together with standard in vitro RBC quality tests, additional parameters were measured that have been reported previously to be useful to discriminate RBC membrane changes.13 These tests included RBC size and shape by flow cytometric light scatter, quantitation of RBC microparticles (MPs) released into the supernatant and adhesion of stored RBCs to endothelium under continuous flow perfusion. Our findings revealed significant differences between RBCs stored in SAGM and AS-1 additive solution that may provide new insights into potential dissimilarities in the function and efficacy of SAGM RBCs and AS-1 RBCs following transfusion.
MATERIALS AND METHODS
Preparation of paired RBC components
RBC components were prepared according to standard procedures from whole blood (WB) collected from healthy volunteer donors (n = 12) attending the Australian Red Cross Blood Service, Melbourne. The study had institutional approval. The WB donors were all blood group O-positive, with a mean age of 45 ± 15 years (range 21 – 64 years; 2 female and 10 male donors) and a mean WB hemoglobin concentration of 147 ± 15 g/L. Briefly, WB (mean volume 458 ± 15 mL) was collected into standard blood collection packs fitted with an in-line WB-leukocyte depletion filter (WBF3; Pall Medical, Portsmouth, UK) and containing 70 mL ± 10% citrate-phosphate-dextrose anticoagulant. All WB packs were held at room temperature and processed within 2.7 ± 0.7 h after collection. A paired, pool-and-split study design was used. Two leukocyte-depleted WB packs were pooled, mixed and equally divided into the original collection packs. The paired WB packs were centrifuged at 5,000 x g for 10 min at room temperature and the RBCs were extracted into fresh packs by an automated blood component processor (Optipress II; Baxter Healthcare, Maurepas, France). One of the paired RBC packs was resuspended in 100 mL of SAGM (Pall) and the other paired RBC pack was resuspended in 100 mL of AS-1 (Adsol; Baxter, Jiutepec, Morelos, Mexico), by sterile-connecting the AS-1 solution pack on to the RBC storage pack (Pall). The RBC packs were stored according to standard blood banking conditions at 2 – 6 °C and 10 mL samples were collected aseptically at days 1, 14, 21, 28 and 42 of storage.
Routine RBC Quality Assessment
Residual leukocyte counts were determined on day 1 by an absolute bead count assay using flow cytometry (TruCount tubes, BD Biosciences, San Jose, CA). All RBC components met the Council of Europe guideline of less than 1 × 106 leukocytes/unit. On each day of sample collection full blood examinations were performed on an automated hematology analyzer (Cell Dyn 3200; Abbott, Santa Clara, CA) and extracellular pH was measured at 22 °C with a pH meter (PHM210; Radiometer, France). The RBC supernatant was collected following centrifugation at 5,000 x g for 5 min at 4 °C and was used to determine the level of hemolysis and microparticle content (see below). The level of supernatant hemoglobin (Hb) was determined by a low Hb analyser (Hemocue, Angelholm, Sweden) and percentage (%) hemolysis was calculated according to standard procedures.
RBC size and shape by flow cytometry
RBC size and shape were determined using flow cytometric light scatter (FACSCanto II, BD Biosciences) as described previously.13 Briefly, samples of stored RBCs were diluted in phosphate-buffered saline containing bovine serum albumin (BSA) (0.5% w/v). A logarithmic forward scatter (FSC) versus side scatter (SSC) plot was used to set a region around the RBC population and 20,000 gated events were collected. The mean FSC and SSC values were used as measures of the relative size and shape/cell surface unevenness, respectively.
Microparticle (MP) quantitation
The number of MPs in the supernatant of the stored RBC packs was quantitated by a flow cytometric absolute bead count assay essentially as previously described.14 Glycophorin A (GPA)+ MPs were quantitated by mixing RBC supernatant (25 μL) with 1 μL of phycoerythrin (PE)-conjugated anti-CD235a (anti-GPA; clone GA-R2 (HIR2)), or matched PE-conjugated IgG2b isotype control (both from BD Biosciences) at the same immunoglobulin concentration, in an absolute count tube (TruCount tubes, BD Biosciences) and adjusted to 50 μL reaction volume. Phosphatidylserine (PS)+ MPs were quantitated by mixing RBC supernatant (10 μL) with 5 μL of allophycocyanin (APC)-conjugated annexin V (BD Biosciences) and adjusted to 100 μL reaction volume with annexin V binding buffer (BD Biosciences). Following incubation, the final volume was adjusted to 300 μL with the same buffer used for labelling and the samples were analyzed immediately by flow cytometry. A total of 10,000 bead events were collected. The number of MPs/μL of supernatant was calculated according to the manufacturer's instructions for the absolute count tubes.
RBC adhesion and strength of adhesion to endothelial cells
Adhesion of stored RBCs to endothelial cells (ECs) under continuous flow perfusion to simulate in vivo microvascular blood flow was performed essentially as described previously.15,16 Briefly, primary human umbilical vein ECs were cultured to confluence on gelatin-coated glass coverslips and mounted into a microvolume perfusion chamber. The ECs were perfused with a 1.5 % (v/v) suspension of stored RBCs suspended in M-199 medium supplemented with 1 % (final concentration) human serum albumin at a shear stress of 0.5 dyne/cm2 at 37 °C for 5 min. Perfusion was visualized with an inverted microscope and recorded by a CCD camera. Following wash-out with perfusion medium for 5 min, the number of adhered RBCs was determined by scoring 15 randomly selected fields. Strength of adhesion of RBCs was determined by increasing the shear stress of the wash-out perfusion to 3 dyne/cm2 and re-scoring 15 randomly selected fields. Adherent RBCs were defined as cells that remained tethered to the EC layer for at least 20 sec under continuous flow perfusion. The results were calculated as the mean number of adherent RBCs/mm2.
Statistical analysis
Results presented are mean ± standard deviation (SD), unless stated otherwise. The two-tailed paired Student's t-test was used to determine statistical difference between the SAGM and AS-1 groups. Repeated measures analysis of variance (RM-ANOVA) with Holm-Sidak or Tukey post-hoc tests were used to determine the effect of storage within groups. Statistical analysis software (SigmaStat Version 3.0; Systat Software, Richmond, CA) was used. Significance was defined as p < 0.05.
RESULTS
Routine RBC component quality parameters
There were no significant differences in the physical parameters of the SAGM and AS-1 RBCs at the beginning of storage, except that AS-1 RBCs had significantly larger mean cell volume (MCV) compared to the paired SAGM RBCs (p = 0.001) (Table 2). Consequently AS-1 RBCs had significantly lower mean cell hemoglobin concentration (MCHC) at day 1 compared to the paired SAGM RBCs (p = 0.0002).
Table 2.
Parameters of paired SAGM and AS-1 RBC components at Day 1
| Parameter | Paired RBC components (n = 6) |
p value | |
|---|---|---|---|
| SAGM | AS-1 | ||
| RBC (x1012/L) | 6.91 ± 0.46 | 6.83 ± 0.43 | ns |
| Hb (g/L) | 204 ± 8 | 202 ± 6 | ns |
| HCT (L/L) | 0.618 ± 0.020 | 0.621 ± 0.021 | ns |
| MCV (fL) | 89.6 ± 3.3 | 91.1 ± 3.5 | 0.001 |
| MCHC | 331 ± 10 | 324 ± 9 | 0.0002 |
| Hemolysis (%) | 0.019 ±0.012 | 0.012 ± 0.015 | ns |
| PH | 7.26 ± 0.04 | 7.26 ±0.03 | ns |
|
| |||
| Mean ± SD | |||
ns; not significant
pH – similar for SAGM and AS-1 RBCs
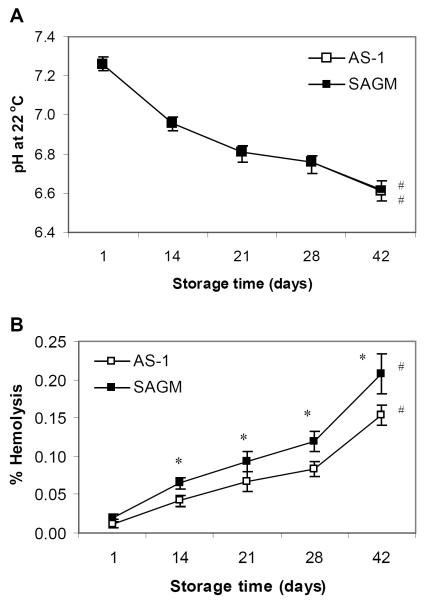
All the RBC components maintained acceptable routine quality parameters throughout 42 days storage. As expected, the extracellular pH of SAGM RBCs and AS-1 RBCs significantly declined during 42 days storage (RM-ANOVA, p < 0.0001) (Fig 1A), however all RBC components remained above the minimum acceptance limit of pH 6.5. There was no significant difference in the pH of SAGM RBCs and AS-1 RBCs.
Figure 1.
Routine quality parameters of paired RBC components stored refrigerated in SAGM or AS-1 additive solutions for 42 days. (A) pH and (B) % hemolysis. SAGM (closed symbol); AS-1 (open symbol). * Significant difference (p < 0.04) between SAGM RBCs and AS-1 RBCs, by paired t-test. # Significant change (p < 0.001) across storage period, by RM-ANOVA. Results are mean ± SD (n = 6 pairs).
Hemolysis - lower for AS-1 RBCs
As expected, hemolysis of SAGM RBCs and AS-1 RBCs progressively increased during 42 days storage (RM-ANOVA, p < 0.001) (Fig 1B). The level of hemolysis of all the RBC components remained below the maximum acceptance limit of 0.8 percent stated by the Council of Europe guidelines,17 however AS-1 RBCs had significantly lower hemolysis from day 14 onwards compared to SAGM RBCs (p < 0.04).
RBC size and shape differs
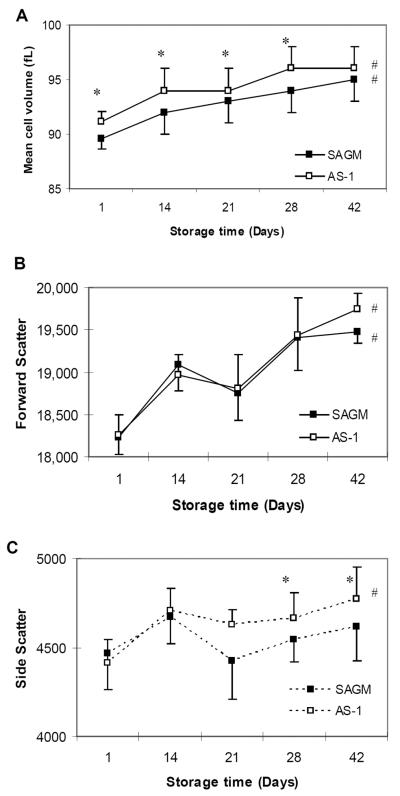
MCV - higher for AS-1 RBCs
The MCV of AS-1 RBCs remained higher compared to SAGM RBCs throughout 42 days storage and was significantly higher from day 1 to day 28 (p < 0.01) (Fig. 2A). The MCV of SAGM RBCs and AS-1 RBCs progressively increased during storage (RM-ANOVA, p < 0.001).
Figure 2.
Size and shape of RBCs stored in SAGM or AS-1 additive solutions. RBC mean cell volume (MCV) was calculated by an automated hematology analyzer (A). The change in arbitrary size of the RBCs during storage was measured by flow cytometric forward light scatter (FSC) (B); and the change in unevenness of the RBC surface membrane during storage was measured by flow cytometric side scatter (SSC) (C). SAGM (closed symbol); AS-1 (open symbol). * Significant difference (p < 0.02) between SAGM RBCs and AS-1 RBCs, by paired t-test. # Significant change (p < 0.001) across storage period, by RM-ANOVA. Results are mean ± standard error of the mean (n = 6 pairs).
Forwards Scatter – similar for SAGM and AS-1 RBCs
The flow cytometric FSC profile showed a progressive increase in the size of SAGM and AS-1 RBCs during 42 days storage (RM-ANOVA, p < 0.006) (Fig 2B). There were no significant differences in the FSC profile of SAGM and AS-1 RBCs. These results are consistent with the MCV data (Fig 2A) and indicate that the RBCs swelled during storage.
Side Scatter - greater for AS-1 RBCs
The flow cytometric 90 degree angle SSC of AS-1 RBCs significantly increased during 42 days storage (RM-ANOVA, p = 0.001), but not for SAGM RBCs (Fig 2C). The increased SSC profile of AS-1 RBCs was significantly different compared to SAGM RBCs at day 28 and day 42 of storage (p < 0.02). These results suggest that the cell surface of AS-1 RBCs became more irregular during storage, whereas the cell surface of SAGM RBCs was comparatively smoother.
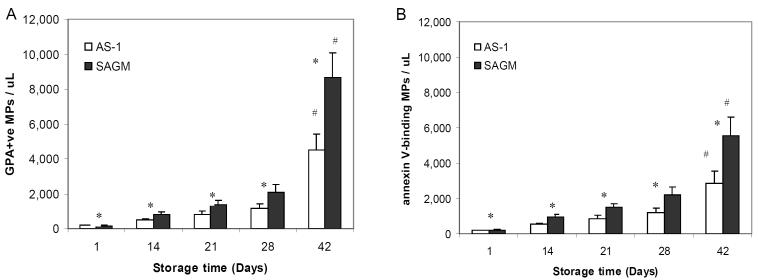
Accumulation of MPs in the supernatant - lower for AS-1 RBCs
From day 14 of storage onwards, the supernatant from SAGM RBCs contained significantly greater numbers of GPA+ MPs and annexin V-binding MPs compared to the supernatant from AS-1 RBCs (p < 0.03) (Fig 3A and B respectively). An exponential increase in the numbers of MPs was seen between day 28 and day 42. SAGM RBCs had a 61-fold increase in the number of GPA+ MPs shed into the supernatant at day 42 of storage compared to day 1, whereas AS-1 RBCs had a 24-fold increase (RM-ANOVA p < 0.001 for both) (Fig 3A). Similarly, SAGM RBCs had a 25-fold increase in the number of annexin V-binding MPs shed into the supernatant at day 42 of storage compared to day 1, whilst AS-1 RBCs had a 14-fold increase (RM-ANOVA, p < 0.001 for both) (Fig 3B).
Figure 3.
Accumulation of MPs in the supernatant of RBCs stored in SAGM or AS-1 additive solutions. MPs were quantitated by flow cytometric absolute bead count assay. Glycophorin A+ (GPA) MPs were detected by staining with PE-conjugated anti-CD235a (A); and MPs with exposed phosphatidylserine were determined by binding of APC-conjugated annexin V (B). SAGM (closed bars); AS-1 (open bars). *Significant difference (p < 0.03) between SAGM RBCs and AS-1 RBCs, by paired t-test. # Significant change (p < 0.001) across storage period, by RM-ANOVA. Results are mean ± standard error of the mean (n = 6 pairs).
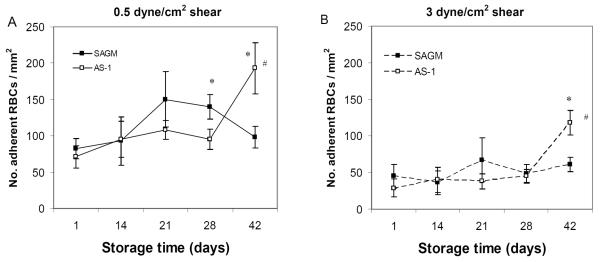
RBC adhesion to ECs differs
Under conditions of continuous flow perfusion designed to mimic microvascular blood flow (i.e. at a shear stress of 0.5 dyne/cm2), the adhesion of AS-1 RBCs to ECs significantly increased with longer RBC storage duration (RM-ANOVA p < 0.001), with a notable marked increase between day 28 and day 42 (p = 0.03) (Fig 4A). In contrast, SAGM RBCs reached a maximum level of adhesiveness earlier and were significantly more adherent at day 28 of storage compared to AS-1 RBCs (p = 0.04) (Fig 4A). After day 28, the adhesion profile reversed and AS-1 RBCs showed a marked increase in adhesiveness that was significantly higher compared to SAGM RBCs (p= 0.025).
Figure 4.
Adhesion of RBCs stored in SAGM or AS-1 additive solutions to ECs under continuous flow conditions. RBCs were perfused across ECs at a shear stress of 0.5 dyne/cm2 (A). Strength of adhesion of RBCs to ECs was determined by increasing the shear stress to 3 dyne/cm2 and the number of remaining adherent RBCs was scored (B). SAGM (closed symbol); AS-1 (open symbol). * Significant difference (p < 0.04) between SAGM RBCs and AS-1 RBCs, by paired t-test. # Significant change (p < 0.002) across storage period, by RM-ANOVA. Results are mean ± standard error of the mean (n = 6 pairs).
Strength of RBC adhesion to ECs differs
Strength of RBC adhesion to ECs was determined by scoring the number of RBCs that remained adherent after the flow shear stress was raised to 3 dyne/cm2 from 0.5 dyne/cm2. As expected, the number of RBCs that remained adherent at 3 dyne/cm2 shear stress was significantly less than at 0.5 dyne/cm2 (p < 0.05) (Fig 4B). However, the adhesion profile of SAGM RBCs and AS-1 RBCs across the 42 day storage period was similar at 3 dyne/cm2 as seen at 0.5 dyne/cm2 shear stress. At day 42 of storage, AS-1 RBCs were significantly more adherent at 3 dyne/cm2 compared to SAGM RBCs (p= 0.02) (Fig 4B).
DISCUSSION
The results reported here suggest that the manifestation of storage-related changes may be different for RBCs preserved in SAGM or AS-1 additive solutions. In particular, differences in membrane-related changes of SAGM RBCs and AS-1 RBCs were identified, including RBC size and shape, loss of RBC membrane via vesiculation, hemolysis, and interaction of RBCs with endothelium under continuous flow perfusion.
The apparent increased MCV of RBCs suspended in hyper-osmotic AS-1 solution compared to SAGM RBCs was contrary to expected. The MCV was determined by an automated hematology analyzer. The automated sample preparation includes dilution of the RBCs in a proprietary diluent. The diluent may affect the apparent shape and size of RBCs suspended in different additive solutions. Measurement of RBC shape and size by flow cytometric light scatter in which the RBC samples were diluted in physiological PBS-albumin did not identify significant differences in the shape and size of SAGM RBCs and AS-1 RBCs until later storage times.
Our findings confirmed a previous report from an unpaired study12 that AS-1 RBCs have significantly lower hemolysis compared to RBCs stored in SAGM. Consistent with this was our finding that AS-1 RBCs had significantly lower levels of vesiculation compared to SAGM RBCs. These results support the premise that the modest increased concentrations of mannitol and glucose in AS-1 additive solution help to protect the RBC from excessive loss of membrane and eventual hemolysis.8,12 Vesiculation occurs during normal RBC aging and is thought to be a mechanism by which RBCs rid themselves of damaged or potentially toxic constituents, such as externalised PS, oxidized lipids and aggregated proteins, thereby protecting the RBC from premature senescence and clearance from the circulation.18,19 It is not known whether the array of proteins and lipids contained in MPs shed during refrigerated storage of RBC components is similar to those shed by RBCs during normal aging in vivo. Nevertheless, our finding of significantly greater numbers of MPs shed during storage of SAGM RBCs compared to AS-1 RBCs may provide important insight into potential differences between these RBC components when transfused. Other investigators have reported that MPs from stored AS-1 RBCs,20 and SAGM RBCs,21 promote thrombin generation. To our knowledge no studies have directly compared the procoagulant potential of AS-1 RBCs and SAGM RBCs.
MPs shed by RBCs during storage contain Hb,22–24 and have been shown to be potent scavengers of nitric oxide (NO),25 an important vasodilator for increased vascular relaxation and blood flow. Using rodent in vivo vasoactivity models, supernatant from stored RBCs has been shown to induce significant vasoconstriction that correlated with heme concentration and NO scavenging.25,26 It was postulated that the ferrous oxyhemoglobin encapsulated within RBC MPs would be the major source of vasoconstrictive activity rather than free heme in the RBC supernatant, which would be rapidly cleared from the circulation by haptoglobin.25 In addition to potential hemostatic and vascular effects, MPs from stored RBC components have been reported to suppress monocyte function,27,28 whilst on the other hand induced activation of neutrophils.29 The effect of the additive solution used was not specifically addressed in these published reports. Further investigations are warranted to determine whether different additive solutions influence the bioactivity of MPs generated during storage of RBC components.
The lower rate of vesiculation by AS-1 RBCs, and thus the retention of membrane, suggests that storage-related membrane changes progress at a slower rate or are different to those that occur to SAGM RBCs. Consistent with this notion was the increased SSC of AS-1 RBCs, which suggested that AS-1 RBCs had a more uneven cell surface compatible with echinocytic shape changes. Differences in the retention of RBC membrane may be associated with the different profiles of RBC-EC adhesion reported here for AS-1 RBCs and SAGM RBCs. SAGM RBCs were more adherent up to day 28 compared to AS-1 RBCs, after which AS-1 RBCs became significantly more adherent.
Adhesion of RBCs to vascular endothelium can disturb blood flow, decrease oxygen delivery to the organs and peripheral tissues, and in severe cases, cause vascular occlusion.30,31 The mechanisms of adhesion of stored RBCs to ECs and the identity of the ligands involved are yet to be elucidated. It is likely that adhesion of stored RBCs to ECs can be induced by more than one mechanism and the predominant mechanism may be influenced variously by the length of storage and storage conditions (i.e. additive solutions) of the RBC components.32 Externalized PS on RBCs has been suggested to be a mechanism of adherence of stored RBCs to ECs,33 although in our hands PS exposure does not account for all adherent RBCs (unpublished observations). Burger and colleagues34 have shown that overnight 37 °C incubation of stored SAGM RBCs induced the exposure of PS and vesiculation and suggested that a similar phenomenon could occur upon transfusion of stored RBCs. Whether differences exist between SAGM RBCs and AS-1 RBCs in the extent of exposure of PS or vesiculation when subjected to 37 °C has not been reported. Zhu and colleagues31 reported that storage of RBCs inhibited the release of RBC-derived ATP, a vasodilator, and thereby promoted the adhesion of stored RBCs to endothelium. Other investigators have proposed that transfusion of stored RBCs can exacerbate endothelial dysfunction through increased NO scavenging and iron-mediated oxidative damage by RBCs.26,35–38 Further studies are needed to investigate the relationship of stored RBCs and endothelial adherence and the influence of RBC component manufacture.
The effect, if any, of the in vitro differences reported here between SAGM RBCs and AS-1 RBCs on their in vivo function following transfusion is unknown. However, it is noteworthy that the concern about the “age of blood” has come predominantly from North American clinical studies,5 where SAGM is not used. The definition of “fresh” and “old” blood is a matter of conjecture and is different between published “age of blood” clinical studies.4 The results reported here suggest that SAGM RBCs may undergo a somewhat accelerated or different storage lesion compared to AS-1 RBCs, which could mean that in order to observe an “age of blood” effect, if it exists, in a clinical study of SAGM RBC transfusion may require “fresh” RBCs to be defined as less than 7 days of storage. Other differences in the manufacture of RBC components in the USA and Europe, such as centrifugation conditions, amount of residual plasma, average whole blood hold time, type of leukocyte-reduction filtration (i.e. filter types, whole blood or component filtration), may contribute to the “continental divide” in the outcome of the reported “age of blood” clinical studies. Manufacturing details are rarely provided in reported clinical studies of RBC transfusion. A recommendation from our findings is that the manufacturing details of RBC components should be included in the published reports of RBC transfusion clinical studies to enable valid comparisons of study outcomes to be made.
In conclusion, using a paired study design and tests that examined RBC membrane integrity and interaction of stored RBCs with ECs, we have reported here new findings that demonstrate significant differences between RBCs stored in SAGM and its variant solution, AS-1. To our knowledge this is the first report of a paired comparison of the in vitro parameters of RBCs stored in SAGM and AS-1, despite the fact that these additive solutions have been in wide use for over 30 years. Further studies are warranted to determine whether the differential membrane changes during storage of SAGM-RBCs and AS-1-RBCs results in differences in their in vivo function.
ACKNOWLEDGEMENTS
We thank the Donor Services and Processing laboratory staff at Australian Red Cross Blood Service, Melbourne for assistance with the collection and processing of whole blood packs and the staff at the BMDI Cord Blood Bank, Melbourne for umbilical cords for EC preparation.
Funding support: Australian governments fully fund the Australian Red Cross Blood Service for the provision of blood products and services to the Australian community. This work was funded in part by NIH Grant 1R01 HL095470-01A1.
Abbreviations
- FSC
forward scatter
- GPA
glycophorin A
- MP
microparticle
- PS
phosphatidylserine
- SSC
side scatter
- WB
whole blood
Footnotes
Disclosures: No conflict of interest to disclose.
REFERENCES
- 1.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91:13–9. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 2.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–59. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Högman CF, Meryman HT. Storage parameters affecting red blood cell survival and function after transfusion. Transfus Med Rev. 1999;13:275–96. doi: 10.1016/s0887-7963(99)80058-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Watering Red cell storage and prognosis. Vox Sang. 2011;100:36–45. doi: 10.1111/j.1423-0410.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 6.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10(Suppl 2):s7–11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Högman CF, Hedlund K, Sahleström Y. Red cell preservation in protein-poor media. III. Protection against in vitro hemolysis. Vox Sang. 1981;41:274–81. doi: 10.1111/j.1423-0410.1981.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 8.Heaton A, Miripol J, Aster R, Hartman P, Dehart D, Rzad L, Grapka B, Davisson W, Buchholz DH. Use of Adsol® preservation solution for prolonged storage of low viscosity AS-1 red blood cells. Br J Haematol. 1984;57:467–78. doi: 10.1111/j.1365-2141.1984.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann R, Heidenreich D, Weisbach V, Zingsem J, Neidhardt B, Eckstein R. In vitro quality control of red blood cell concentrates outdated in clinical practice. Transfus Clin Biol. 2003;10:275–83. doi: 10.1016/s1246-7820(03)00032-6. [DOI] [PubMed] [Google Scholar]
- 10.Sawant RB, Jathar SK, Rajadhyaksha SB, Kadam PT. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007;1:47–51. doi: 10.4103/0973-6247.33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makroo RN, Raina V, Bhatia A, Gupta R, Majid A, Thakur UK, Roamma NL. Evaluation of the red cell hemolysis in packed red cells during processing and storage. Asian J Transfus Sci. 2011;5:15–7. doi: 10.4103/0973-6247.75970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess JR, Sparrow RL, van der Meer PF, Acker JP, Cardigan RA, Devine DV, BEST Collaborative Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion. 2009;49:2599–603. doi: 10.1111/j.1537-2995.2009.02275.x. [DOI] [PubMed] [Google Scholar]
- 13.Veale MF, Healey G, Sparrow RL. Effect of additive solutions on red blood cell (RBC) membrane properties of stored RBCs prepared from whole blood held for 24 hours at room temperature. Transfusion. 2011;51:25S–33S. doi: 10.1111/j.1537-2995.2010.02960.x. [DOI] [PubMed] [Google Scholar]
- 14.Sparrow RL, Chan KS. Microparticle content of plasma for transfusion is influenced by the whole blood hold conditions: pre-analytical considerations for proteomic investigations. J Proteomics. 2012;76:211–9. doi: 10.1016/j.jprot.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anniss AM, Sparrow RL. Storage duration and leukocyte content of red cell products increases adhesion of stored red blood cells to endothelium under flow conditions. Transfusion. 2006;46:1561–7. doi: 10.1111/j.1537-2995.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 16.Anniss AM, Sparrow RL. Variable adhesion of different red blood cell products to activated vascular endothelium under flow conditions. Am J Hematol. 2007;82:439–45. doi: 10.1002/ajh.20837. [DOI] [PubMed] [Google Scholar]
- 17.Council of Europe . Guide for the preparation, use and quality assurance of blood components. 16th Council of Europe Publishing; Strasbourg, Cedex: 2010. [Google Scholar]
- 18.Willekens FLA, Werre JM, Groenen-Döpp YAM, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJCGM. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 19.Bosman GJCGM, Were JM, Willekens FLA, Novotny VMJ. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney J, Kouttab N, Kurtis J. Stored red blood cell supernatant facilitates thrombin generation. Transfusion. 2009;49:1569–79. doi: 10.1111/j.1537-2995.2009.02196.x. [DOI] [PubMed] [Google Scholar]
- 21.Rubin O, Delobel J, Prudent M, Lion N, Kohl K, Tucker EI, Tissot JD, Angelillo-Scherrer A. Red blood cell–derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2012 Dec 11; doi: 10.1111/trf.12008. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 22.Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46:143–52. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 23.Kriebardis AG, Antonelou MH, Stamolulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 24.Bosman GJCGM, Lasonder E, Luten M, Roerdinkholder-Stoelwinder B, Novotný VMJ, Bos H, De Grip WJ. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 25.Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012;52:1410–22. doi: 10.1111/j.1537-2995.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leuk Biol. 2008;84:1316–25. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 28.Ozment CP, Mamo LB, Campbell ML, Lokhnygina Y, Ghio AJ, Turi JL. Transfusion-related biologic effects and free hemoglobin, heme, and iron. Transfusion. 2013;53:732–40. doi: 10.1111/j.1537-2995.2012.03837.x. [DOI] [PubMed] [Google Scholar]
- 29.Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards MJ, Caldwell CC, Lentsch AB, Pritts TA. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg. 2012;214:648–57. doi: 10.1016/j.jamcollsurg.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somani A, Steiner ME, Hebbel RP. The dynamic regulation of microcirculatory conduit function: Features relevant to transfusion medicine. Transfus Aph Sci. 2010;43:61–8. doi: 10.1016/j.transci.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5'-triphosphate release from red blood cells promotes their adhesion to endothelial cells: A mechanism of hypoxemia after transfusion. Crit Care Med. 2011;39:2478–86. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spinella PC, Sparrow RL, Hess JR, Norris PJ. Properties of stored RBCs: Understanding immune and vascular reactivity. Transfusion. 2011;51(4):894–900. doi: 10.1111/j.1537-2995.2011.03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking- induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–46. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 34.Burger P, Kostova E, Bloem E, Hilarius-Stokman P, Meijer AB, van den Berg TK, Verhoeven AJ, de Korte D, van Bruggen R. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br J Haematol. 2013;160:377–86. doi: 10.1111/bjh.12133. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman GA. Hypoxic erythrocytes spark lung leukocyte adhesion. Blood. 2008;111:4831–2. doi: 10.1182/blood-2008-02-140947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roback JD. Vascular effects of the red blood cell storage lesion. Hemat Am Soc Hemat Educ Program. 2011;2011:475–9. doi: 10.1182/asheducation-2011.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapley R, Owusu BY, Brandon A, Cusick M, Rodriguez C, Marques MB, Kerby JD, Barnum SR, Weinberg JA, Lancaster JR, Patel RP. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. Biochem J. 2012;446:499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon SB, Wang D, Sun J, Kanias T, Feng J, Helms CC, Solomon MA, Alimchandani M, Quezado M, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–72. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]